Abstract
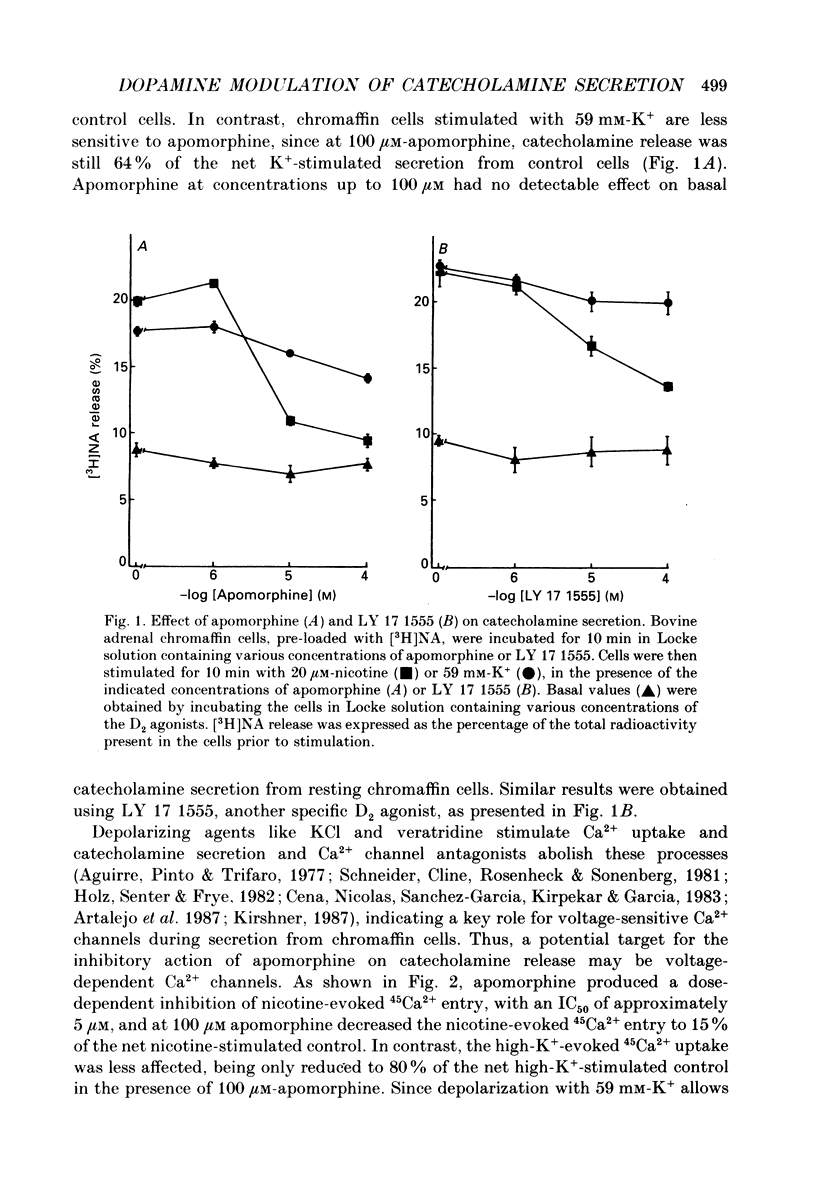
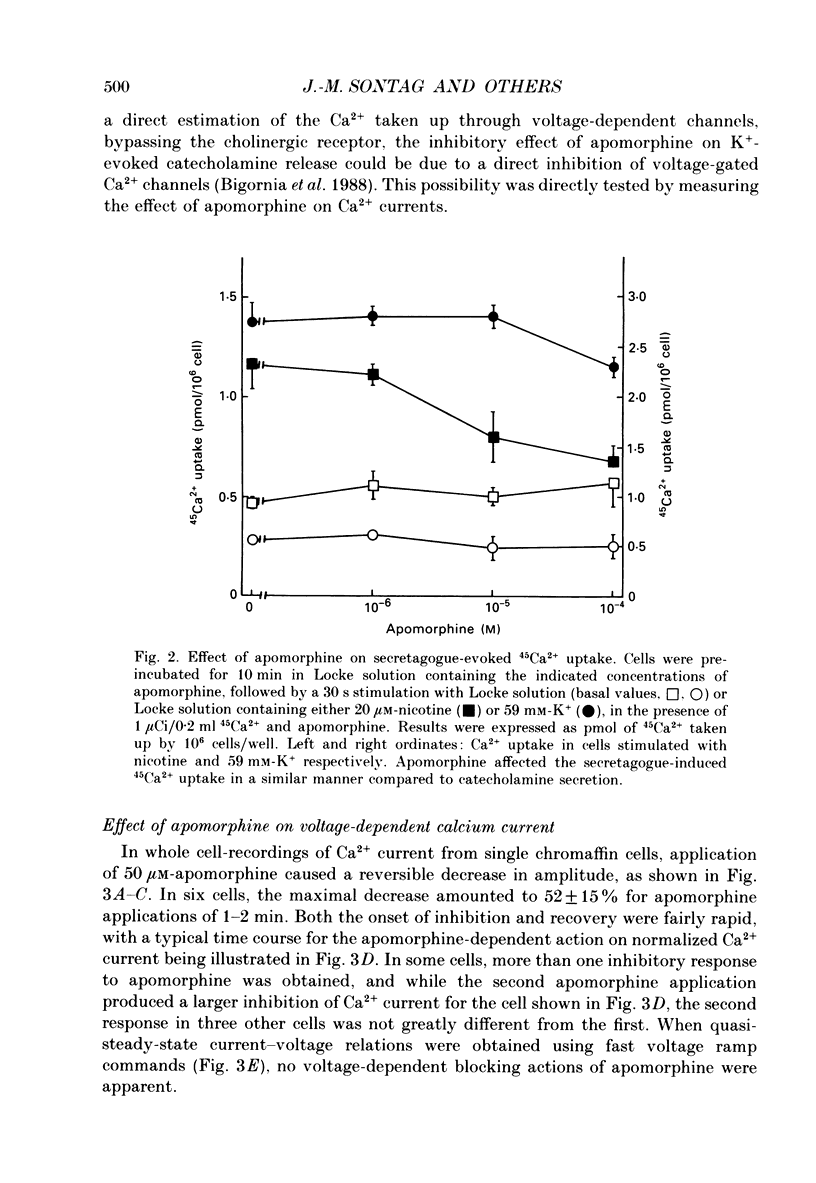
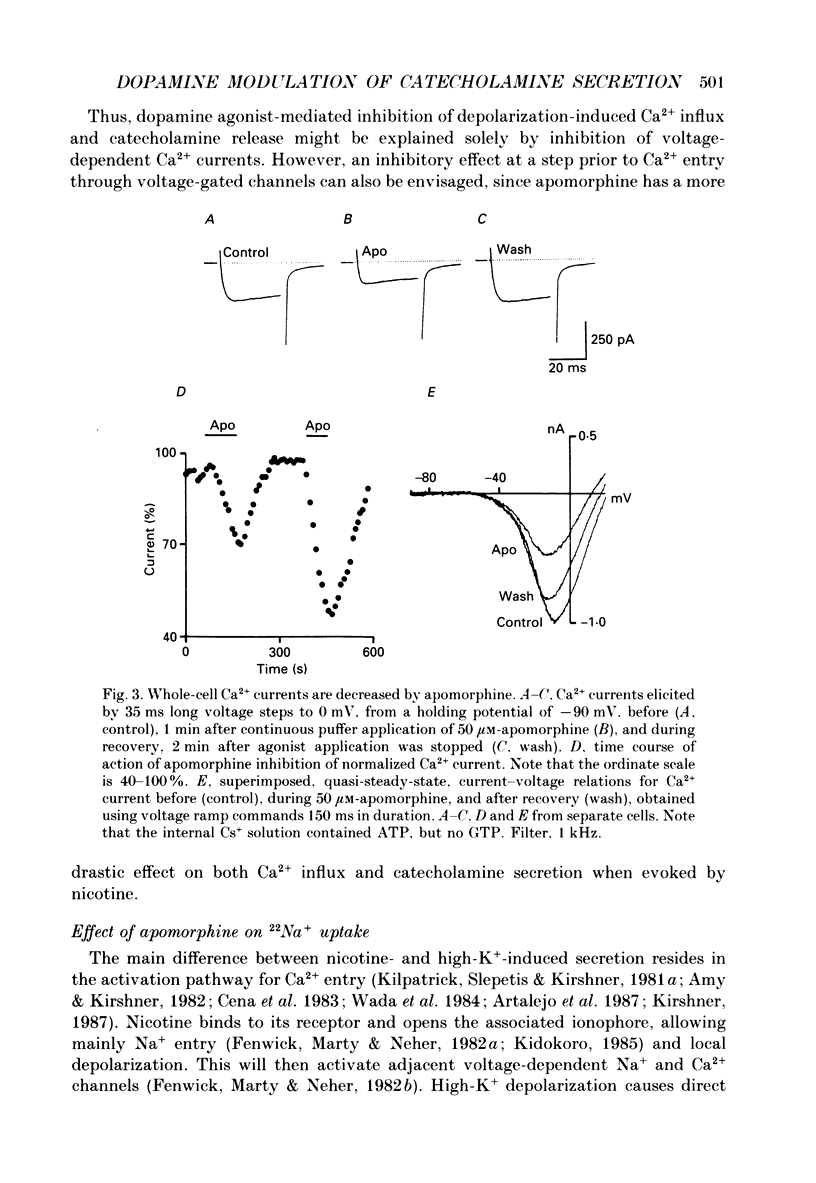
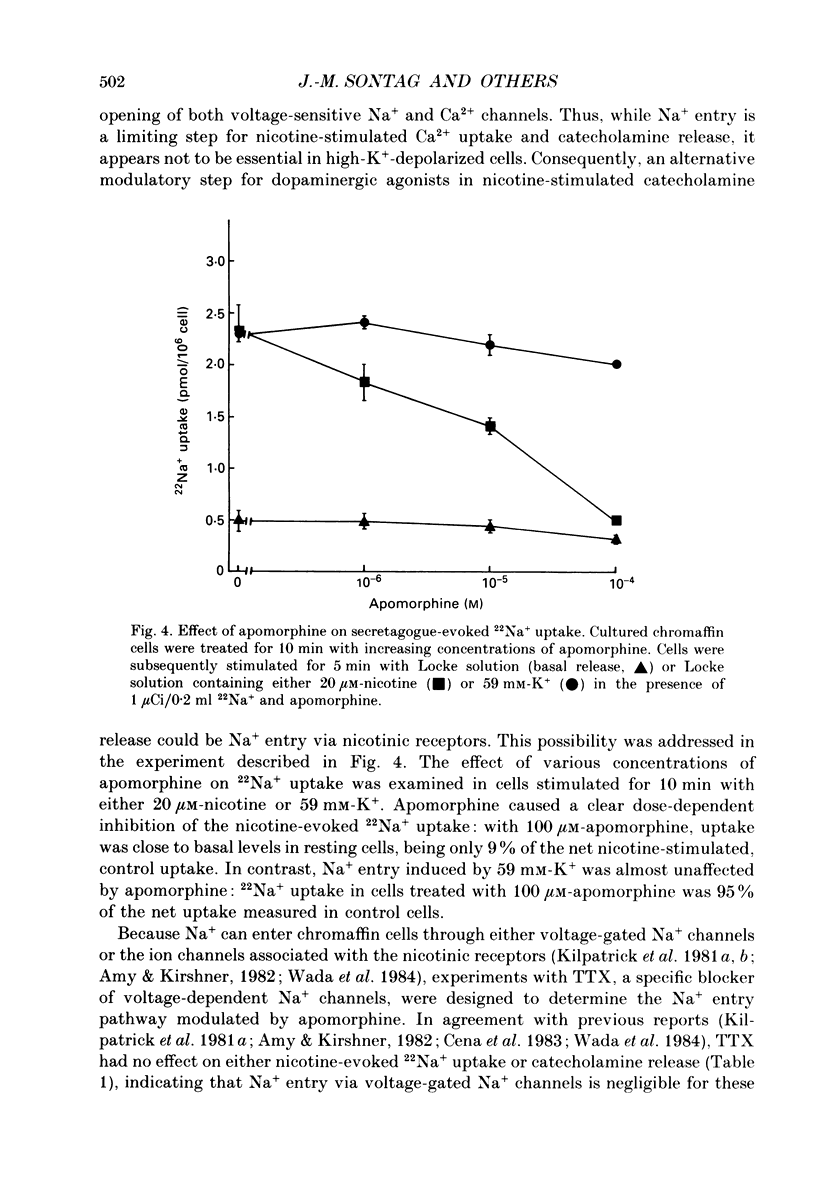
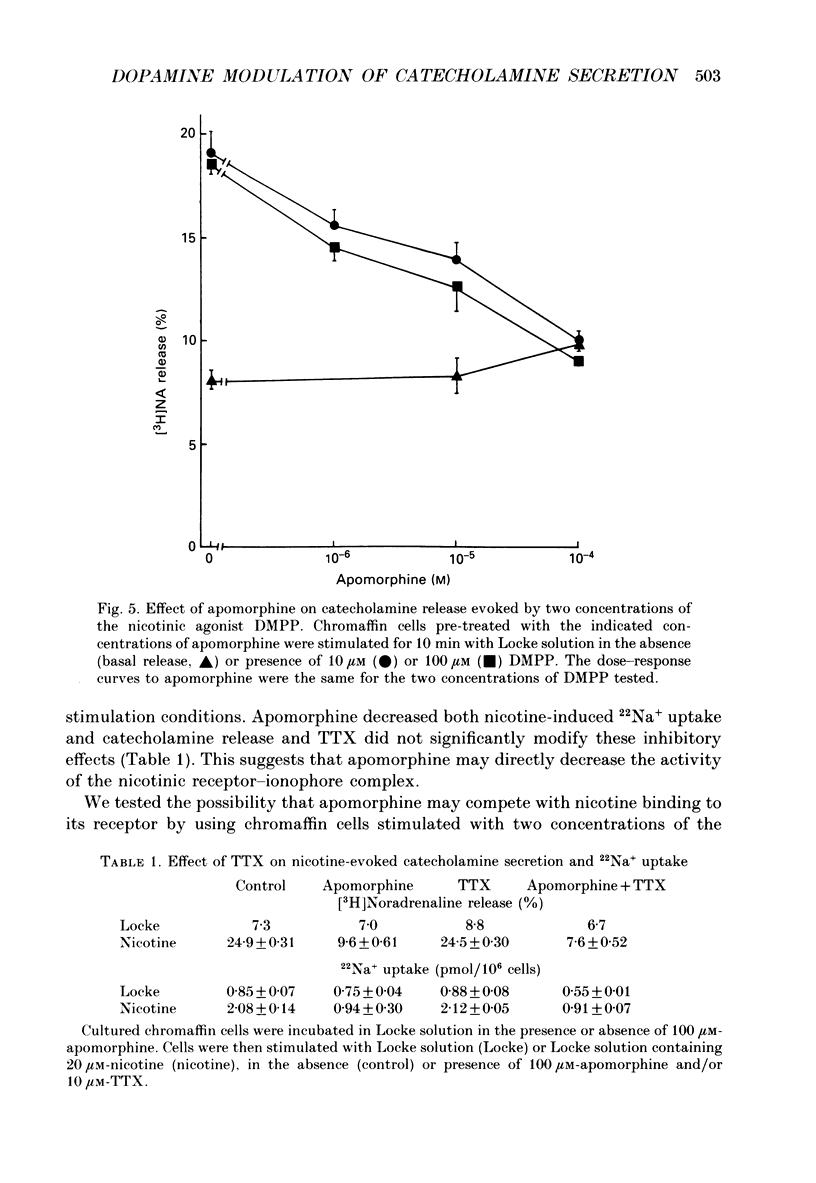
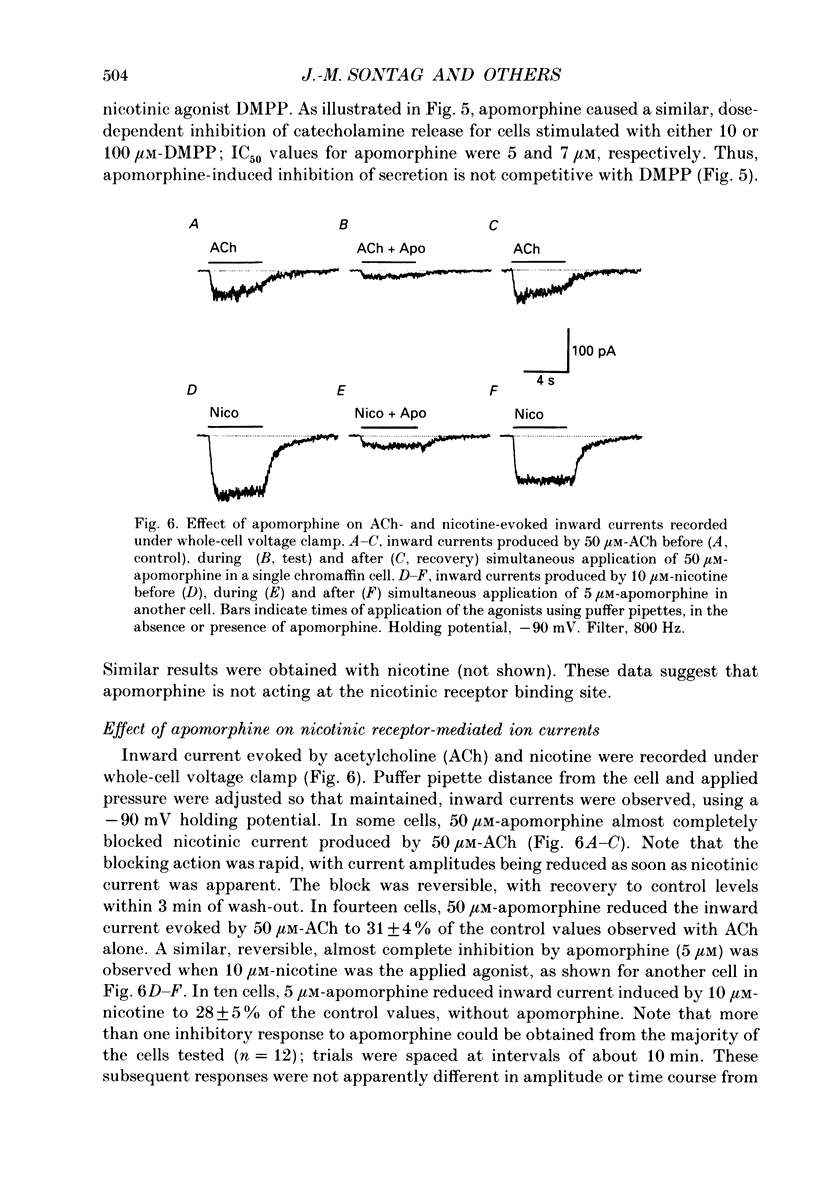
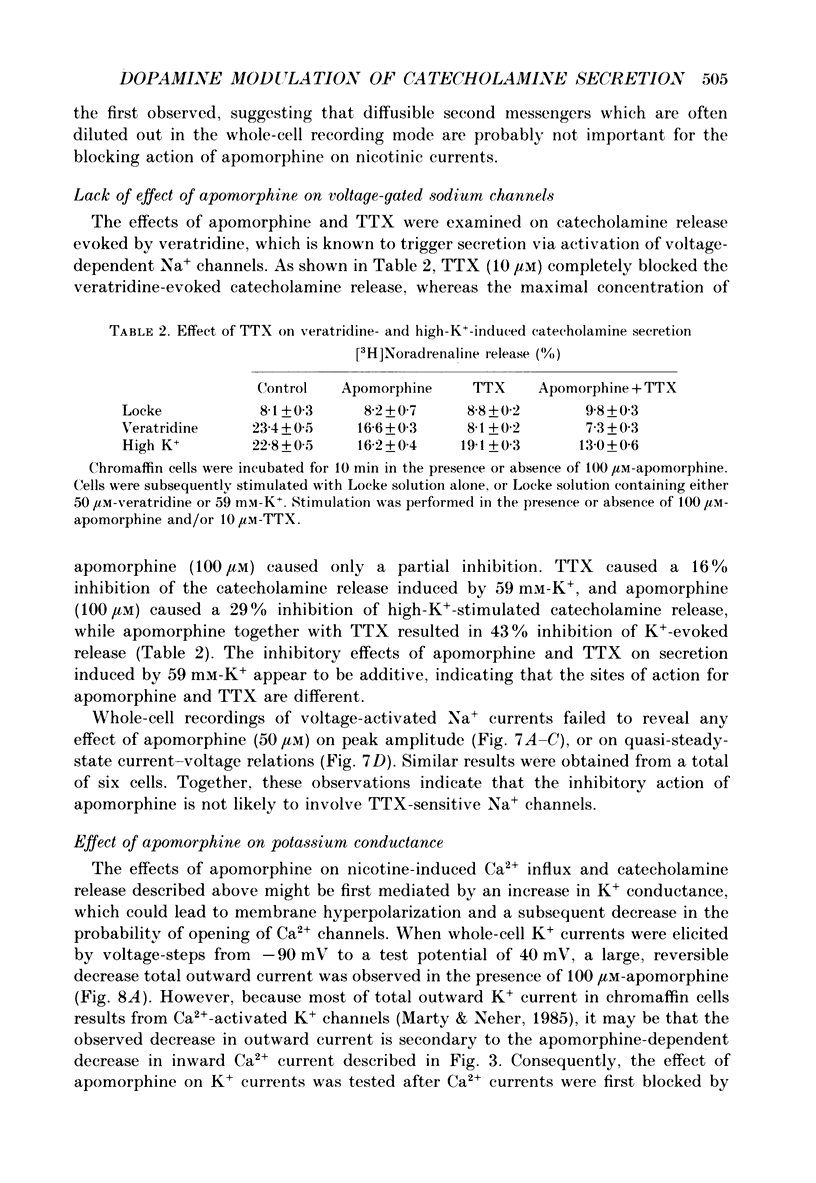
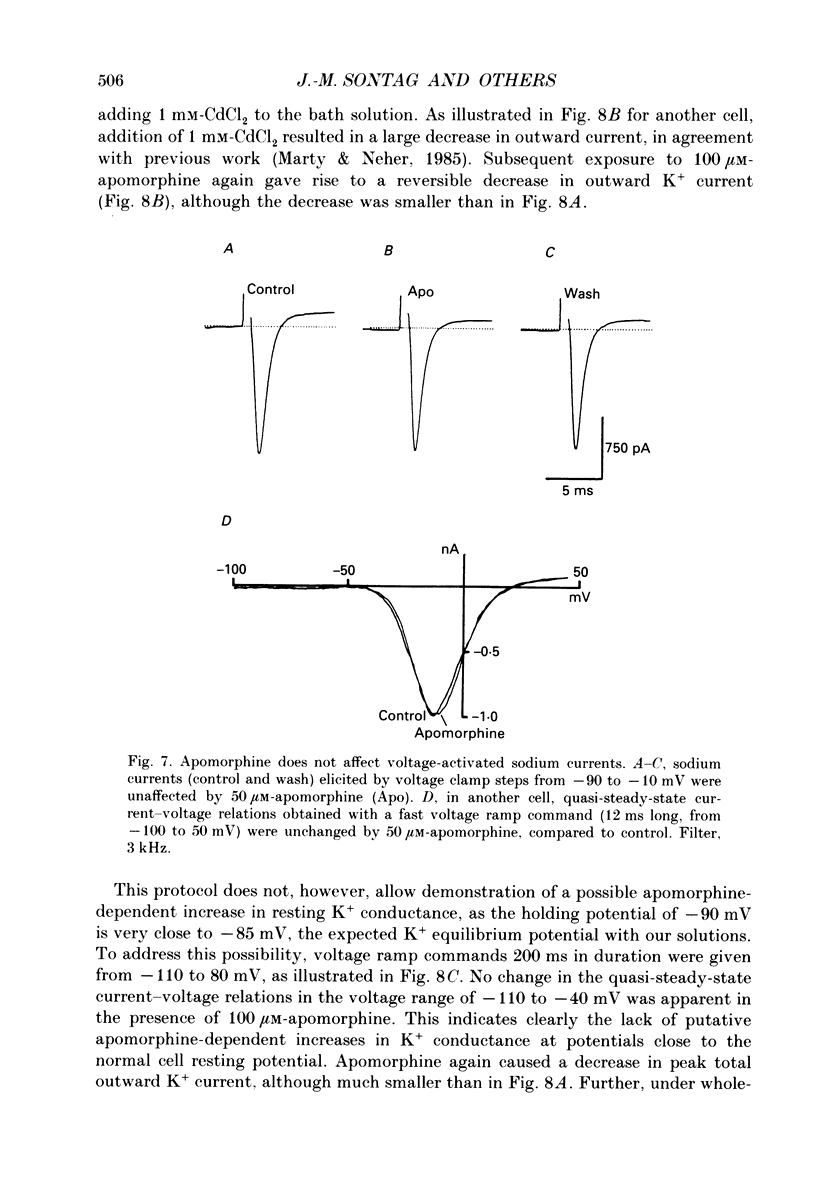
1. Catecholamine secretion from cultured bovine adrenal chromaffin cells was decreased in a dose-dependent manner by the D2 dopamine agonists apomorphine and LY 17 1555. 2. 45Ca2+ uptake was similarly inhibited and whole-cell Ca2+ currents were reduced by apomorphine. 3. These inhibitory effects of D2 agonists depended on the secretagogue used, being much more pronounced for nicotine-evoked responses compared to high K+ stimulation, indicating another possible site of action of apomorphine up-stream of Ca2+ entry. 4. Inhibition by apomorphine of nicotine-evoked responses could not be explained by competitive antagonism against nicotine or DMPP (1,1-dimethyl-4-phenyl-piperazinium iodide). 5. Apomorphine caused reductions of inward whole-cell nicotinic current evoked by ACh and nicotine. 6. Inhibition of nicotine-evoked secretion and 22Na+ influx by apomorphine were not affected by tetrodotoxin, and voltage-dependent, whole-cell Na+ currents were unaltered by apomorphine. 7. No evidence was obtained for increases in K+ conductance by apomorphine. 8. Action potentials recorded in whole-cell current clamp were blocked by apomorphine when they were triggered by nicotinic depolarization but not when they were elicited by direct electrical stimulation. 9. Inclusion of GDP-beta-S in the pipette internal solution did not affect apomorphine-dependent inhibition of nicotinic-evoked responses, while the decrease in whole-cell Ca2+ current induced by apomorphine was completely inhibited in the presence of GDP-beta-S. 10. Increases in cyclic AMP caused by cholera toxin and forskolin did not change the apomorphine-dependent inhibitory effects on nicotine-evoked secretion, indicating that changes in cyclic AMP levels caused by dopamine receptor stimulation are probably not involved.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguirre J., Pinto J. E., Trifaró J. M. Calcium movements during the release of catecholamines from the adrenal medulla: effects of methoxyverapamil and external cations. J Physiol. 1977 Jul;269(2):371–394. doi: 10.1113/jphysiol.1977.sp011907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy C., Kirshner N. 22Na+ uptake and catecholamine secretion by primary cultures of adrenal medulla cells. J Neurochem. 1982 Jul;39(1):132–142. doi: 10.1111/j.1471-4159.1982.tb04711.x. [DOI] [PubMed] [Google Scholar]
- Artalejo A. R., García A. G., Montiel C., Sánchez-García P. A dopaminergic receptor modulates catecholamine release from the cat adrenal gland. J Physiol. 1985 May;362:359–368. doi: 10.1113/jphysiol.1985.sp015683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo C. R., García A. G., Aunis D. Chromaffin cell calcium channel kinetics measured isotopically through fast calcium, strontium, and barium fluxes. J Biol Chem. 1987 Jan 15;262(2):915–926. [PubMed] [Google Scholar]
- Ashkenazi A., Peralta E. G., Winslow J. W., Ramachandran J., Capon D. J. Functionally distinct G proteins selectively couple different receptors to PI hydrolysis in the same cell. Cell. 1989 Feb 10;56(3):487–493. doi: 10.1016/0092-8674(89)90251-1. [DOI] [PubMed] [Google Scholar]
- Bader M. F., Trifaró J. M., Langley O. K., Thiersé D., Aunis D. Secretory cell actin-binding proteins: identification of a gelsolin-like protein in chromaffin cells. J Cell Biol. 1986 Feb;102(2):636–646. doi: 10.1083/jcb.102.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C. Dopamine as a postganglionic autonomic neurotransmitter. Neuroscience. 1982 Jan;7(1):1–8. doi: 10.1016/0306-4522(82)90147-6. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N. Dopamine: a prolactin-inhibiting hormone. Endocr Rev. 1985 Fall;6(4):564–589. doi: 10.1210/edrv-6-4-564. [DOI] [PubMed] [Google Scholar]
- Bigornia L., Suozzo M., Ryan K. A., Napp D., Schneider A. S. Dopamine receptors on adrenal chromaffin cells modulate calcium uptake and catecholamine release. J Neurochem. 1988 Oct;51(4):999–1006. doi: 10.1111/j.1471-4159.1988.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Bittner M. A., Holz R. W., Neubig R. R. Guanine nucleotide effects on catecholamine secretion from digitonin-permeabilized adrenal chromaffin cells. J Biol Chem. 1986 Aug 5;261(22):10182–10188. [PubMed] [Google Scholar]
- Bormann J., Flügge G., Fuchs E. Effect of atrial natriuretic factor (ANF) on nicotinic acetylcholine receptor channels in bovine chromaffin cells. Pflugers Arch. 1989 May;414(1):11–14. doi: 10.1007/BF00585620. [DOI] [PubMed] [Google Scholar]
- Canonico P. L., Valdenegro C. A., Macleod R. M. Dopamine inhibits 32P incorporation into phosphatidylinositol in the anterior pituitary gland of the rat. Endocrinology. 1982 Jul;111(1):347–349. doi: 10.1210/endo-111-1-347. [DOI] [PubMed] [Google Scholar]
- Castelletti L., Memo M., Missale C., Spano P. F., Valerio A. Potassium channels involved in the transduction mechanism of dopamine D2 receptors in rat lactotrophs. J Physiol. 1989 Mar;410:251–265. doi: 10.1113/jphysiol.1989.sp017531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceña V., Nicolas G. P., Sanchez-Garcia P., Kirpekar S. M., Garcia A. G. Pharmacological dissection of receptor-associated and voltage-sensitive ionic channels involved in catecholamine release. Neuroscience. 1983 Dec;10(4):1455–1462. doi: 10.1016/0306-4522(83)90126-4. [DOI] [PubMed] [Google Scholar]
- Corcoran J. J., Kirshner N. Inhibition of calcium uptake, sodium uptake, and catecholamine secretion by methoxyverapamil (D600) in primary cultures of adrenal medulla cells. J Neurochem. 1983 Apr;40(4):1106–1109. doi: 10.1111/j.1471-4159.1983.tb08099.x. [DOI] [PubMed] [Google Scholar]
- Cota G., Hiriart M. Hormonal and neurotransmitter regulation of Ca channel activity in cultured adenohypophyseal cells. Soc Gen Physiol Ser. 1989;44:143–165. [PubMed] [Google Scholar]
- Cull-Candy S. G., Mathie A., Powis D. A. Acetylcholine receptor channels and their block by clonidine in cultured bovine chromaffin cells. J Physiol. 1988 Aug;402:255–278. doi: 10.1113/jphysiol.1988.sp017203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Macconi D., Spada A. Dopamine inhibits adenylate cyclase in human prolactin-secreting pituitary adenomas. Nature. 1979 Mar 15;278(5701):252–254. doi: 10.1038/278252a0. [DOI] [PubMed] [Google Scholar]
- Delbeke D., Dannies P. S. Stimulation of the adenosine 3',5'-monophosphate and the Ca2+ messenger systems together reverse dopaminergic inhibition of prolactin release. Endocrinology. 1985 Aug;117(2):439–446. doi: 10.1210/endo-117-2-439. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Taraskevich P. S. Slowing effects of dopamine and calcium-channel blockers on frequency of sodium spikes in rat pars intermedia cells. J Physiol. 1982 May;326:201–211. doi: 10.1113/jphysiol.1982.sp014186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert A., Sladeczek F., Guillon G., Bertrand P., Shu C., Epelbaum J., Garcia-Sainz A., Jard S., Lombard C., Kordon C. Angiotensin II and dopamine modulate both cAMP and inositol phosphate productions in anterior pituitary cells. Involvement in prolactin secretion. J Biol Chem. 1986 Mar 25;261(9):4071–4075. [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman J. E., Weight F. F. Single K+ channels activated by D2 dopamine receptors in acutely dissociated neurons from rat corpus striatum. Proc Natl Acad Sci U S A. 1988 May;85(10):3618–3622. doi: 10.1073/pnas.85.10.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González M. C., Artalejo A. R., Montiel C., Hervás P. P., García A. G. Characterization of a dopaminergic receptor that modulates adrenomedullary catecholamine release. J Neurochem. 1986 Aug;47(2):382–388. doi: 10.1111/j.1471-4159.1986.tb04513.x. [DOI] [PubMed] [Google Scholar]
- Gregerson K. A., Einhorn L., Smith M. M., Oxford G. S. Modulation of potassium channels by dopamine in rat pituitary lactotrophs: a role in the regulation of prolactin secretion? Soc Gen Physiol Ser. 1989;44:123–141. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick R. M., Hammond C., Paupardin-Tritsch D., Homburger V., Rouot B., Bockaert J., Gerschenfeld H. M. An alpha 40 subunit of a GTP-binding protein immunologically related to Go mediates a dopamine-induced decrease of Ca2+ current in snail neurons. Neuron. 1988 Mar;1(1):27–32. doi: 10.1016/0896-6273(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Holz R. W., Senter R. A., Frye R. A. Relationship between Ca2+ uptake and catecholamine secretion in primary dissociated cultures of adrenal medulla. J Neurochem. 1982 Sep;39(3):635–646. doi: 10.1111/j.1471-4159.1982.tb07940.x. [DOI] [PubMed] [Google Scholar]
- Hope W., McCulloch M. W., Story D. F., Rand M. J. Effects of pimozide on noradrenergic transmission in rabbit isolated ear arteries. Eur J Pharmacol. 1977 Nov 15;46(2):101–111. doi: 10.1016/0014-2999(77)90245-x. [DOI] [PubMed] [Google Scholar]
- Ingram C. D., Bicknell R. J., Mason W. T. Intracellular recordings from bovine anterior pituitary cells: modulation of spontaneous activity by regulators of prolactin secretion. Endocrinology. 1986 Dec;119(6):2508–2518. doi: 10.1210/endo-119-6-2508. [DOI] [PubMed] [Google Scholar]
- Israel J. M., Kirk C., Vincent J. D. Electrophysiological responses to dopamine of rat hypophysial cells in lactotroph-enriched primary cultures. J Physiol. 1987 Sep;390:1–22. doi: 10.1113/jphysiol.1987.sp016682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y., Miyazaki S., Ozawa S. Acetylcholine-induced membrane depolarization and potential fluctuations in the rat adrenal chromaffin cell. J Physiol. 1982 Mar;324:203–220. doi: 10.1113/jphysiol.1982.sp014107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y., Ritchie A. K. Chromaffin cell action potentials and their possible role in adrenaline secretion from rat adrenal medulla. J Physiol. 1980 Oct;307:199–216. doi: 10.1113/jphysiol.1980.sp013431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Slepetis R., Kirshner N. Inhibition of catecholamine secretion from adrenal medulla cells by neurotoxins and cholinergic antagonists. J Neurochem. 1981 Jul;37(1):125–131. doi: 10.1111/j.1471-4159.1981.tb05299.x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Slepetis R., Kirshner N. Ion channels and membrane potential in stimulus-secretion coupling in adrenal medulla cells. J Neurochem. 1981 Mar;36(3):1245–1255. doi: 10.1111/j.1471-4159.1981.tb01724.x. [DOI] [PubMed] [Google Scholar]
- Koch B. D., Dorflinger L. J., Schonbrunn A. Pertussis toxin blocks both cyclic AMP-mediated and cyclic AMP-independent actions of somatostatin. Evidence for coupling of Ni to decreases in intracellular free calcium. J Biol Chem. 1985 Oct 25;260(24):13138–13145. [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987 Nov;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackovic Z., Neff N. H. Minireview. Evidence that dopamine is a neurotransmitter in peripheral tissues. Life Sci. 1983 Apr 11;32(15):1665–1674. doi: 10.1016/0024-3205(83)90827-5. [DOI] [PubMed] [Google Scholar]
- Levitan I. B. Phosphorylation of ion channels. J Membr Biol. 1985;87(3):177–190. doi: 10.1007/BF01871217. [DOI] [PubMed] [Google Scholar]
- Long J. P., Heintz S., Cannon J. G., Kim J. Inhibition of the sympathetic nervous system by 5,6-dihydroxy-2-dimethylamino tetralin (M-7), apomorphine and dopamine. J Pharmacol Exp Ther. 1975 Feb;192(2):336–342. [PubMed] [Google Scholar]
- Lyon R. A., Titeler M., Bigornia L., Schneider A. S. D2 dopamine receptors on bovine chromaffin cell membranes: identification and characterization by [3H]N-methylspiperone binding. J Neurochem. 1987 Feb;48(2):631–635. doi: 10.1111/j.1471-4159.1987.tb04139.x. [DOI] [PubMed] [Google Scholar]
- Malgaroli A., Vallar L., Elahi F. R., Pozzan T., Spada A., Meldolesi J. Dopamine inhibits cytosolic Ca2+ increases in rat lactotroph cells. Evidence of a dual mechanism of action. J Biol Chem. 1987 Oct 15;262(29):13920–13927. [PubMed] [Google Scholar]
- Marchetti C., Carbone E., Lux H. D. Effects of dopamine and noradrenaline on Ca channels of cultured sensory and sympathetic neurons of chick. Pflugers Arch. 1986 Feb;406(2):104–111. doi: 10.1007/BF00586670. [DOI] [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menniti F. S., Diliberto E. J., Jr Newly synthesized dopamine as the precursor for norepinephrine synthesis in bovine adrenomedullary chromaffin cells. J Neurochem. 1989 Sep;53(3):890–897. doi: 10.1111/j.1471-4159.1989.tb11788.x. [DOI] [PubMed] [Google Scholar]
- Ohara K., Haga K., Berstein G., Haga T., Ichiyama A., Ohara K. The interaction between D-2 dopamine receptors and GTP-binding proteins. Mol Pharmacol. 1988 Mar;33(3):290–296. [PubMed] [Google Scholar]
- Quik M., Bergeron L., Mount H., Philie J. Dopamine D2 receptor binding in adrenal medulla: characterization using [3H]spiperone. Biochem Pharmacol. 1987 Nov 1;36(21):3707–3713. doi: 10.1016/0006-2952(87)90024-4. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Sato M. A single GTP-binding protein regulates K+-channels coupled with dopamine, histamine and acetylcholine receptors. Nature. 1987 Jan 15;325(6101):259–262. doi: 10.1038/325259a0. [DOI] [PubMed] [Google Scholar]
- Schneider A. S., Cline H. T., Rosenheck K., Sonenberg M. Stimulus-secretion coupling in isolated adrenal chromaffin cells: calcium channel activation and possible role of cytoskeletal elements. J Neurochem. 1981 Sep;37(3):567–575. doi: 10.1111/j.1471-4159.1982.tb12524.x. [DOI] [PubMed] [Google Scholar]
- Senogles S. E., Benovic J. L., Amlaiky N., Unson C., Milligan G., Vinitsky R., Spiegel A. M., Caron M. G. The D2-dopamine receptor of anterior pituitary is functionally associated with a pertussis toxin-sensitive guanine nucleotide binding protein. J Biol Chem. 1987 Apr 5;262(10):4860–4867. [PubMed] [Google Scholar]
- Steinsland O. S., Hieble J. P. Dopaminergic inhibition of adrenergic neurotransmission as a model for studies on dopamine receptor mechanisms. Science. 1978 Jan 27;199(4327):443–445. doi: 10.1126/science.22933. [DOI] [PubMed] [Google Scholar]
- Swennen L., Denef C. Physiological concentrations of dopamine decrease adenosine 3',5'-monophosphate levels in cultured rat anterior pituitary cells and enriched populations of lactotrophs: evidence for a causal relationship to inhibition of prolactin release. Endocrinology. 1982 Aug;111(2):398–405. doi: 10.1210/endo-111-2-398. [DOI] [PubMed] [Google Scholar]
- Vallar L., Meldolesi J. Mechanisms of signal transduction at the dopamine D2 receptor. Trends Pharmacol Sci. 1989 Feb;10(2):74–77. doi: 10.1016/0165-6147(89)90082-5. [DOI] [PubMed] [Google Scholar]
- Wada A., Yashima N., Izumi F., Kobayashi H., Yanagihara N. Involvement of Na influx in acetylcholine receptor mediated secretion of catecholamines from cultured bovine adrenal medulla cells. Neurosci Lett. 1984 Jun 1;47(1):75–80. doi: 10.1016/0304-3940(84)90389-6. [DOI] [PubMed] [Google Scholar]


