Abstract
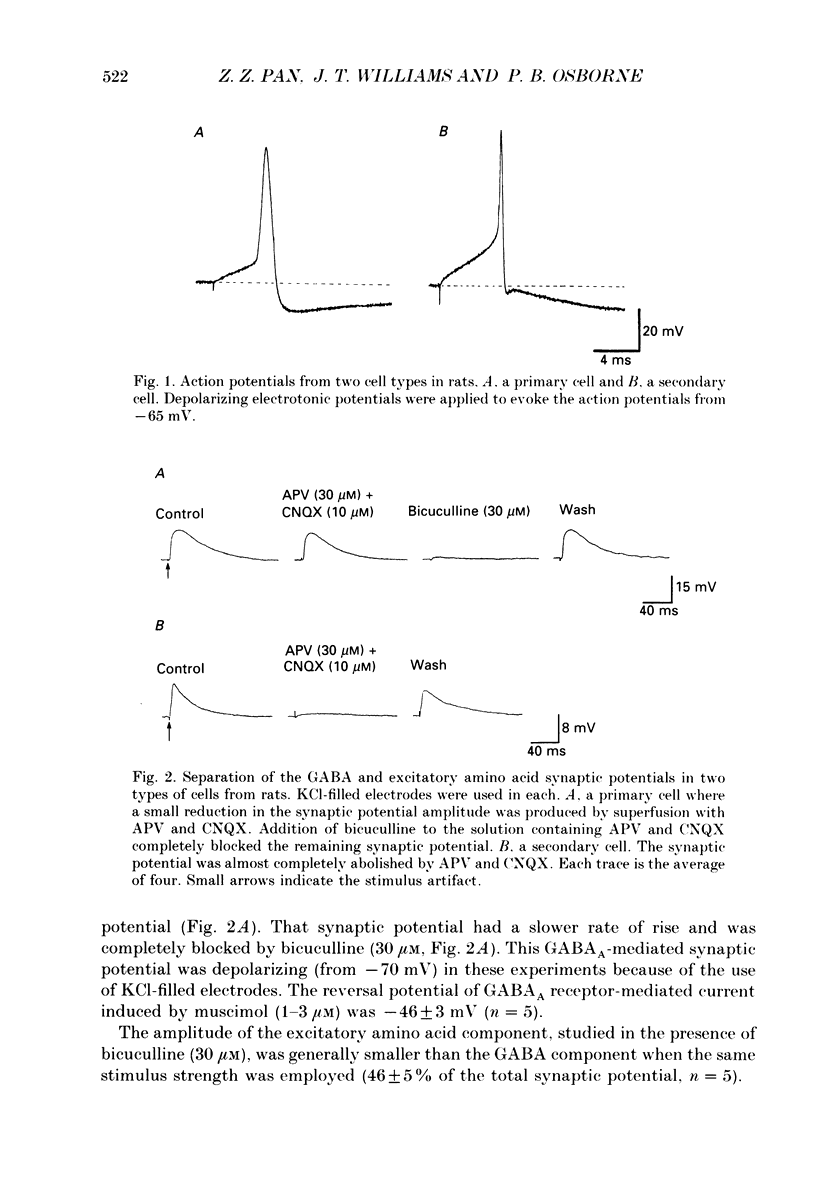
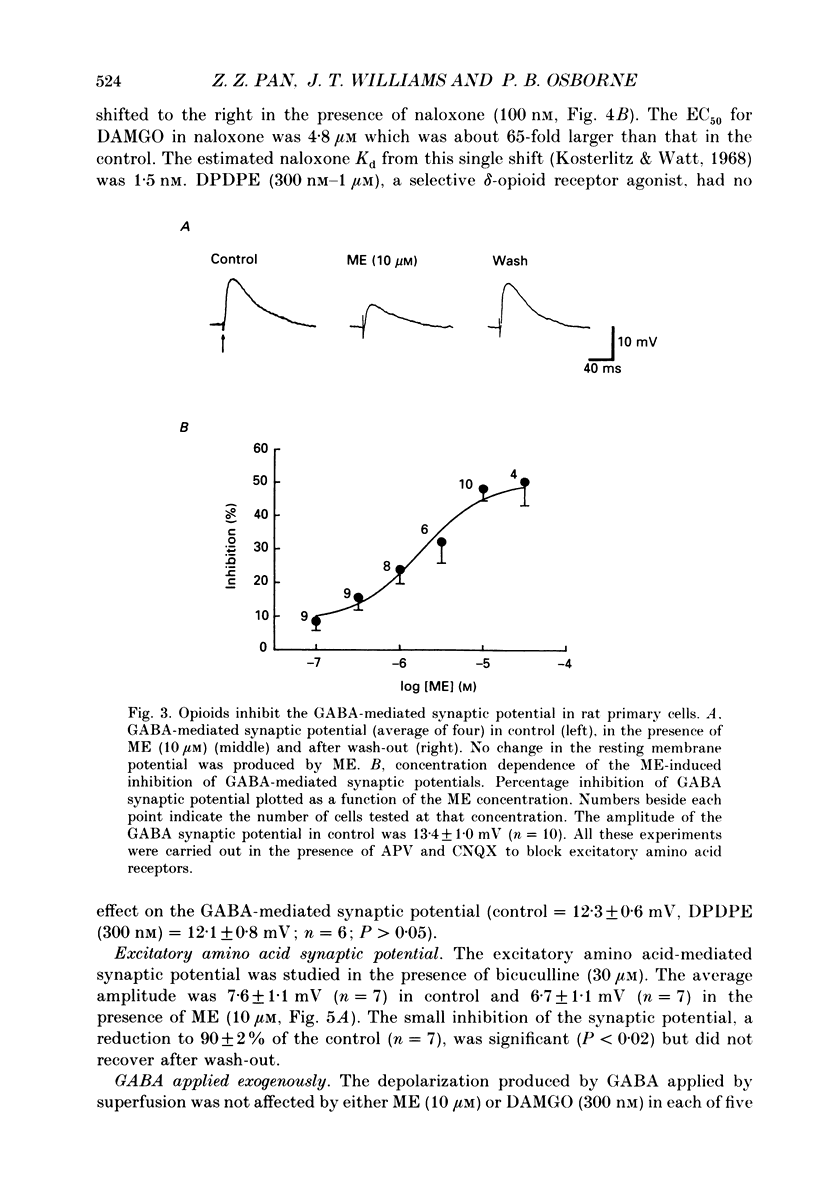
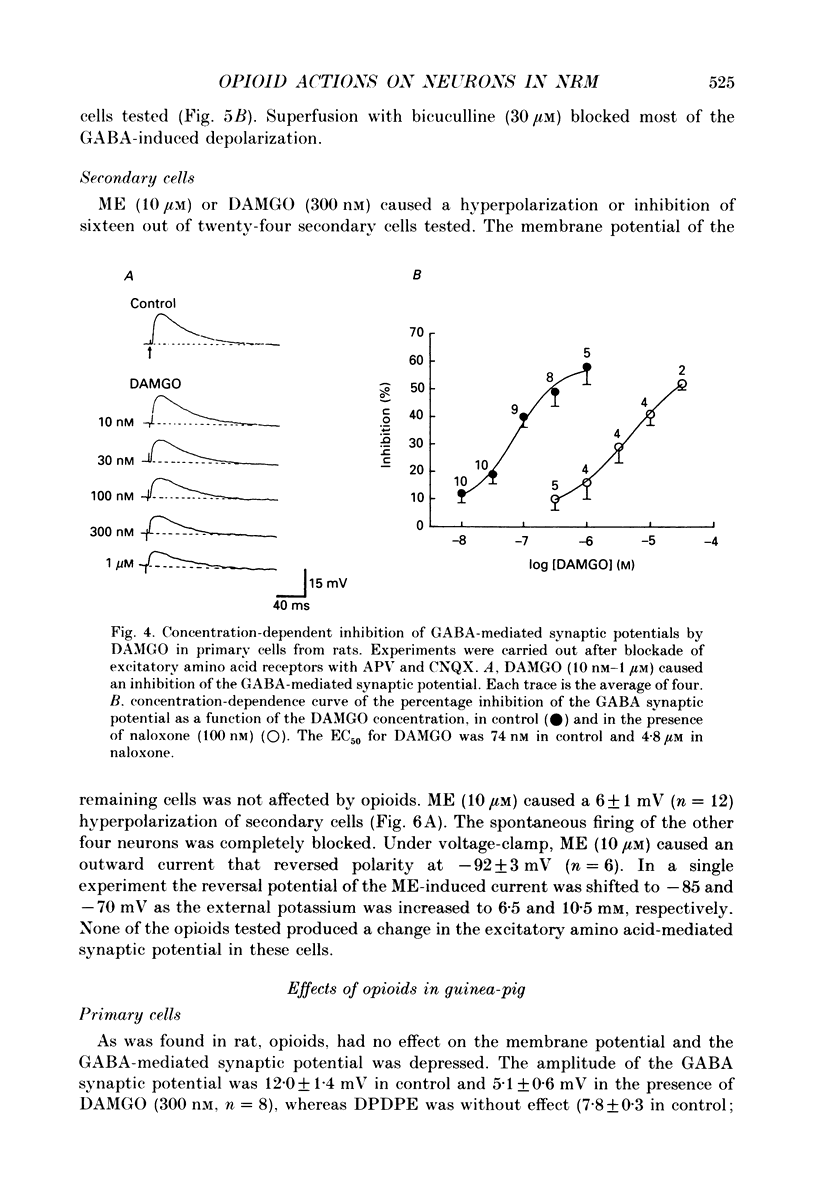
1. Intracellular recordings were made from neurons of the nucleus raphe magnus (NRM) from rat (n = 128) and guinea-pig (n = 115). Two types of cells were found in each, primary (103 in rat, 27 in guinea-pig) and secondary cells (25 in rat, 88 in guinea-pig). 2. Primary cells had input resistances of 186 +/- 9 M omega (n = 9) in rat and 255 +/- 50 M omega (n = 11) in guinea-pig. The action potential in each was about 1.5 ms in duration. Synaptic potentials were evoked by focal electrical stimulation and consisted of both gamma-aminobutyric acid (GABA) and excitatory amino acid components. 3. Morphine, [Met5]enkephalin (ME) and [D-Ala2,N-Me-Phe4, Gly5-ol]enkephalin (DAMGO) depressed the amplitude of the GABA-mediated synaptic potential by a maximum of 50-65% and had little effect on the excitatory amino acid-mediated synaptic potential. There was no effect of these opioids on the resting membrane potential or input resistance of primary cells in rat or guinea-pig. 4. Secondary cells had short duration action potentials (less than 1 ms) and an input resistance of 354 +/- 47 M omega in rat (n = 6) and 290 +/- 40 M omega in guinea-pig (n = 15). The synaptic potential observed in the cells of this group was mediated by activation of only excitatory amino acid receptors. 5. ME hyperpolarized and/or abolished the spontaneous firing in sixteen out of twenty-four neurons in the secondary group from rat and eight out of eighty-four neurons from guinea-pig. ME induced an outward current at -60 mV that reversed polarity at potentials more negative than -92 +/- 3 mV in rat (n = 6) and -98 +/- 2 mV in guinea-pig (n = 18). The reversal potential of the opioid current was shifted to less negative potentials when the external potassium concentration was increased, as predicted by the Nernst equation. 6. The morphology of the two types of cells were distinguishable in that primary cells were oval (29 x 18 microns in rat; 36 x 19 microns in guinea-pig) with two to four thick tapering dendrites that branched within 50 microns of the cell body. Secondary cells were generally round or oval (about 24 x 13 microns in rat; 27 x 17 microns in guinea-pig) with two to five thin non-tapering dendrites.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
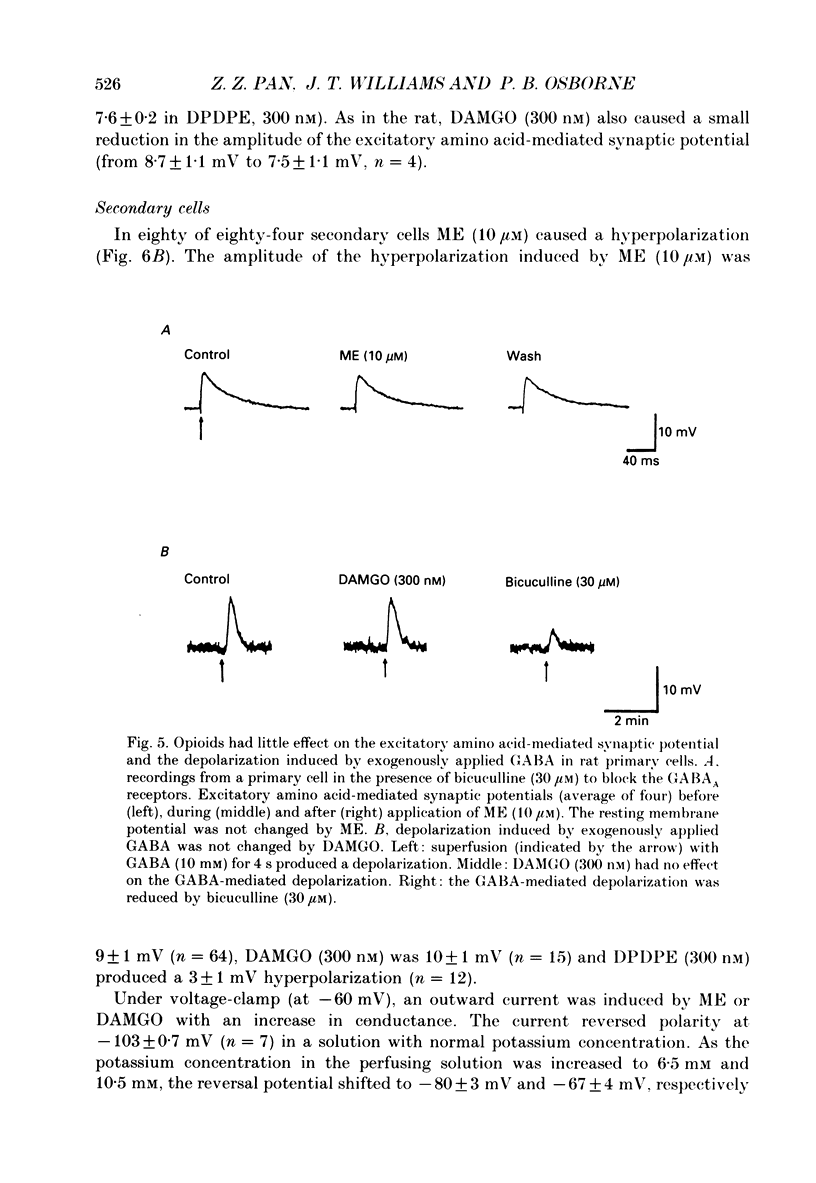
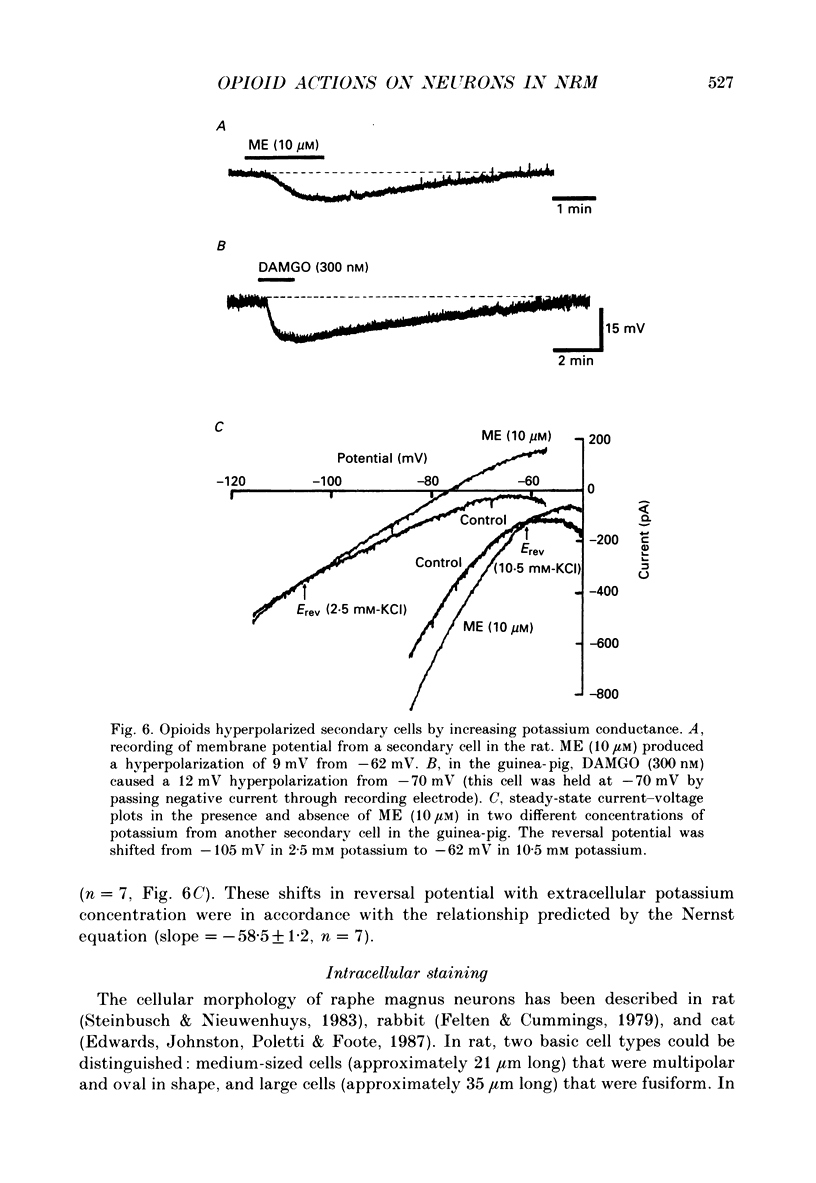
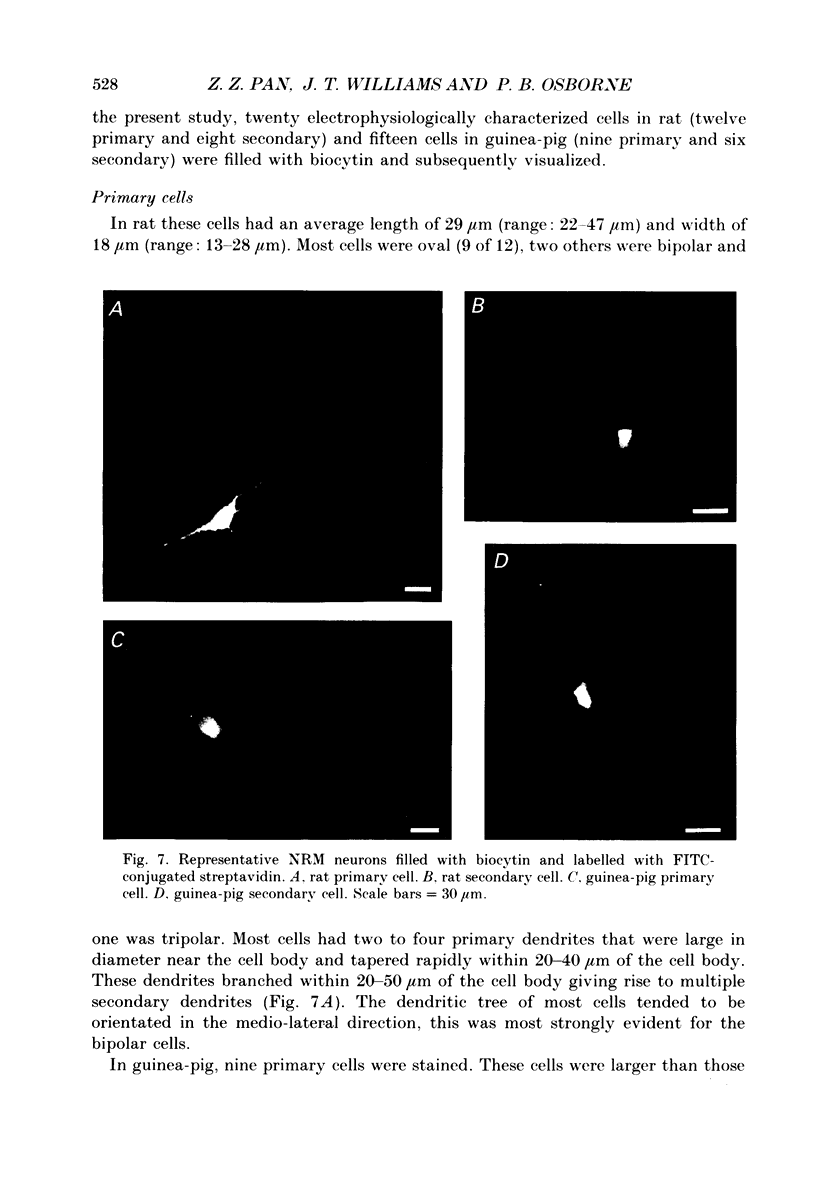
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. D., Basbaum A. I., Fields H. L. Response of medullary raphe neurons to peripheral stimulation and to systemic opiates. Brain Res. 1977 Mar 11;123(2):363–368. doi: 10.1016/0006-8993(77)90487-5. [DOI] [PubMed] [Google Scholar]
- Basbaum A. I., Fields H. L. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Behbehani M. M., Fields H. L. Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res. 1979 Jul 6;170(1):85–93. doi: 10.1016/0006-8993(79)90942-9. [DOI] [PubMed] [Google Scholar]
- Bowker R. M., Dilts R. P. Distribution of mu-opioid receptors in the nucleus raphe magnus and nucleus gigantocellularis: a quantitative autoradiographic study. Neurosci Lett. 1988 Jun 7;88(3):247–252. doi: 10.1016/0304-3940(88)90218-2. [DOI] [PubMed] [Google Scholar]
- Chiang C. Y., Pan Z. Z. Differential responses of serotonergic and non-serotonergic neurons in nucleus raphe magnus to systemic morphine in rats. Brain Res. 1985 Jun 24;337(1):146–150. doi: 10.1016/0006-8993(85)91620-8. [DOI] [PubMed] [Google Scholar]
- Drower E. J., Hammond D. L. GABAergic modulation of nociceptive threshold: effects of THIP and bicuculline microinjected in the ventral medulla of the rat. Brain Res. 1988 May 31;450(1-2):316–324. doi: 10.1016/0006-8993(88)91570-3. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Griersmith B. T. Inhibition of the spinal transmission of nociceptive information by supraspinal stimulation in the cat. Pain. 1979 Apr;6(2):149–161. doi: 10.1016/0304-3959(79)90122-2. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., North R. A. Electrophysiology of opioids. Pharmacol Rev. 1983 Dec;35(4):219–281. [PubMed] [Google Scholar]
- Edwards D. L., Johnston K. M., Poletti C. E., Foote W. E. Morphology of pontomedullary raphe and reticular formation neurons in the brainstem of the cat: an intracellular HRP study. J Comp Neurol. 1987 Feb 8;256(2):257–273. doi: 10.1002/cne.902560206. [DOI] [PubMed] [Google Scholar]
- Felten D. L., Cummings J. P. The raphe nuclei of the rabbit brain stem. J Comp Neurol. 1979 Sep 1;187(1):199–243. doi: 10.1002/cne.901870112. [DOI] [PubMed] [Google Scholar]
- Fields H. L., Anderson S. D. Evidence that raphe-spinal neurons mediate opiate and midbrain stimulation-produced analgesias. Pain. 1978 Dec;5(4):333–349. doi: 10.1016/0304-3959(78)90002-7. [DOI] [PubMed] [Google Scholar]
- Fields H. L., Barbaro N. M., Heinricher M. M. Brain stem neuronal circuitry underlying the antinociceptive action of opiates. Prog Brain Res. 1988;77:245–257. doi: 10.1016/s0079-6123(08)62792-2. [DOI] [PubMed] [Google Scholar]
- Fields H. L., Vanegas H., Hentall I. D., Zorman G. Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature. 1983 Dec 15;306(5944):684–686. doi: 10.1038/306684a0. [DOI] [PubMed] [Google Scholar]
- Giesler G. J., Jr, Gerhart K. D., Yezierski R. P., Wilcox T. K., Willis W. D. Postsynaptic inhibition of primate spinothalamic neurons by stimulation in nucleus raphe magnus. Brain Res. 1981 Jan 5;204(1):184–188. doi: 10.1016/0006-8993(81)90661-2. [DOI] [PubMed] [Google Scholar]
- Horikawa K., Armstrong W. E. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods. 1988 Aug;25(1):1–11. doi: 10.1016/0165-0270(88)90114-8. [DOI] [PubMed] [Google Scholar]
- Kosterlitz H. W., Watt A. J. Kinetic parameters of narcotic agonists and antagonists, with particular reference to N-allylnoroxymorphone (naloxone). Br J Pharmacol Chemother. 1968 Jun;33(2):266–276. doi: 10.1111/j.1476-5381.1968.tb00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light A. R., Casale E. J., Menétrey D. M. The effects of focal stimulation in nucleus raphe magnus and periaqueductal gray on intracellularly recorded neurons in spinal laminae I and II. J Neurophysiol. 1986 Sep;56(3):555–571. doi: 10.1152/jn.1986.56.3.555. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Enkephalin hyperpolarizes interneurones in the rat hippocampus. J Physiol. 1988 Apr;398:123–130. doi: 10.1113/jphysiol.1988.sp017033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A., Khachaturian H., Lewis M. E., Akil H., Watson S. J. Anatomy of CNS opioid receptors. Trends Neurosci. 1988 Jul;11(7):308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Mason P., Strassman A., Maciewicz R. Serotonin immunocytochemistry of physiologically characterized raphe magnus neurons. Exp Brain Res. 1988;73(1):1–7. doi: 10.1007/BF00279654. [DOI] [PubMed] [Google Scholar]
- Mayer D. J., Price D. D. Central nervous system mechanisms of analgesia. Pain. 1976 Dec;2(4):379–404. doi: 10.1016/0304-3959(76)90080-4. [DOI] [PubMed] [Google Scholar]
- Nagai T., Maeda T., Imai H., McGeer P. L., McGeer E. G. Distribution of GABA-T-intensive neurons in the rat hindbrain. J Comp Neurol. 1985 Jan 8;231(2):260–269. doi: 10.1002/cne.902310213. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E., Jahr C. E. Enkephalin blocks inhibitory pathways in the vertebrate CNS. Nature. 1980 Sep 4;287(5777):22–25. doi: 10.1038/287022a0. [DOI] [PubMed] [Google Scholar]
- Nishikawa T., Scatton B. Inhibitory influence of GABA on central serotonergic transmission. Raphé nuclei as the neuroanatomical site of the GABAergic inhibition of cerebral serotonergic neurons. Brain Res. 1985 Apr 1;331(1):91–103. doi: 10.1016/0006-8993(85)90718-8. [DOI] [PubMed] [Google Scholar]
- Pan Z. Z., Williams J. T. GABA- and glutamate-mediated synaptic potentials in rat dorsal raphe neurons in vitro. J Neurophysiol. 1989 Apr;61(4):719–726. doi: 10.1152/jn.1989.61.4.719. [DOI] [PubMed] [Google Scholar]
- Toda K. Responses of raphe magnus neurons to systemic morphine in rats. Brain Res Bull. 1982 Jan;8(1):101–103. doi: 10.1016/0361-9230(82)90033-8. [DOI] [PubMed] [Google Scholar]