Abstract
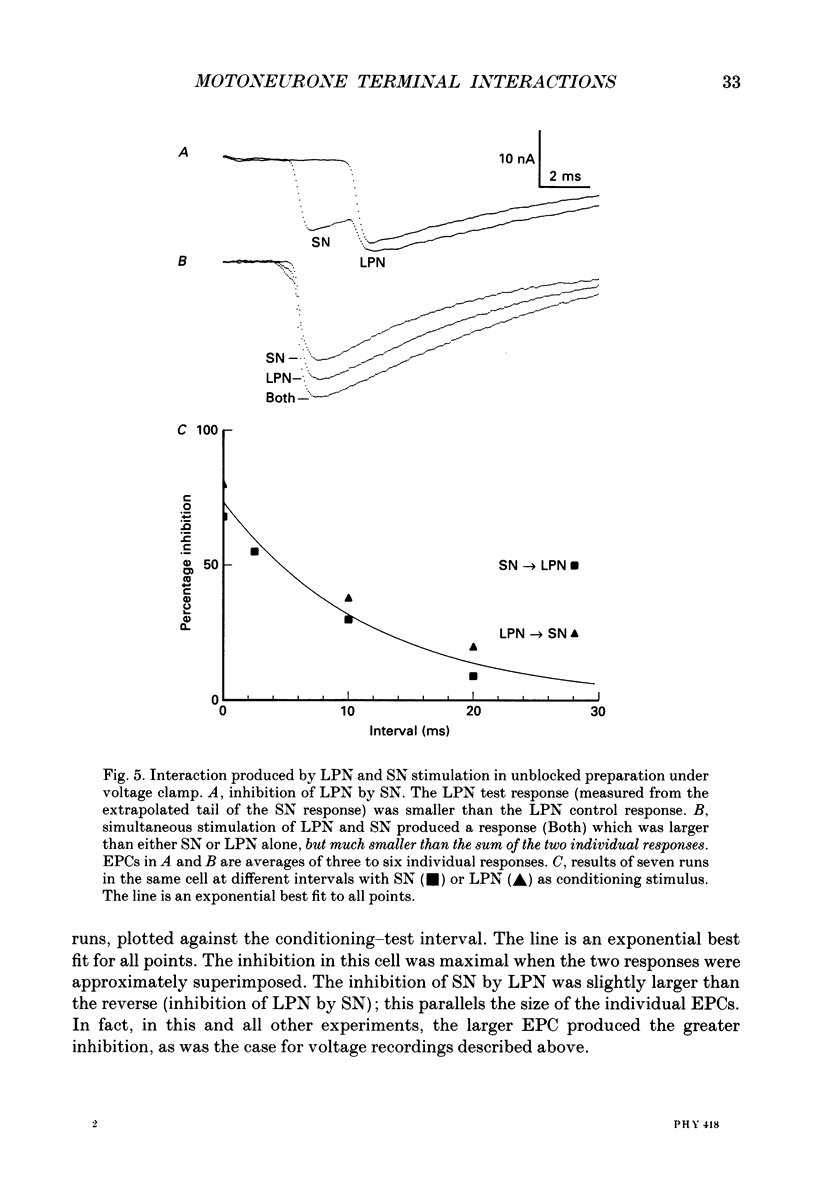
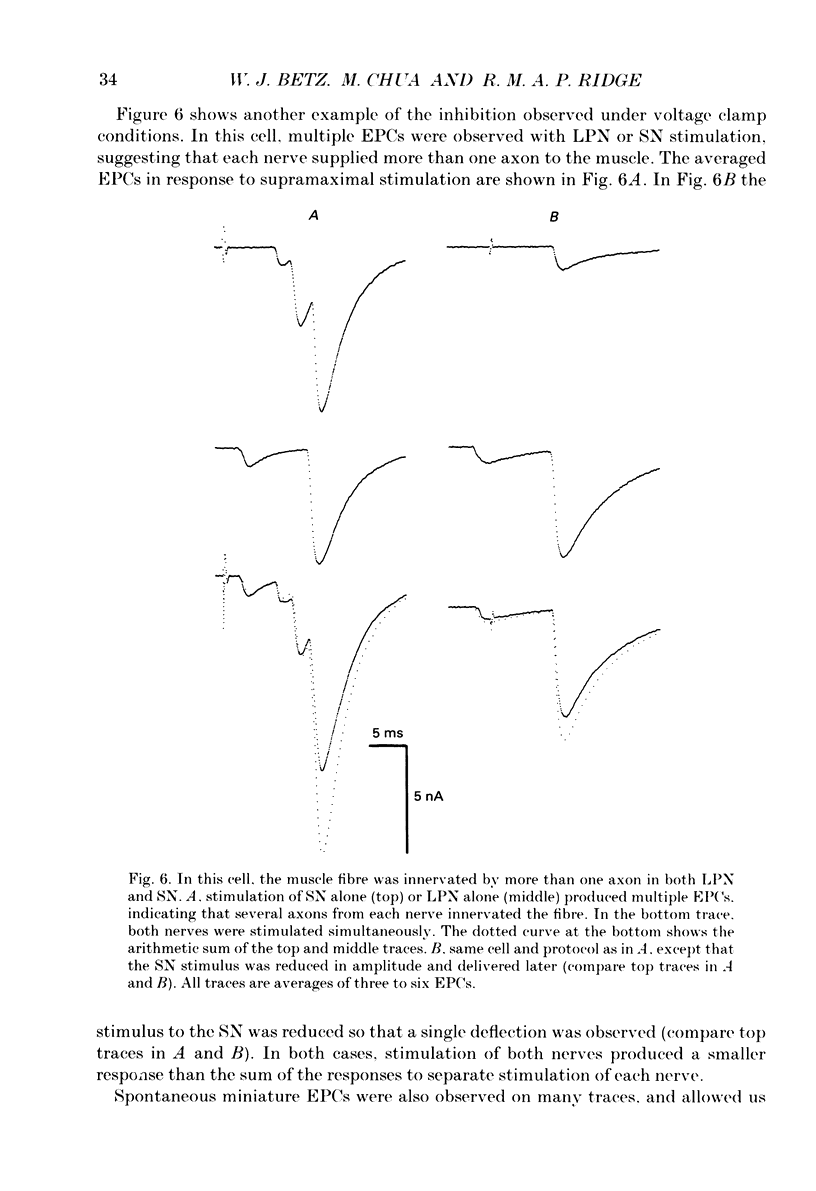
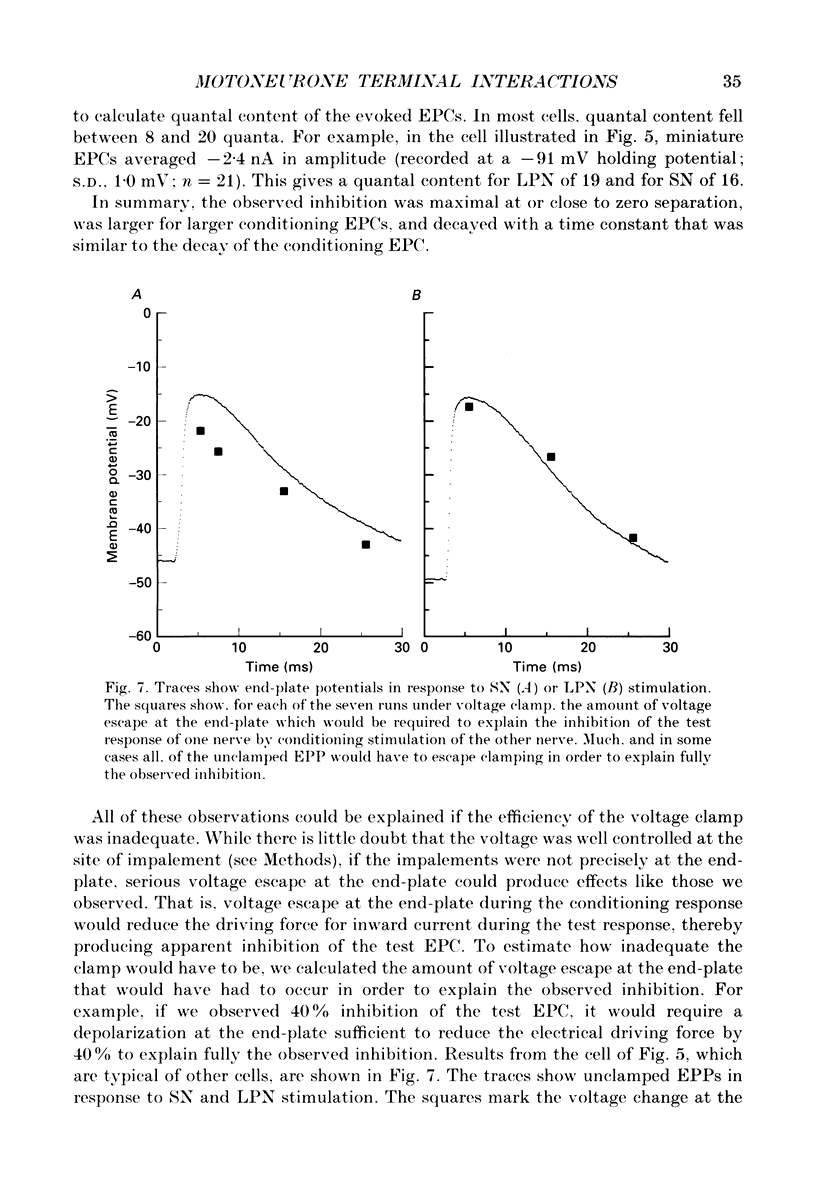
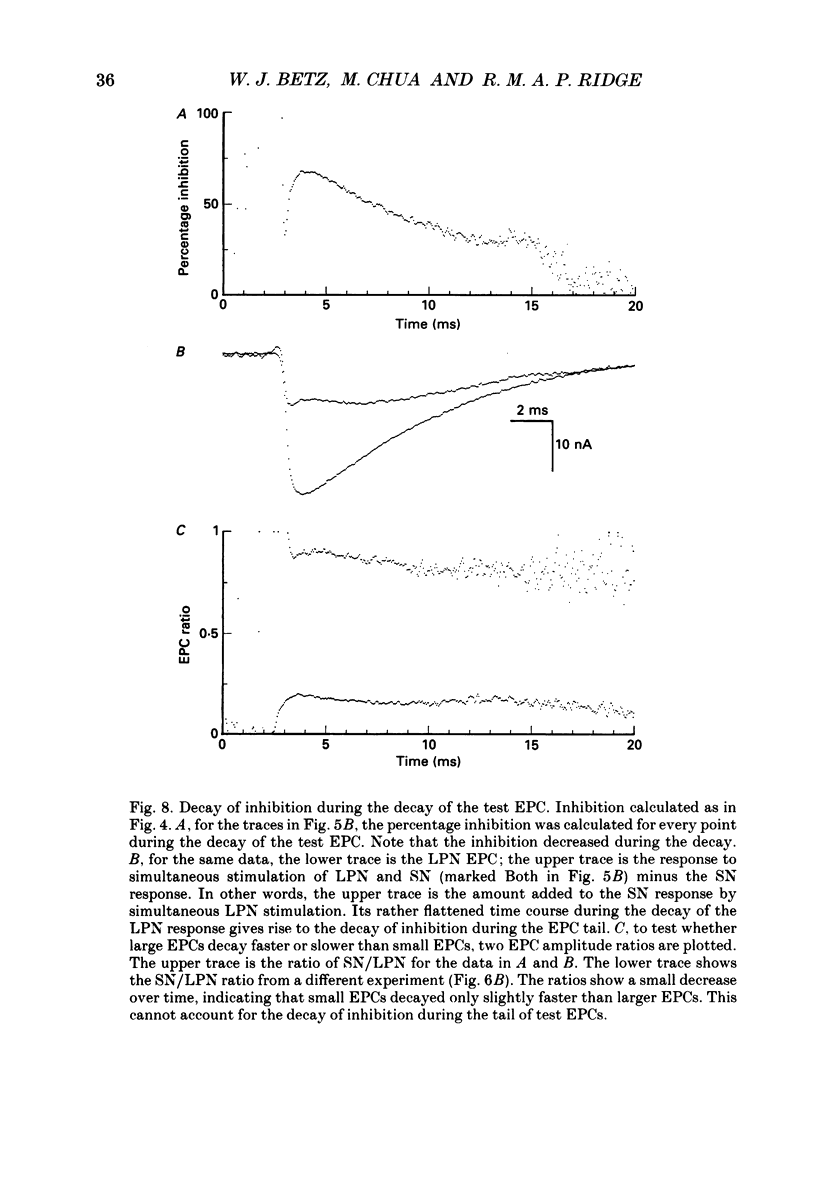
1. Evoked synaptic potentials and currents were recorded in neonatal rat fourth deep lumbrical muscle during the period of polyneuronal innervation. Signs of inhibitory interactions between converging mononeurone terminals were detected. 2. Muscle fibres innervated by axons from the lateral plantar nerve (LPN) and from the sural nerve (SN) were studied. In unblocked preparations the muscle contracted, and electrode tips were mounted on flexible wires to prevent loss of impalements. 3. In voltage recordings from unblocked preparations, paired two-shock stimulation of one nerve revealed synaptic depression: the second response was smaller than the first. When the two stimuli were delivered to different nerves (SN and LPN), the second response was smaller than its own control. 4. In voltage clamped, unblocked preparations, a similar result was obtained. Conditioning stimulation of one nerve (SN, for example) inhibited the response to test stimulation of the other nerve (LPN). The inhibition was greater with larger conditioning responses, was maximal when the conditioning and test stimuli were approximately superimposed, and decayed with a time course of several tens of milliseconds. Several tests showed that the end-plate was well clamped: the observed inhibition could not be explained by voltage escape at the end-plate. 5. The inhibition was not constant during the tail of the test end-plate current (EPC). Instead, it declined during the EPC tail, suggesting that the mechanism of inhibition was active, though diminishing, throughout the time course of the test EPC. 6. The amount of inhibition was not noticeably affected by altering the muscle membrane potential (two cells studied). 7. Treatment with curare or alpha-bungarotoxin to block most ACh receptors reduced the inhibition. In about half of the fibres studied, no inhibition was evident: in the others, up to 50% inhibition was observed. The average inhibition for all receptor-blocked fibres was about 15%. 8. In six alpha-bungarotoxin-treated cells, multiple conditioning stimuli were delivered. In most cases, the amount of inhibition increased with increasing numbers of conditioning stimuli. 9. Several possible mechanisms of inhibition are discussed, including reduction of current through ACh channels during the test response owing to alterations in synaptic cleft ion concentrations produced by the conditioning response, presynaptic inhibition of transmitter release, and postsynaptic receptor saturation.
Full text
PDF


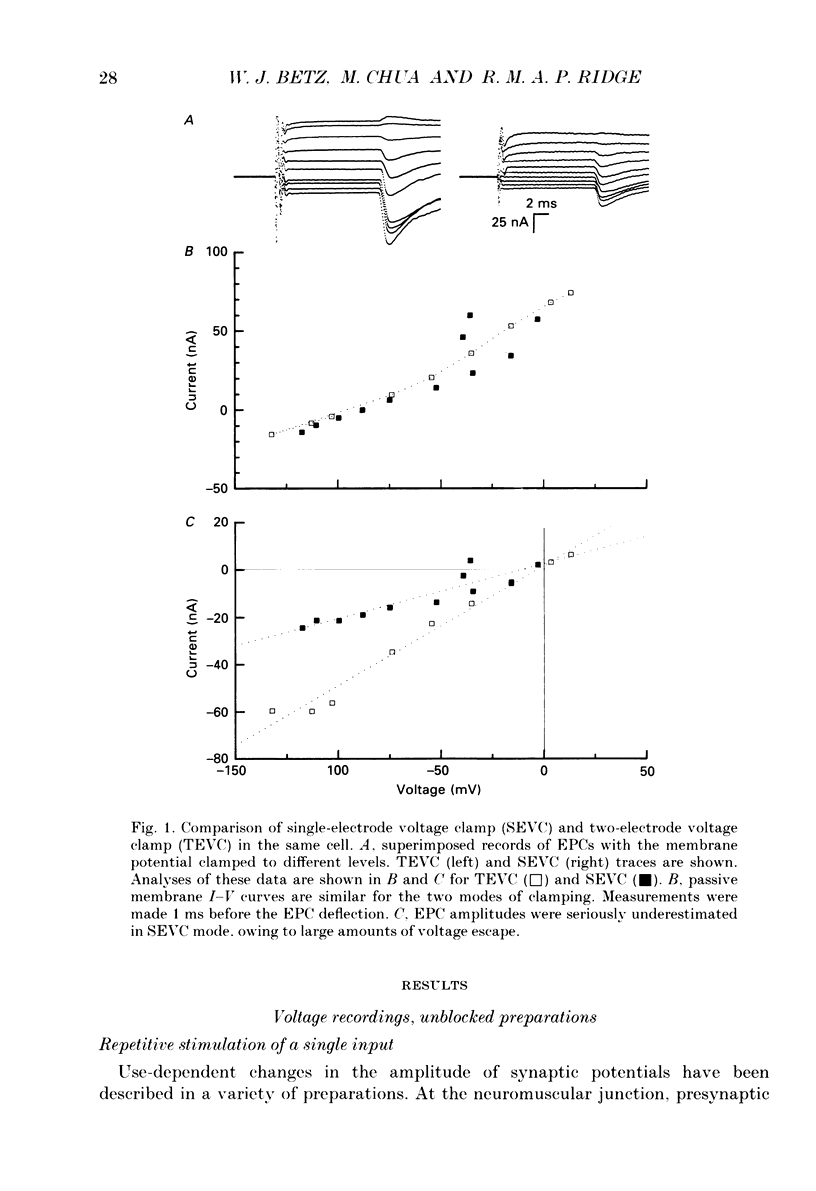
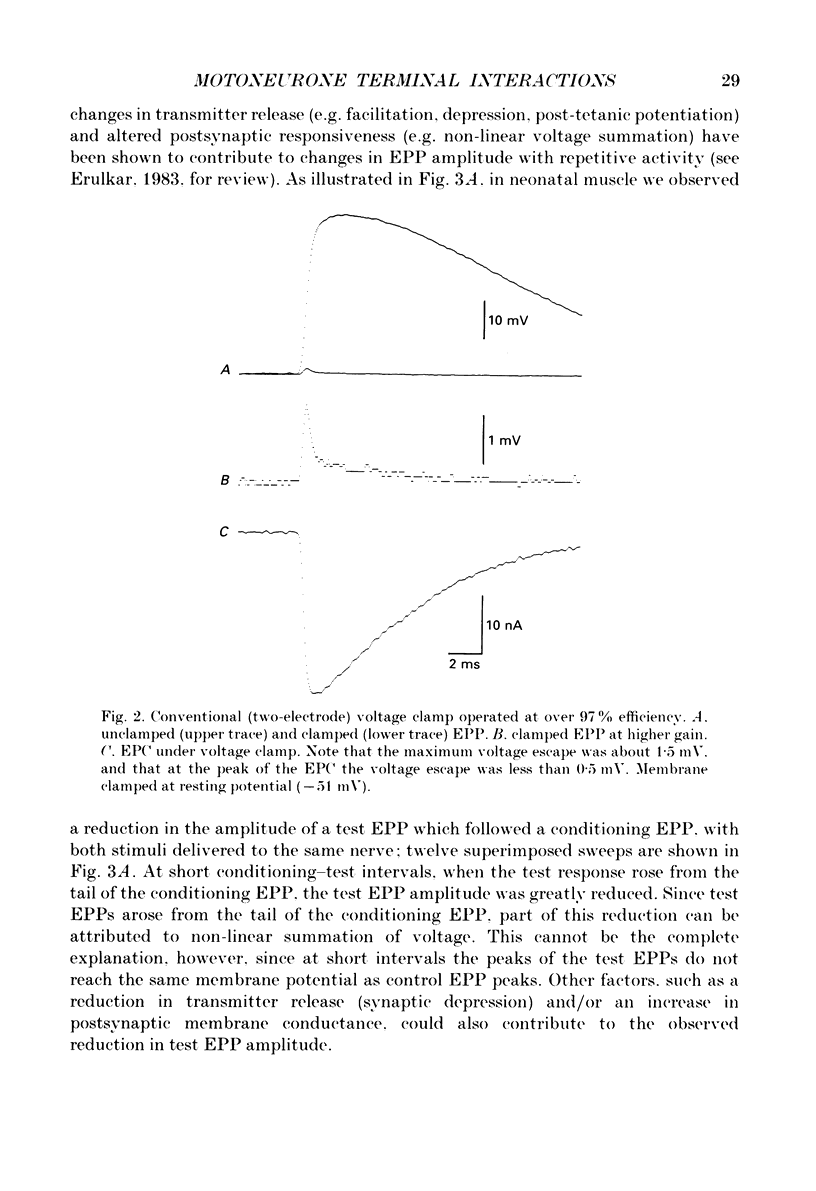
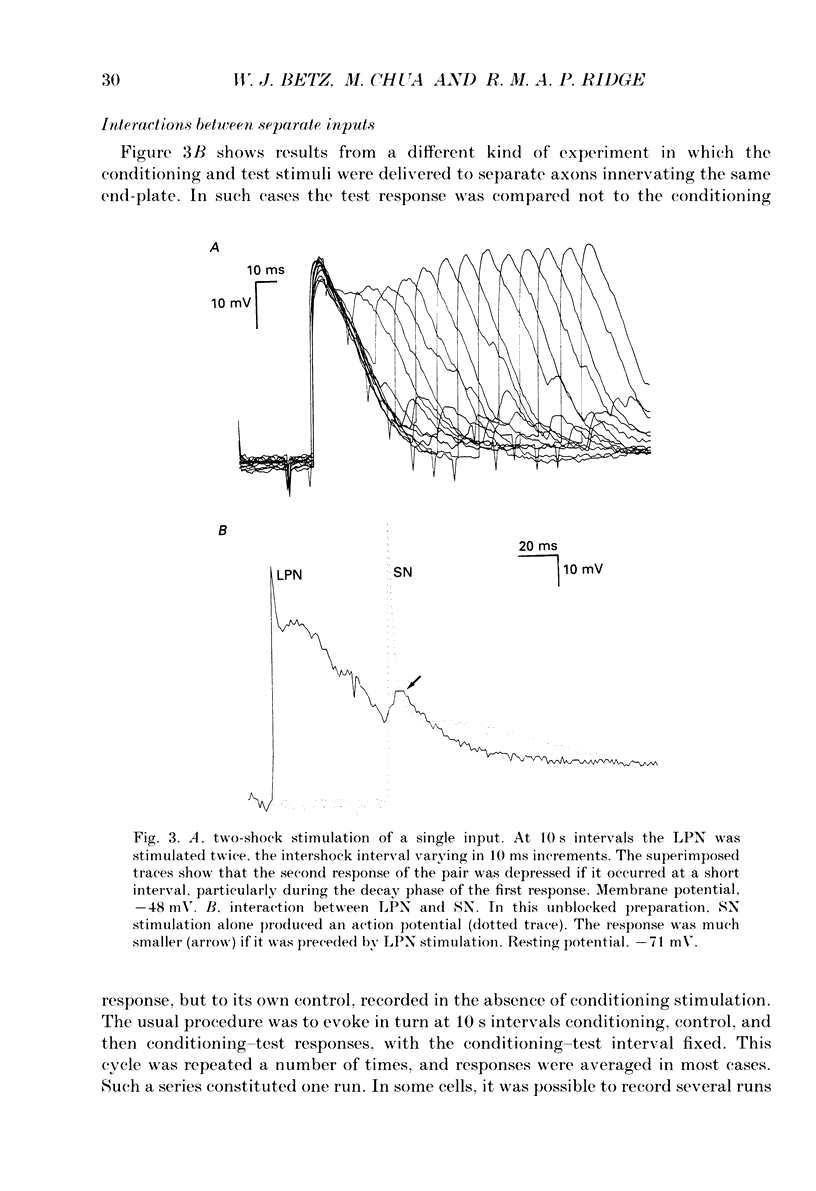

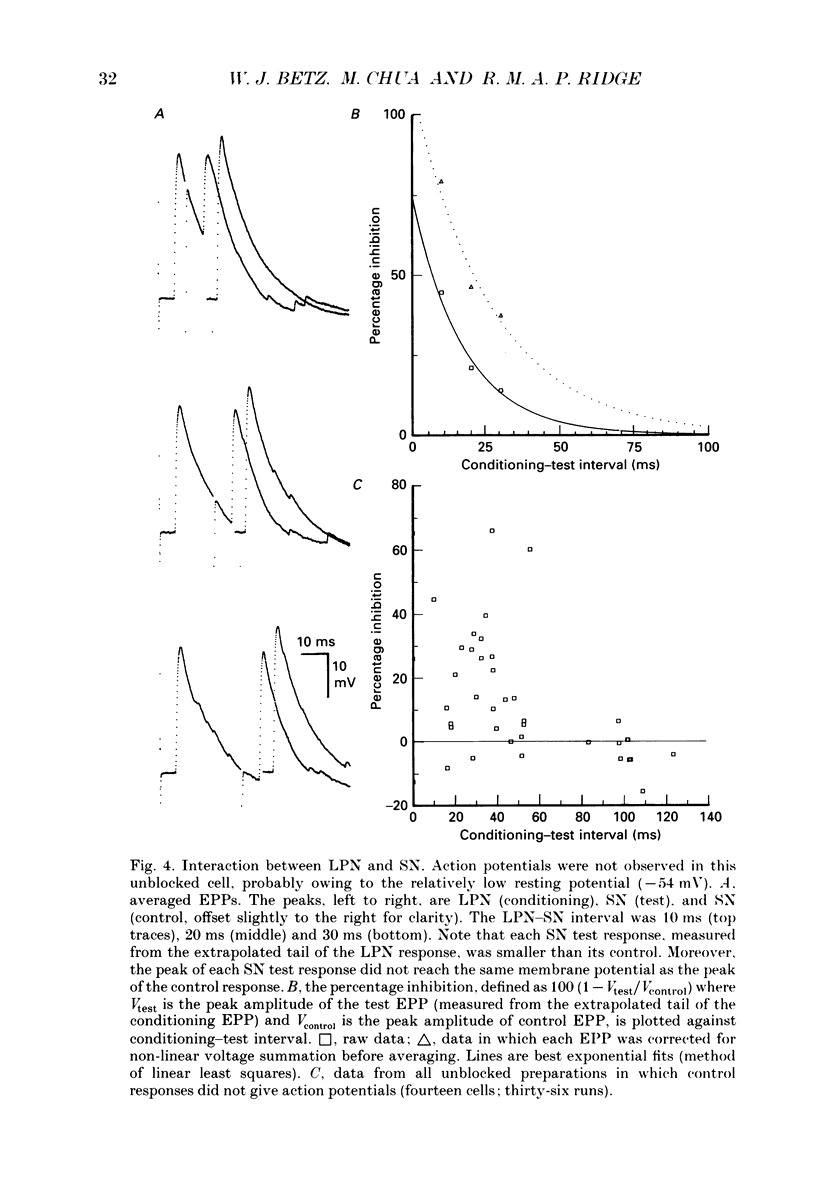






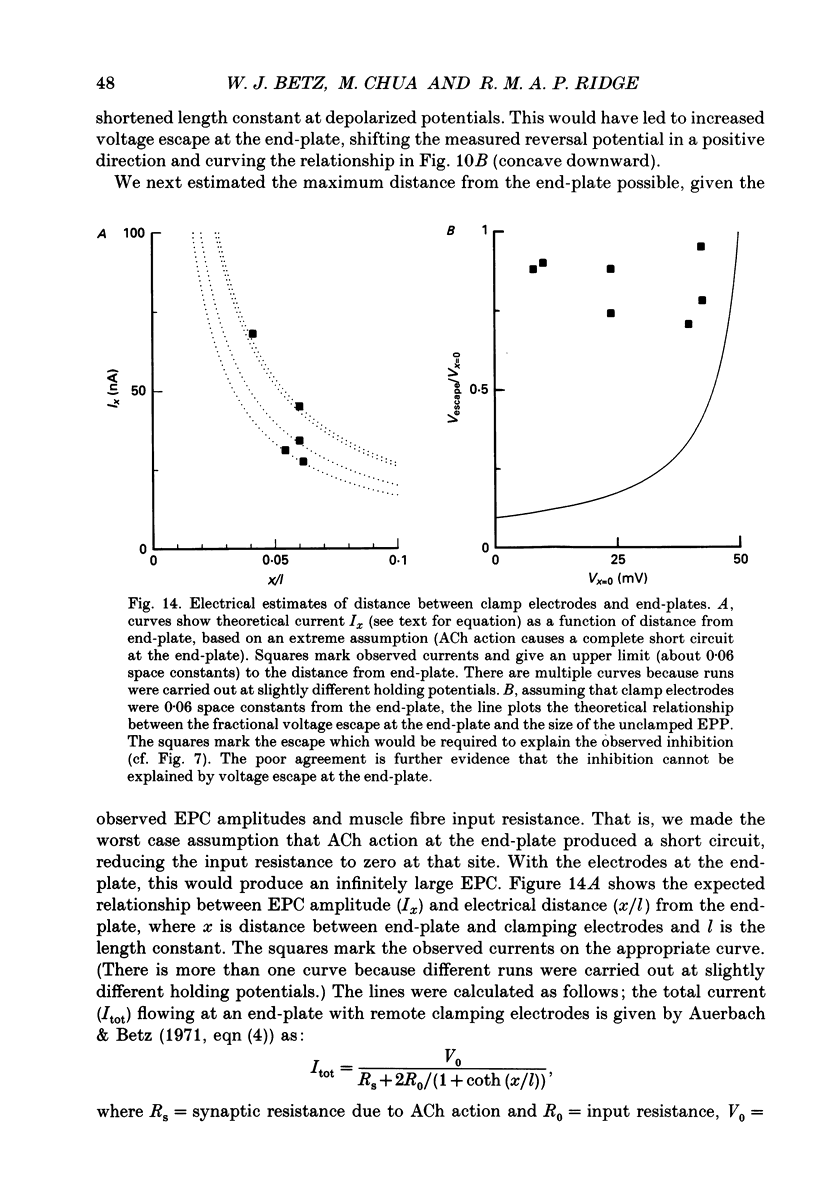



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Iles J. F. Synaptic transmission: ion concentration changes in the synaptic cleft. Proc R Soc Lond B Biol Sci. 1979 Nov 30;206(1162):115–131. doi: 10.1098/rspb.1979.0095. [DOI] [PubMed] [Google Scholar]
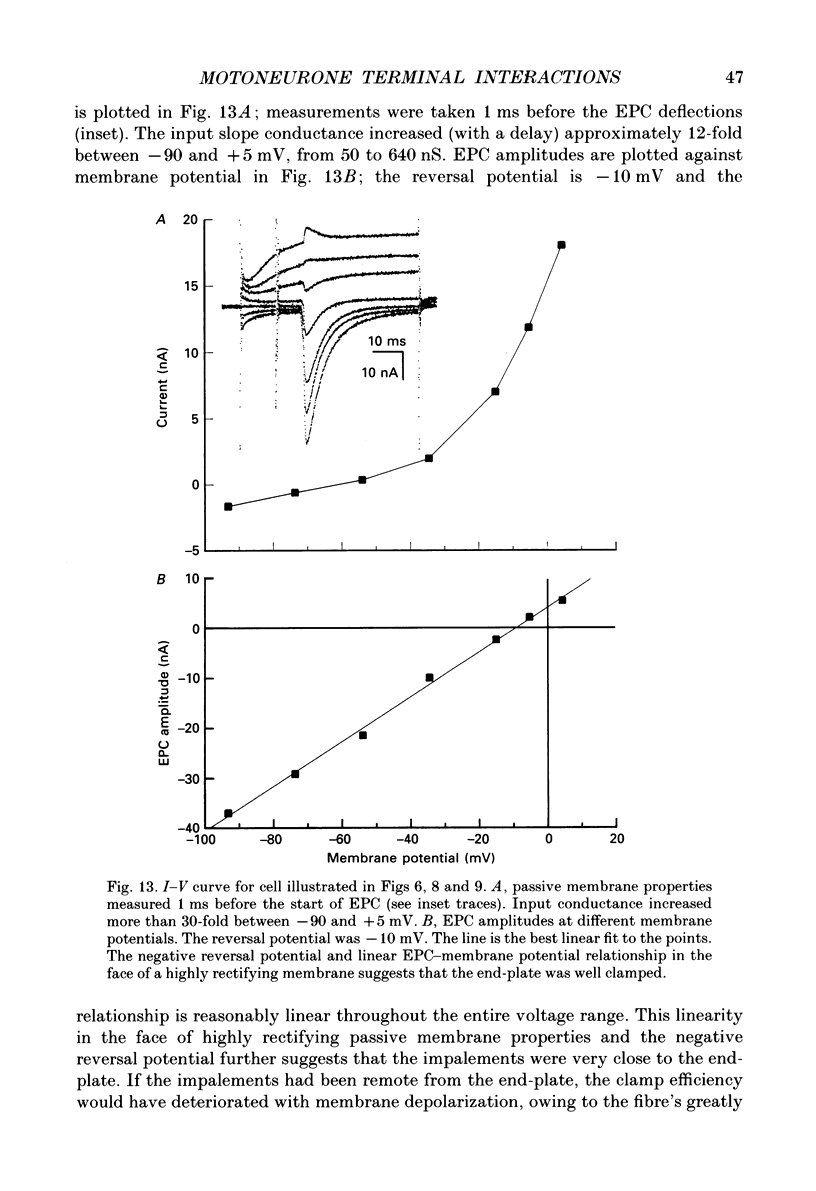
- Auerbach A., Betz W. Does curare affect transmitter release? J Physiol. 1971 Mar;213(3):691–705. doi: 10.1113/jphysiol.1971.sp009409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo G. J., Van der Kloot W. Transient elevation of spontaneous release at the frog neuromuscular junction following acetylcholine iontophoresis. Pflugers Arch. 1988 Feb;411(2):188–194. doi: 10.1007/BF00582313. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. The size of motor units during post-natal development of rat lumbrical muscle. J Physiol. 1979 Dec;297(0):463–478. doi: 10.1113/jphysiol.1979.sp013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby J. L. Ultrastructural observations on synapse elimination in neonatal rabbit skeletal muscle. J Neurocytol. 1981 Feb;10(1):81–100. doi: 10.1007/BF01181746. [DOI] [PubMed] [Google Scholar]
- Callaway E. M., Soha J. M., Van Essen D. C. Competition favouring inactive over active motor neurons during synapse elimination. 1987 Jul 30-Aug 5Nature. 328(6129):422–426. doi: 10.1038/328422a0. [DOI] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. Presynaptic inhibition at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:543–562. doi: 10.1113/jphysiol.1961.sp006646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D. The modulation of neurotransmitter release at synaptic junctions. Rev Physiol Biochem Pharmacol. 1983;98:63–175. doi: 10.1007/BFb0033867. [DOI] [PubMed] [Google Scholar]
- Lichtman J. W., Wilkinson R. S., Rich M. M. Multiple innervation of tonic endplates revealed by activity-dependent uptake of fluorescent probes. 1985 Mar 28-Apr 3Nature. 314(6009):357–359. doi: 10.1038/314357a0. [DOI] [PubMed] [Google Scholar]
- Magrassi L., Purves D., Lichtman J. W. Fluorescent probes that stain living nerve terminals. J Neurosci. 1987 Apr;7(4):1207–1214. doi: 10.1523/JNEUROSCI.07-04-01207.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M., Martin A. R. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol. 1981 Feb;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribchester R. R. Activity-dependent and -independent synaptic interactions during reinnervation of partially denervated rat muscle. J Physiol. 1988 Jul;401:53–75. doi: 10.1113/jphysiol.1988.sp017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribchester R. R., Taxt T. Motor unit size and synaptic competition in rat lumbrical muscles reinnervated by active and inactive motor axons. J Physiol. 1983 Nov;344:89–111. doi: 10.1113/jphysiol.1983.sp014926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribchester R. R., Taxt T. Repression of inactive motor nerve terminals in partially denervated rat muscle after regeneration of active motor axons. J Physiol. 1984 Feb;347:497–511. doi: 10.1113/jphysiol.1984.sp015078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge R. M., Betz W. J. The effect of selective, chronic stimulation on motor unit size in developing rat muscle. J Neurosci. 1984 Oct;4(10):2614–2620. doi: 10.1523/JNEUROSCI.04-10-02614.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer E. Coordinated release of ATP and ACh from cholinergic synaptosomes and its inhibition by calmodulin antagonists. J Neurosci. 1987 Sep;7(9):2948–2956. doi: 10.1523/JNEUROSCI.07-09-02948.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Ginsborg B. L., Hirsh J. K. Modulation of neurotransmitter release by adenosine and ATP. Prog Clin Biol Res. 1987;230:65–75. [PubMed] [Google Scholar]
- Silinsky E. M. On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J Physiol. 1984 Jan;346:243–256. doi: 10.1113/jphysiol.1984.sp015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werle M. J., Herrera A. A. Synaptic competition and the persistence of polyneuronal innervation at frog neuromuscular junctions. J Neurobiol. 1987 Jul;18(4):375–389. doi: 10.1002/neu.480180405. [DOI] [PubMed] [Google Scholar]


