Abstract
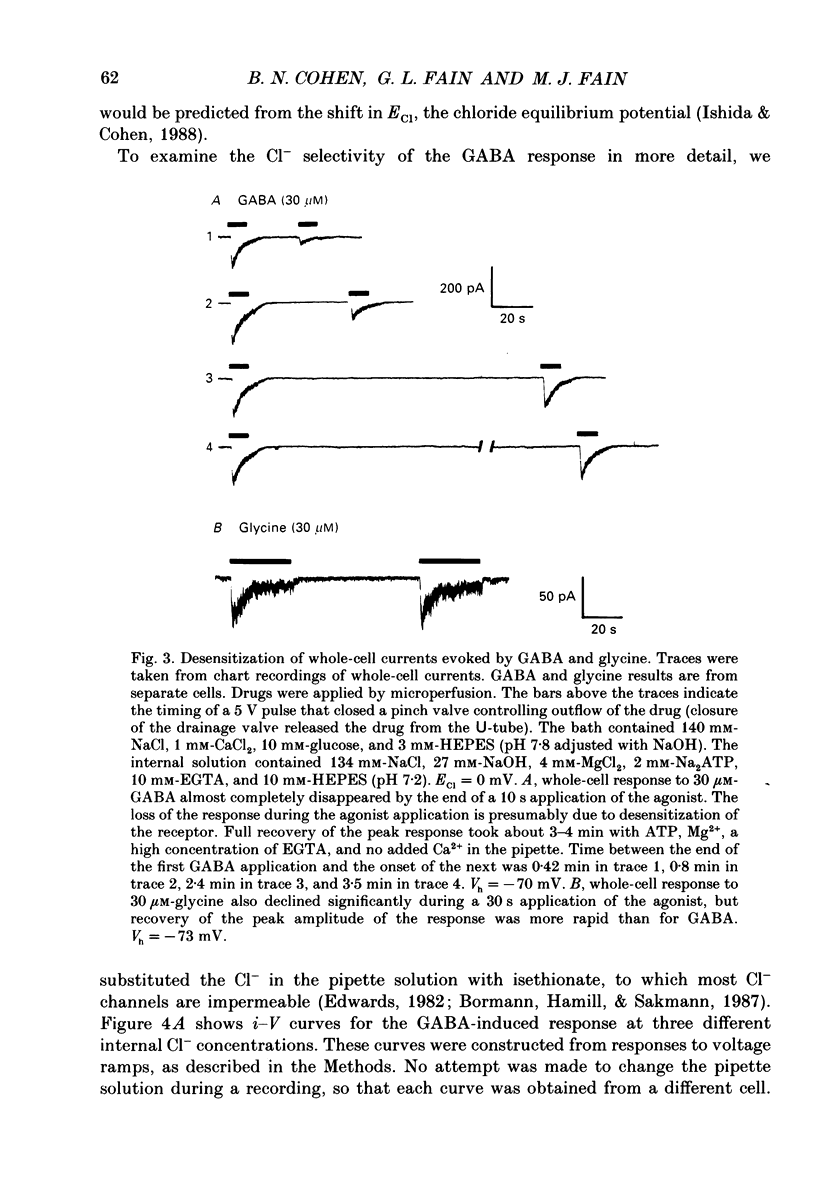
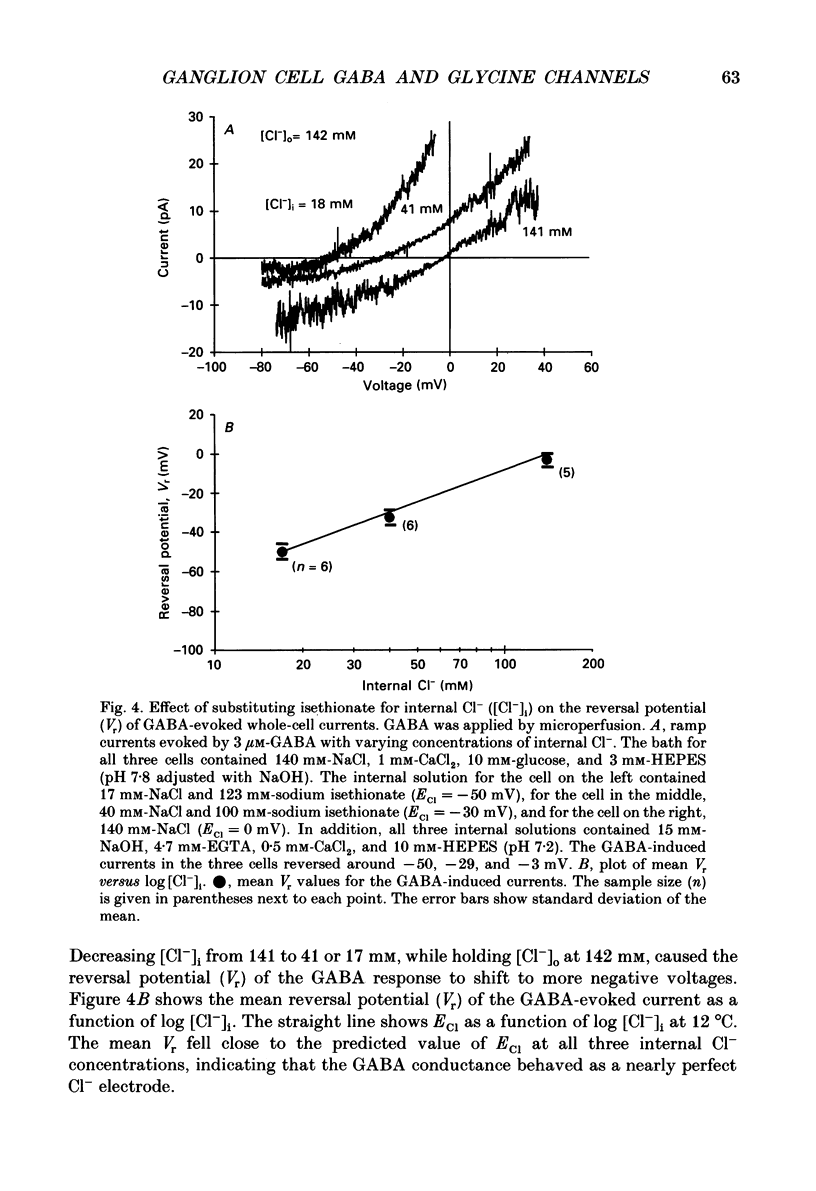
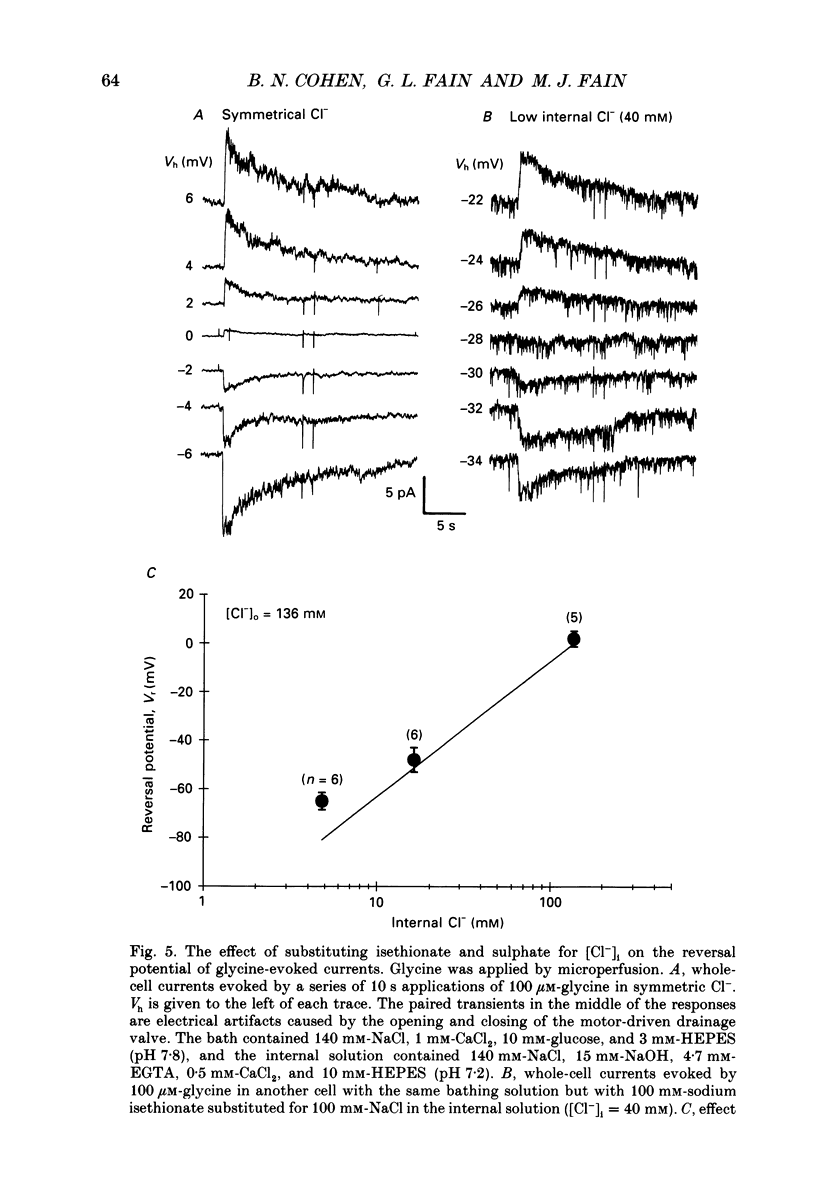
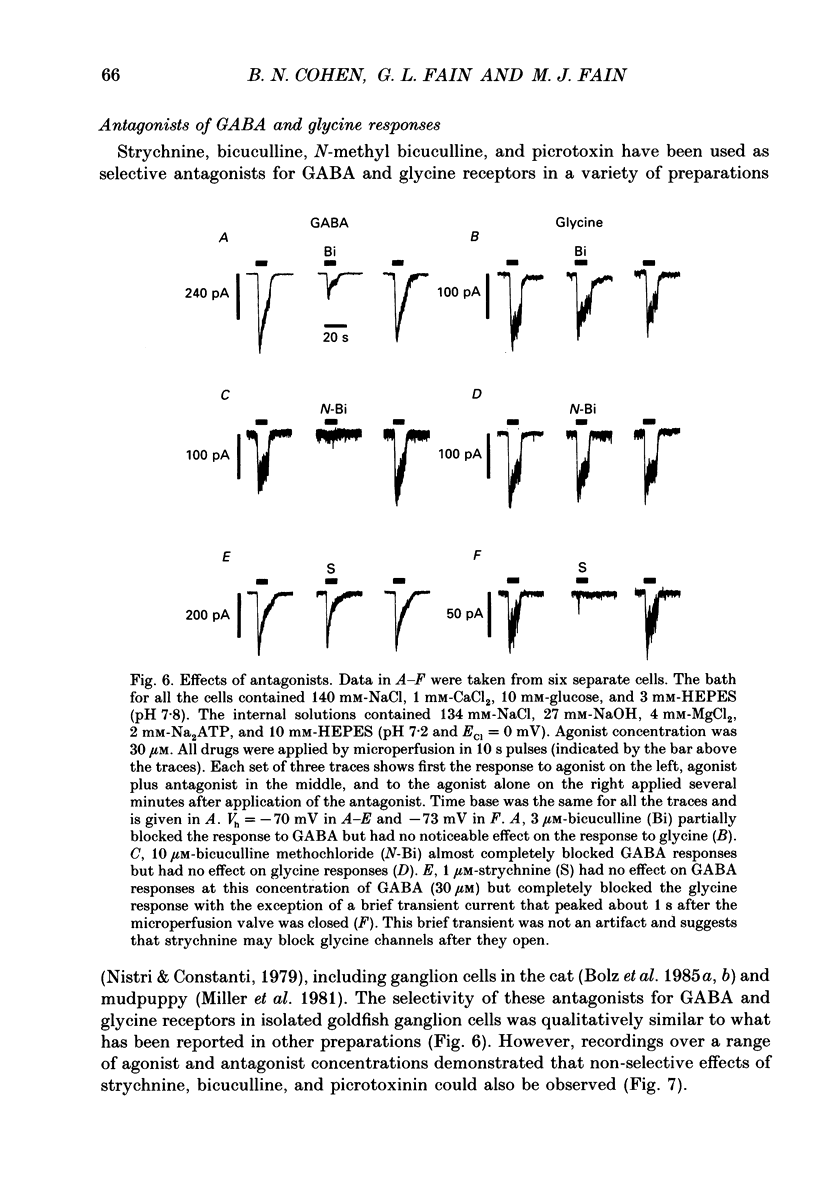
1. Adult goldfish retinas were enzymatically dissociated and ganglion cells were maintained in culture for periods of 1-5 days. Ganglion cells could be identified by their morphology, and this identification was confirmed by retrograde transport of the fluorescent dye Fast Blue injected into the optic nerve stub. 2. All the ganglion cells tested responded to 30 microM-GABA or 100 microM-glycine between 2 and 30 h after enzymatic dissociation of the retina. 3. Whole-cell responses to 30 microM-GABA or glycine declined over a period of seconds during sustained applications of the agonists, probably as a result of desensitization. There was an irreversible decline in the peak whole-cell response to repeated applications of 30 microM-GABA unless the pipette-filling solution contained 2 mM-ATP, 4 mM-Mg2+, 10 mM-EGTA and no added Ca2+. Both GABA and glycine responses also showed an irreversible decline in outside-out patches but, in this case, Mg2+, ATP, and very low Ca2+ failed to stabilize the response. 4. Whole-cell currents activated by both GABA and glycine were demonstrated to be chloride-selective by investigating the dependence of reversal potential (Vr) on internal chloride concentration ([Cl-]i). For GABA responses, the dependence of Vr on [Cl-]i could not be distinguished from that predicted by the Nernst relation. For glycine, deviations from Nernstian dependence were observed, but the permeability to Cl- was at least 20 times greater than to isethionate, SO4(2-), or monovalent cations (Na+ and Cs+). 5. Bicuculline methochloride (10 microM) selectively blocked responses to 3-30 microM-GABA without affecting responses to 30 microM-glycine. Bicuculline itself was not as selective. At agonist concentrations of 30 microM, 3 microM-bicuculline partially blocked the response to GABA but not that to glycine, but bicuculline at 10 microM blocked responses to both GABA and glycine. Strychnine (0.3-1 microM) blocked responses to 30 microM-glycine but also competitively antagonized GABA responses. Picrotoxinin (10 microM) blocked responses to 3 microM-GABA in some cells but also partially antagonized responses to 30 microM-glycine. 6. GABA channels had at least two conductance states at 10-12 degrees C in nearly symmetrical (141 mM in, 142 mM out) chloride. The slope conductance of the most frequently observed (main) state was 16 +/- 2 pS. The reversal potential for the main state was not significantly different from the chloride equilibrium potential (0 mV).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




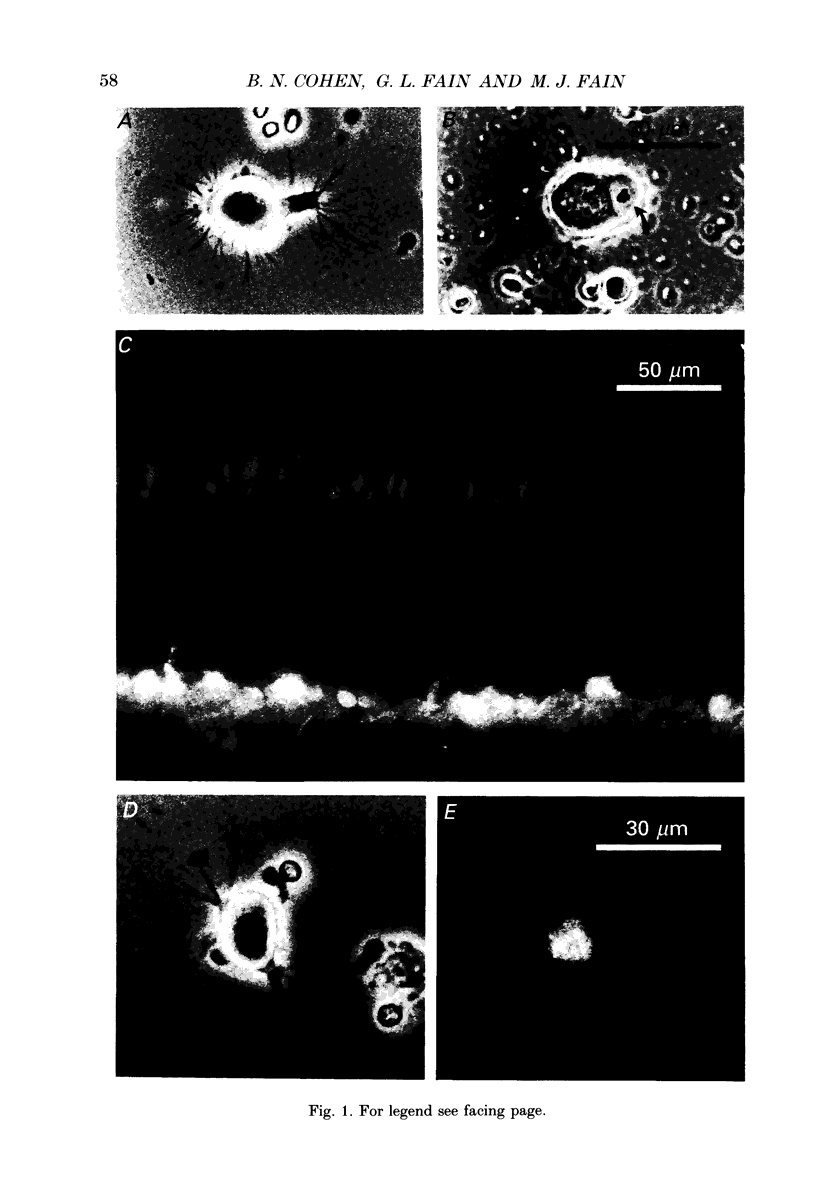

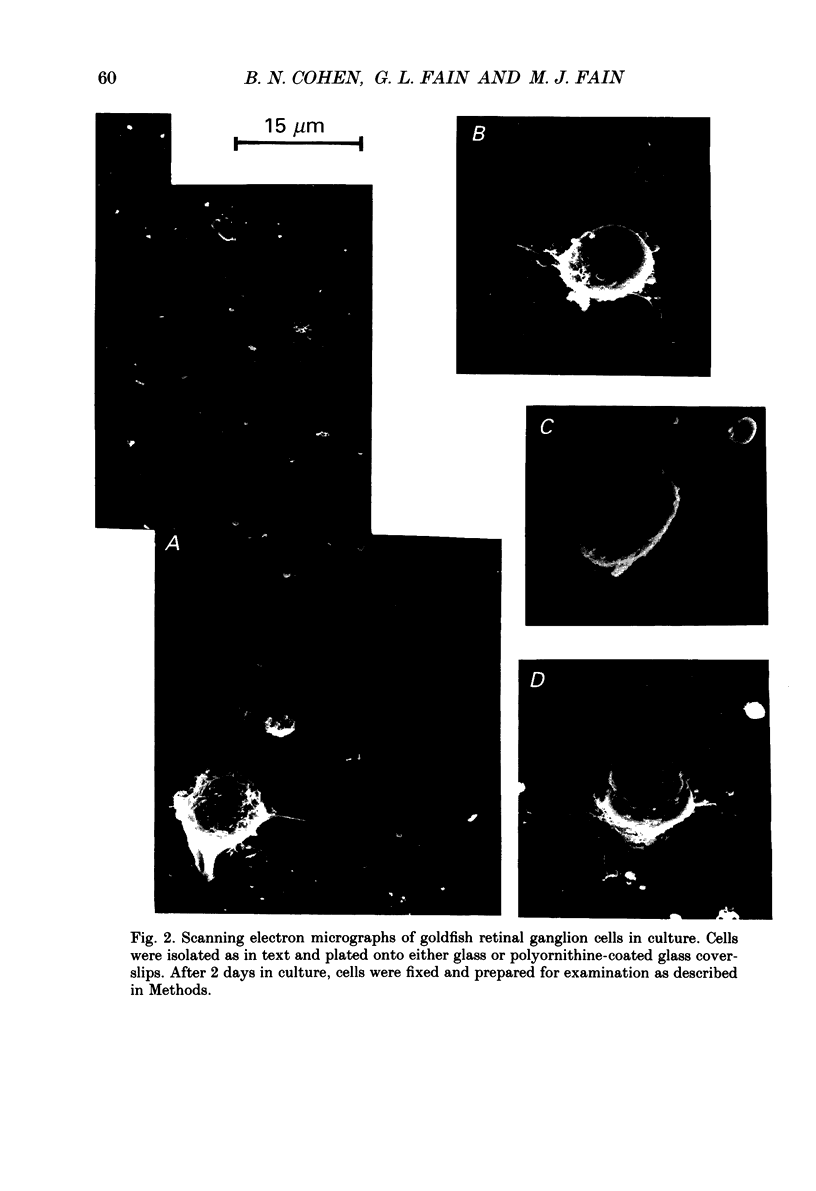




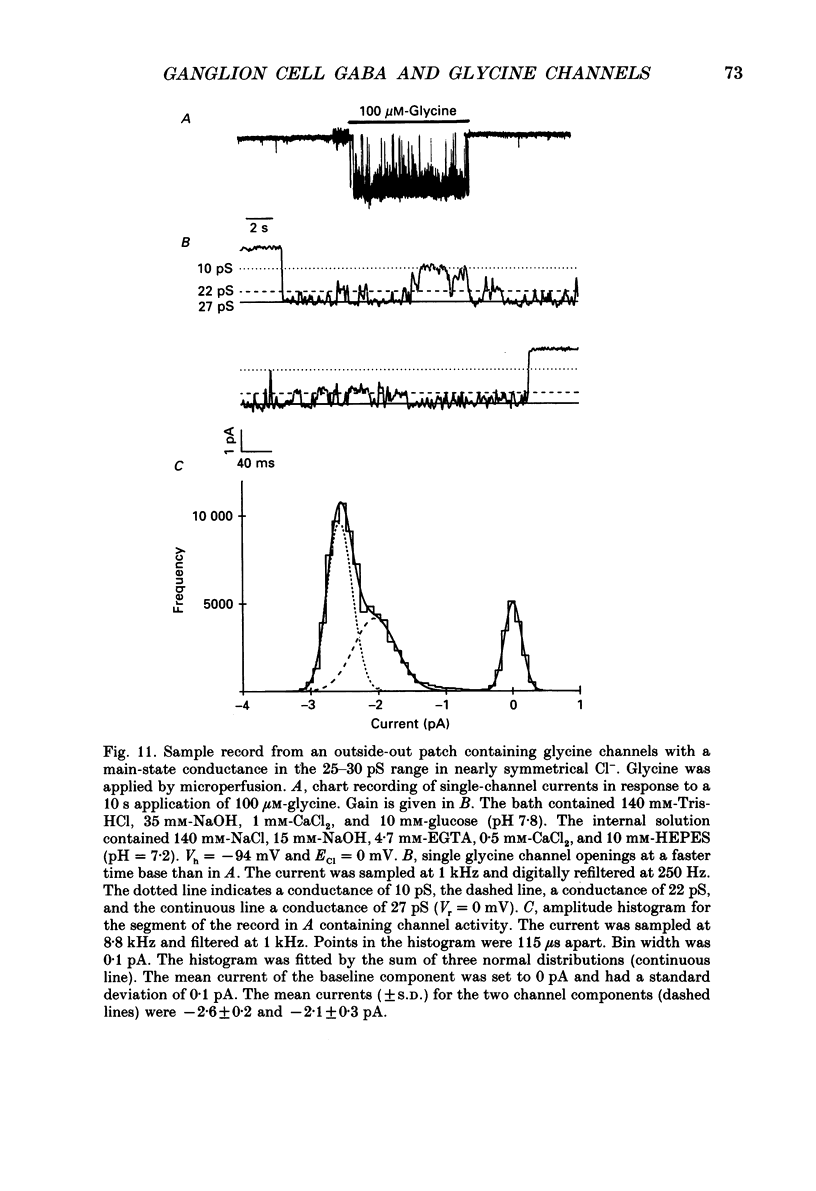
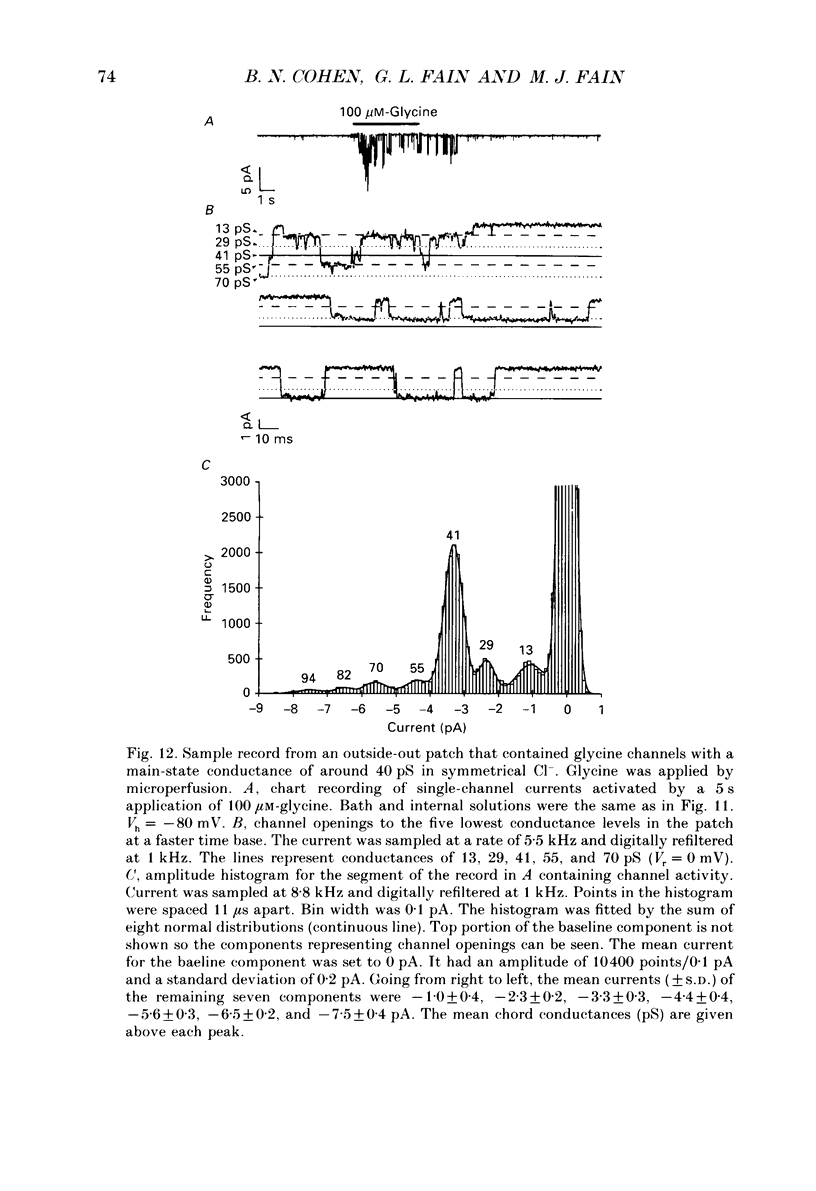

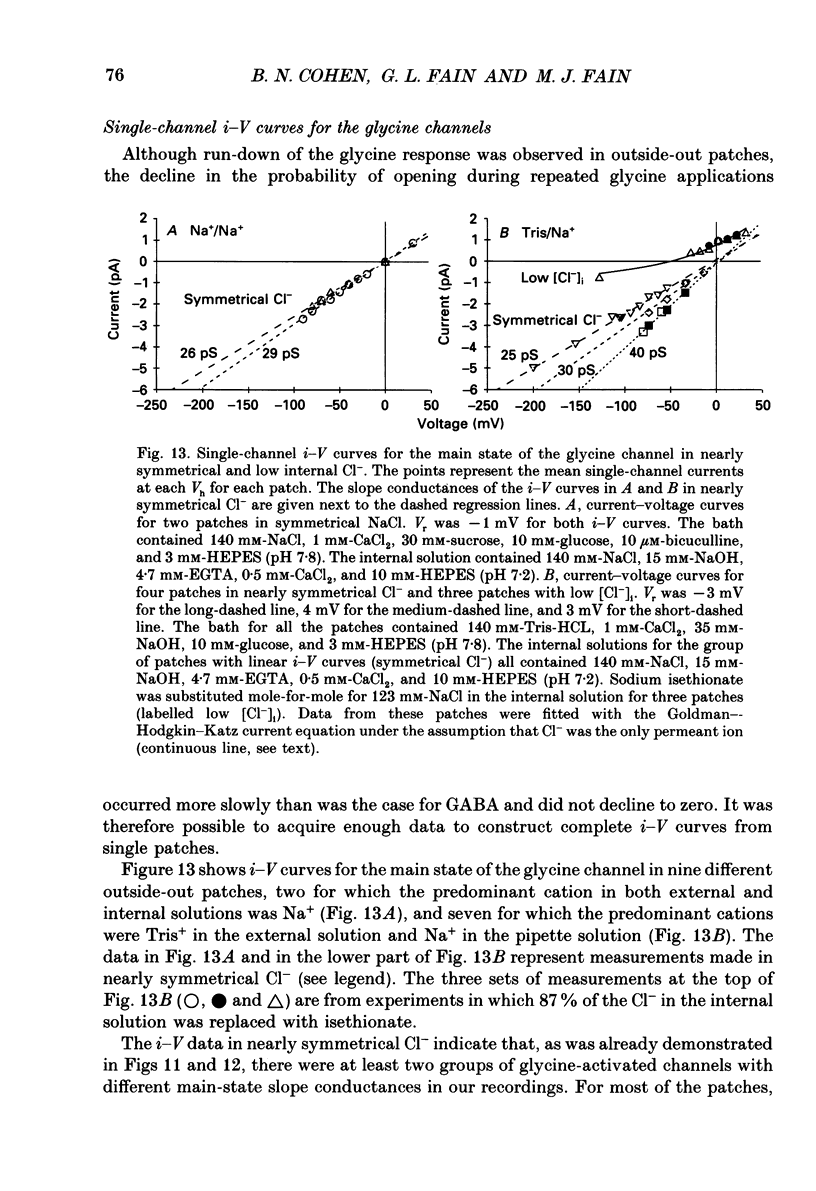






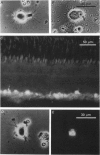
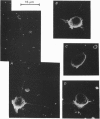
Images in this article
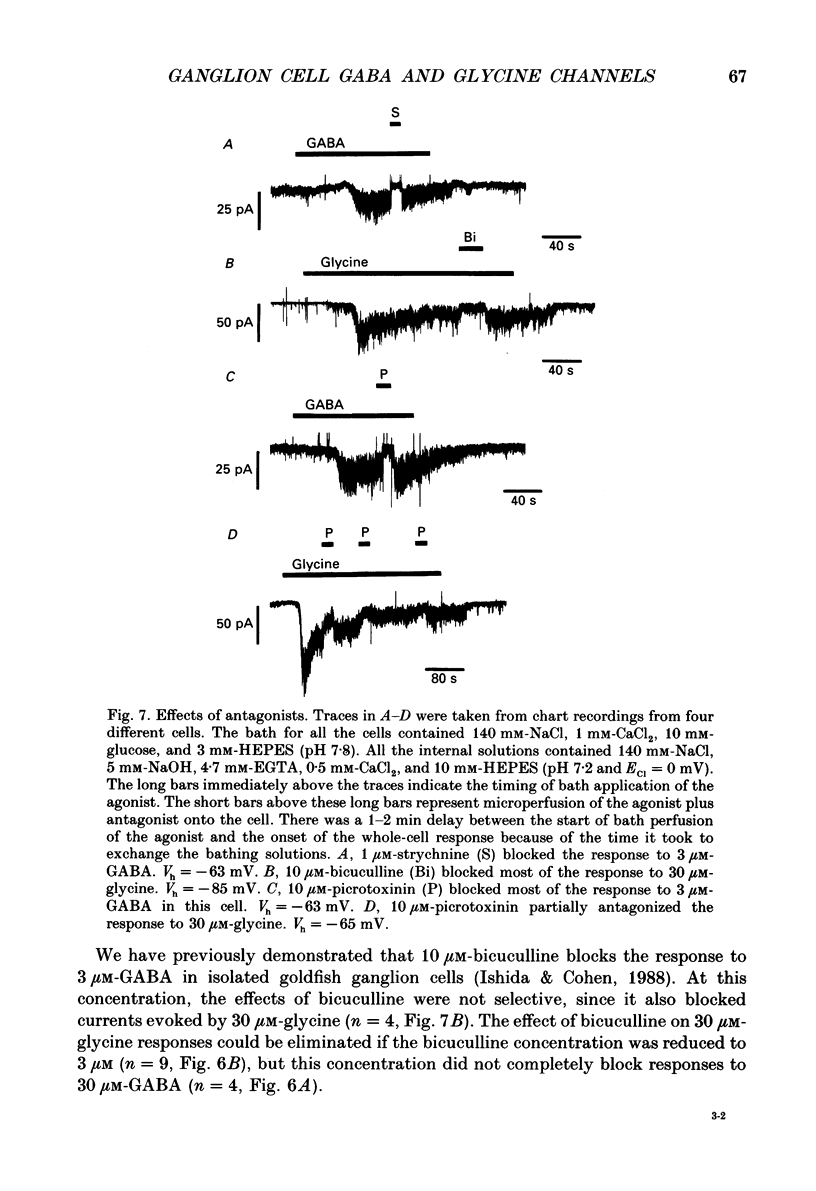
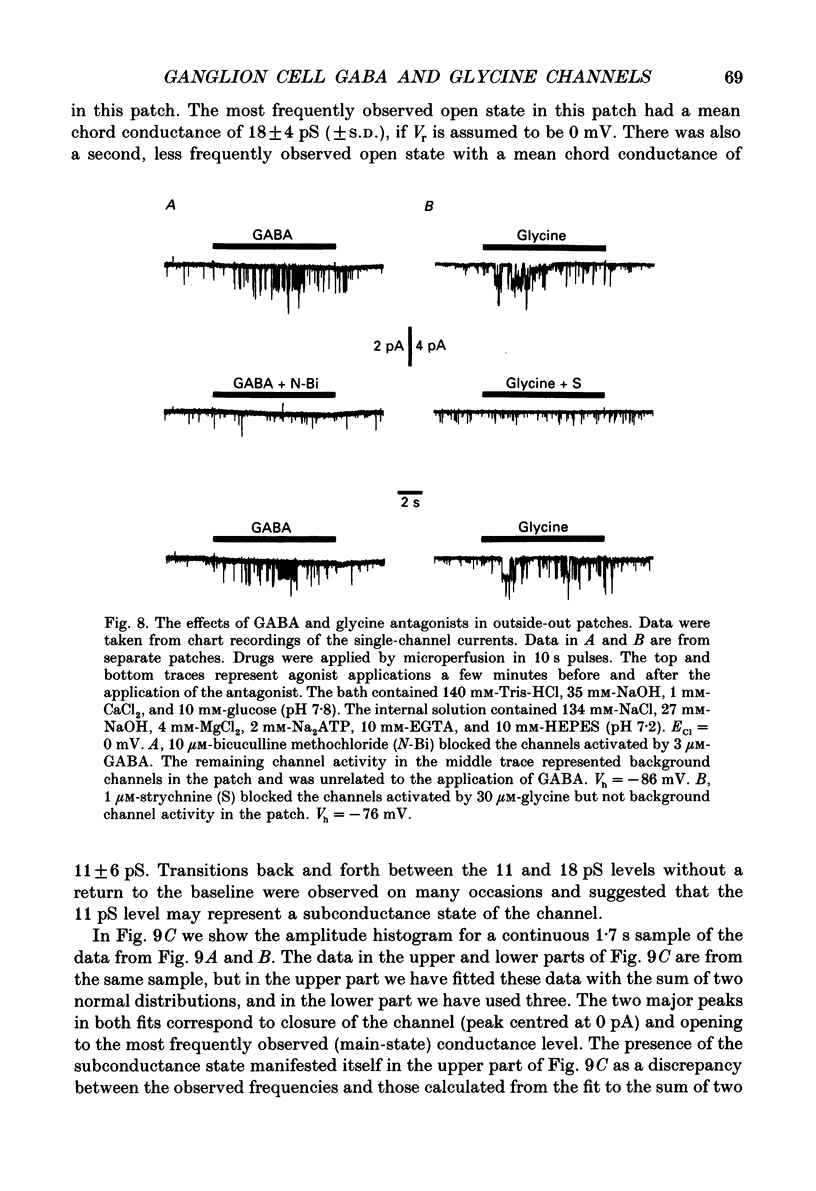
Selected References
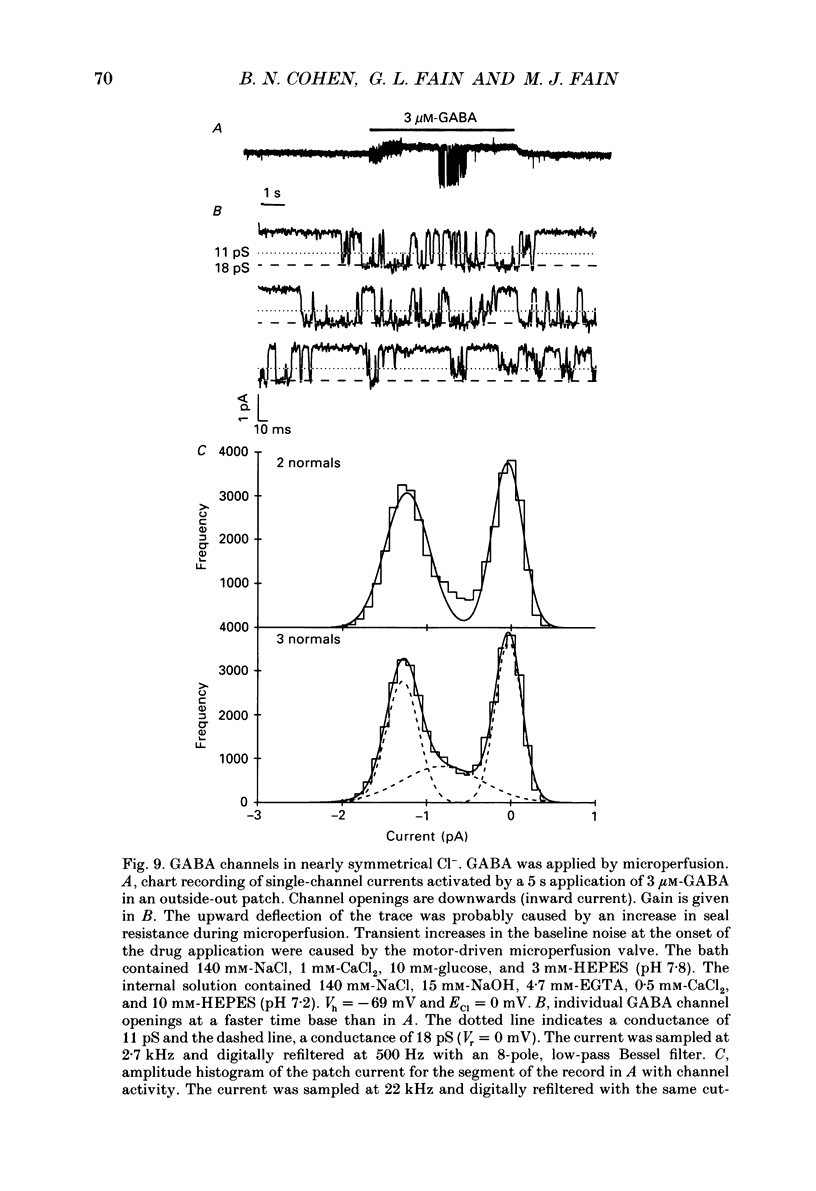
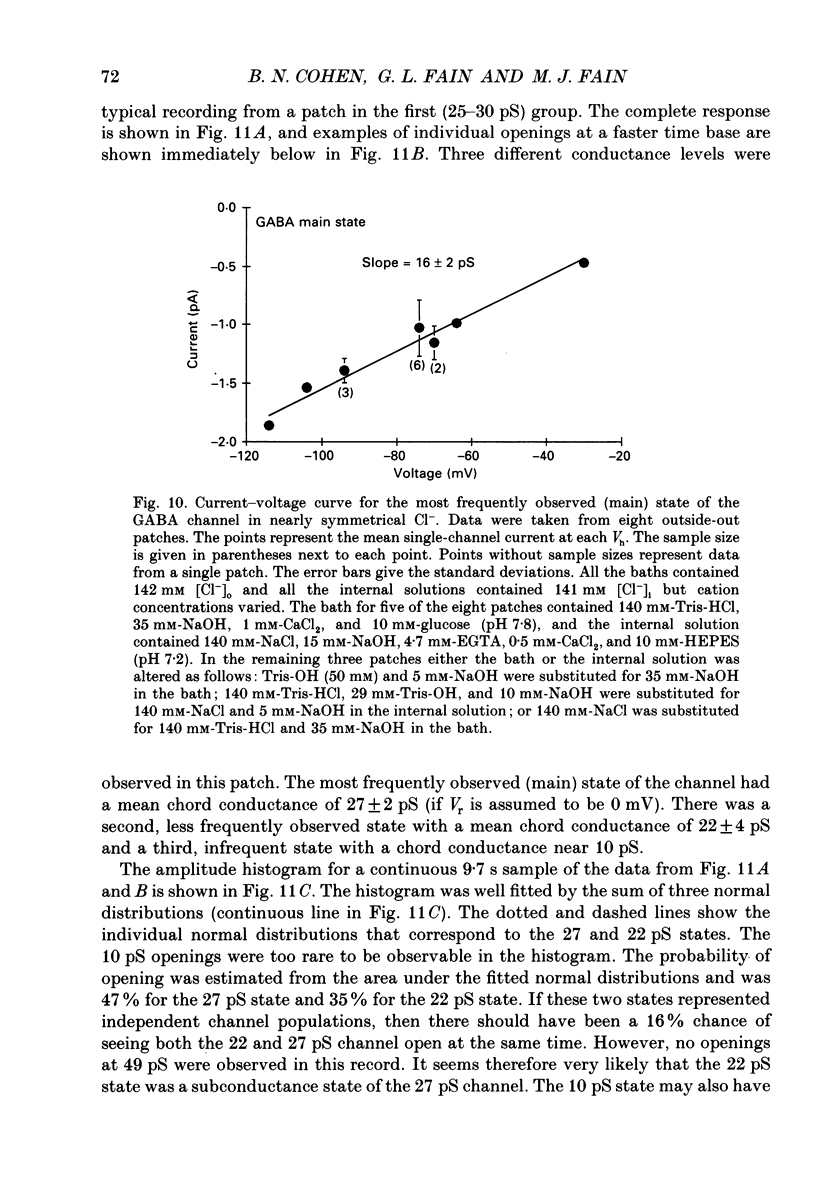
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Inomata N., Tokutomi N. Contribution of chloride shifts to the fade of gamma-aminobutyric acid-gated currents in frog dorsal root ganglion cells. J Physiol. 1987 Oct;391:219–234. doi: 10.1113/jphysiol.1987.sp016735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen C. N., Albuquerque E. X. Conductance properties of GABA-activated chloride currents recorded from cultured hippocampal neurons. Brain Res. 1987 Apr 28;410(1):159–163. doi: 10.1016/s0006-8993(87)80039-2. [DOI] [PubMed] [Google Scholar]
- Belgum J. H., Dvorak D. R., McReynolds J. S. Strychnine blocks transient but not sustained inhibition in mudpuppy retinal ganglion cells. J Physiol. 1984 Sep;354:273–286. doi: 10.1113/jphysiol.1984.sp015375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz J., Frumkes T., Voigt T., Wässle H. Action and localization of gamma-aminobutyric acid in the cat retina. J Physiol. 1985 May;362:369–393. doi: 10.1113/jphysiol.1985.sp015684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz J., Thier P., Voigt T., Wässle H. Action and localization of glycine and taurine in the cat retina. J Physiol. 1985 May;362:395–413. doi: 10.1113/jphysiol.1985.sp015685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Clapham D. E. gamma-Aminobutyric acid receptor channels in adrenal chromaffin cells: a patch-clamp study. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2168–2172. doi: 10.1073/pnas.82.7.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: changes in centre surround receptive fields. J Physiol. 1978 Mar;276:299–310. doi: 10.1113/jphysiol.1978.sp012234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W., Wyatt H. J. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: lateral interactions for cells with more complex receptive fields. J Physiol. 1978 Mar;276:277–298. doi: 10.1113/jphysiol.1978.sp012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. L. Effects of glycine and GABA on the ganglion cels of the retina of the skate Raja erinacea. Brain Res. 1985 Apr 15;332(1):169–173. doi: 10.1016/0006-8993(85)90402-0. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Hökfelt T., Elfvin L. G., Terenius L. Enkephalin-containing sympathetic preganglionic neurons projecting to the inferior mesenteric ganglion: evidence from combined retrograde tracing and immunohistochemistry. Neuroscience. 1982;7(9):2039–2050. doi: 10.1016/0306-4522(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Edwards C. The selectivity of ion channels in nerve and muscle. Neuroscience. 1982 Jun;7(6):1335–1366. doi: 10.1016/0306-4522(82)90249-4. [DOI] [PubMed] [Google Scholar]
- Ehinger B., Ottersen O. P., Storm-Mathisen J., Dowling J. E. Bipolar cells in the turtle retina are strongly immunoreactive for glutamate. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8321–8325. doi: 10.1073/pnas.85.21.8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol. 1986 Sep;378:437–460. doi: 10.1113/jphysiol.1986.sp016229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Ishida A. T., Callery S. Mechanisms of synaptic transmission in the retina. Vision Res. 1983;23(11):1239–1249. doi: 10.1016/0042-6989(83)90099-8. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman R. D., Adolph A. R., Dowling J. E. Inner plexiform circuits in the carp retina: effects of cholinergic agonists, GABA, and substance P on the ganglion cells. Brain Res. 1982 Feb 18;234(1):81–99. doi: 10.1016/0006-8993(82)90474-7. [DOI] [PubMed] [Google Scholar]
- Huck S., Lux H. D. Patch-clamp study of ion channels activated by GABA and glycine in cultured cerebellar neurons of the mouse. Neurosci Lett. 1987 Aug 18;79(1-2):103–107. doi: 10.1016/0304-3940(87)90679-3. [DOI] [PubMed] [Google Scholar]
- Ishida A. T., Cohen B. N. GABA-activated whole-cell currents in isolated retinal ganglion cells. J Neurophysiol. 1988 Aug;60(2):381–396. doi: 10.1152/jn.1988.60.2.381. [DOI] [PubMed] [Google Scholar]
- Massey S. C., Redburn D. A. Transmitter circuits in the vertebrate retina. Prog Neurobiol. 1987;28(1):55–96. doi: 10.1016/0301-0082(87)90005-0. [DOI] [PubMed] [Google Scholar]
- Mathers D. A., Barker J. L. GABA- and glycine-induced Cl- channels in cultured mouse spinal neurons require the same energy to close. Brain Res. 1981 Nov 16;224(2):441–445. doi: 10.1016/0006-8993(81)90875-1. [DOI] [PubMed] [Google Scholar]
- Miller R. F., Frumkes T. E., Slaughter M., Dacheux R. F. Physiological and pharmacological basis of GABA and glycine action on neurons of mudpuppy retina. II. Amacrine and ganglion cells. J Neurophysiol. 1981 Apr;45(4):764–782. doi: 10.1152/jn.1981.45.4.764. [DOI] [PubMed] [Google Scholar]
- Murray M., Grafstein B. Changes in the morphology and amino acid incorporation of regenerating goldfish optic neurons. Exp Neurol. 1969 Apr;23(4):544–560. doi: 10.1016/0014-4886(69)90124-1. [DOI] [PubMed] [Google Scholar]
- Nistri A., Constanti A. Pharmacological characterization of different types of GABA and glutamate receptors in vertebrates and invertebrates. Prog Neurobiol. 1979;13(2):117–235. doi: 10.1016/0301-0082(79)90016-9. [DOI] [PubMed] [Google Scholar]
- Piggott S. M., Kerkut G. A., Walker R. J. The actions of picrotoxin, strychnine, bicuculline and other convulsants and antagonists on the responses to acetylcholine glutamic acid and gamma-aminobutyric acid on Helix neurones. Comp Biochem Physiol C. 1977;57(2):107–116. doi: 10.1016/0306-4492(77)90054-5. [DOI] [PubMed] [Google Scholar]
- Schwartz M., Agranoff B. W. Outgrowth and maintenance of neurites from cultured goldfish retinal ganglion cells. Brain Res. 1981 Feb 16;206(2):331–343. doi: 10.1016/0006-8993(81)90535-7. [DOI] [PubMed] [Google Scholar]
- Soffe S. R. Ionic and pharmacological properties of reciprocal inhibition in Xenopus embryo motoneurones. J Physiol. 1987 Jan;382:463–473. doi: 10.1113/jphysiol.1987.sp016378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer A., Kay A. R., Wong R. K. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science. 1988 Jul 15;241(4863):339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- Sterling P. Microcircuitry of the cat retina. Annu Rev Neurosci. 1983;6:149–185. doi: 10.1146/annurev.ne.06.030183.001053. [DOI] [PubMed] [Google Scholar]
- Tachibana M. Membrane properties of solitary horizontal cells isolated from goldfish retina. J Physiol. 1981 Dec;321:141–161. doi: 10.1113/jphysiol.1981.sp013976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleb O., Trouslard J., Demeneix B. A., Feltz P., Bossu J. L., Dupont J. L., Feltz A. Spontaneous and GABA-evoked chloride channels on pituitary intermediate lobe cells and their internal Ca requirements. Pflugers Arch. 1987 Aug;409(6):620–631. doi: 10.1007/BF00584663. [DOI] [PubMed] [Google Scholar]
- Tanifuji M., Sokabe M., Kasai M. An anion channel of sarcoplasmic reticulum incorporated into planar lipid bilayers: single-channel behavior and conductance properties. J Membr Biol. 1987;99(2):103–111. doi: 10.1007/BF01871230. [DOI] [PubMed] [Google Scholar]
- Weiss D. S., Barnes E. M., Jr, Hablitz J. J. Whole-cell and single-channel recordings of GABA-gated currents in cultured chick cerebral neurons. J Neurophysiol. 1988 Feb;59(2):495–513. doi: 10.1152/jn.1988.59.2.495. [DOI] [PubMed] [Google Scholar]
- Wojtowicz J. M., Marshall K. C., Hendelman W. J. Electrophysiological and pharmacological studies of the inhibitory projection from the cerebellar cortex to the deep cerebellar nuclei in tissue culture. Neuroscience. 1978;3(7):607–618. doi: 10.1016/0306-4522(78)90002-7. [DOI] [PubMed] [Google Scholar]
- Wässle H., Schäfer-Trenkler I., Voigt T. Analysis of a glycinergic inhibitory pathway in the cat retina. J Neurosci. 1986 Feb;6(2):594–604. doi: 10.1523/JNEUROSCI.06-02-00594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]