Abstract
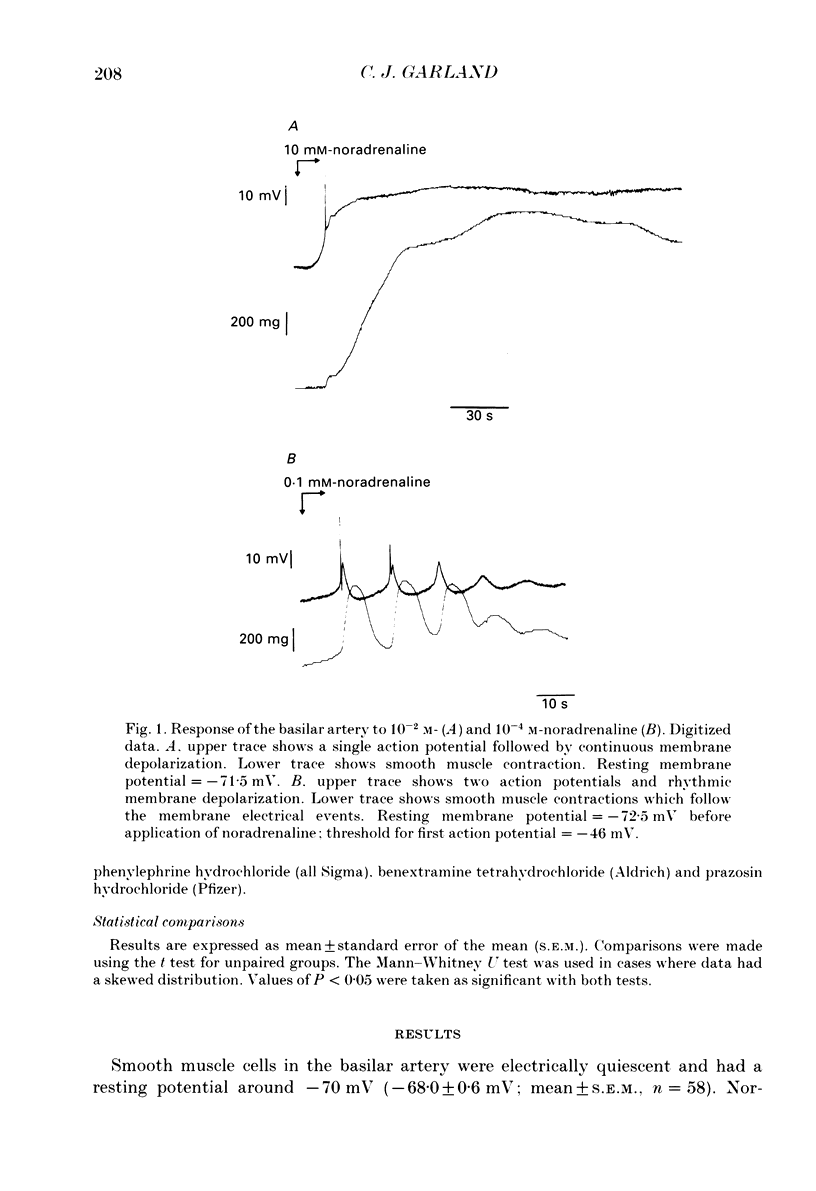
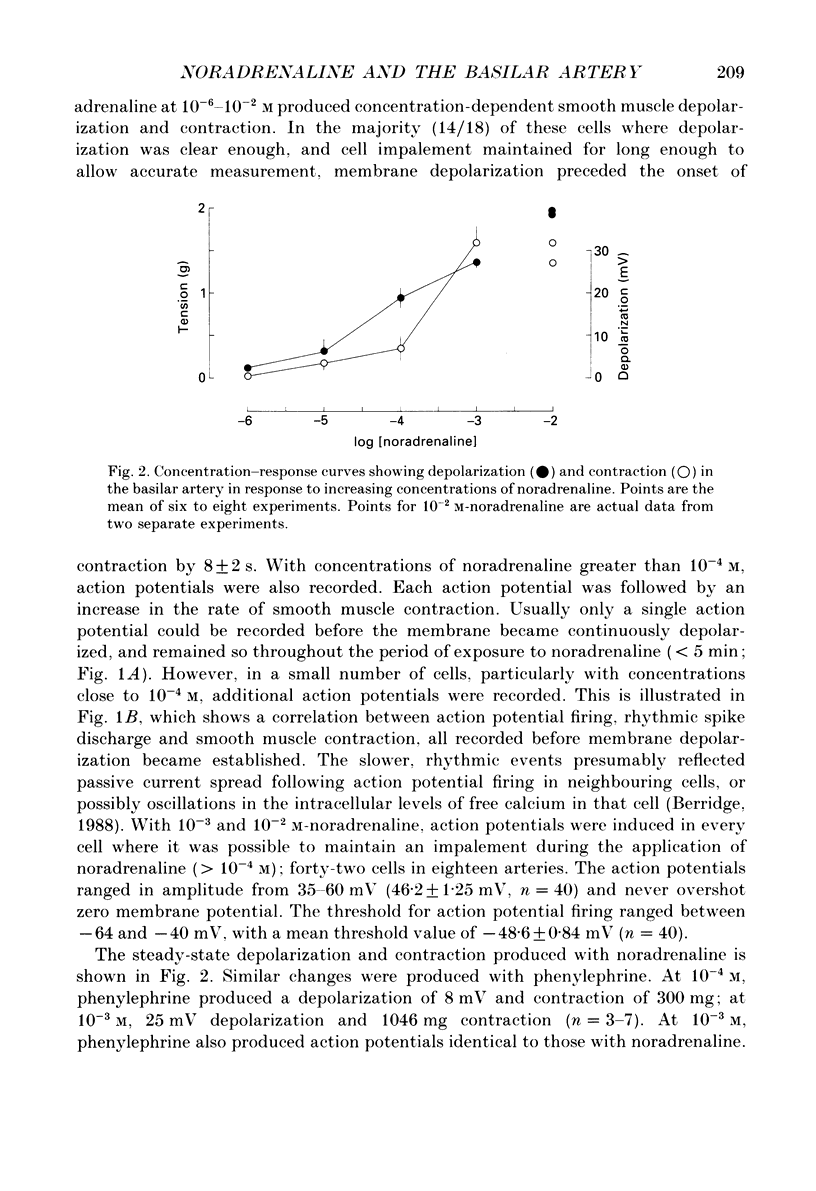
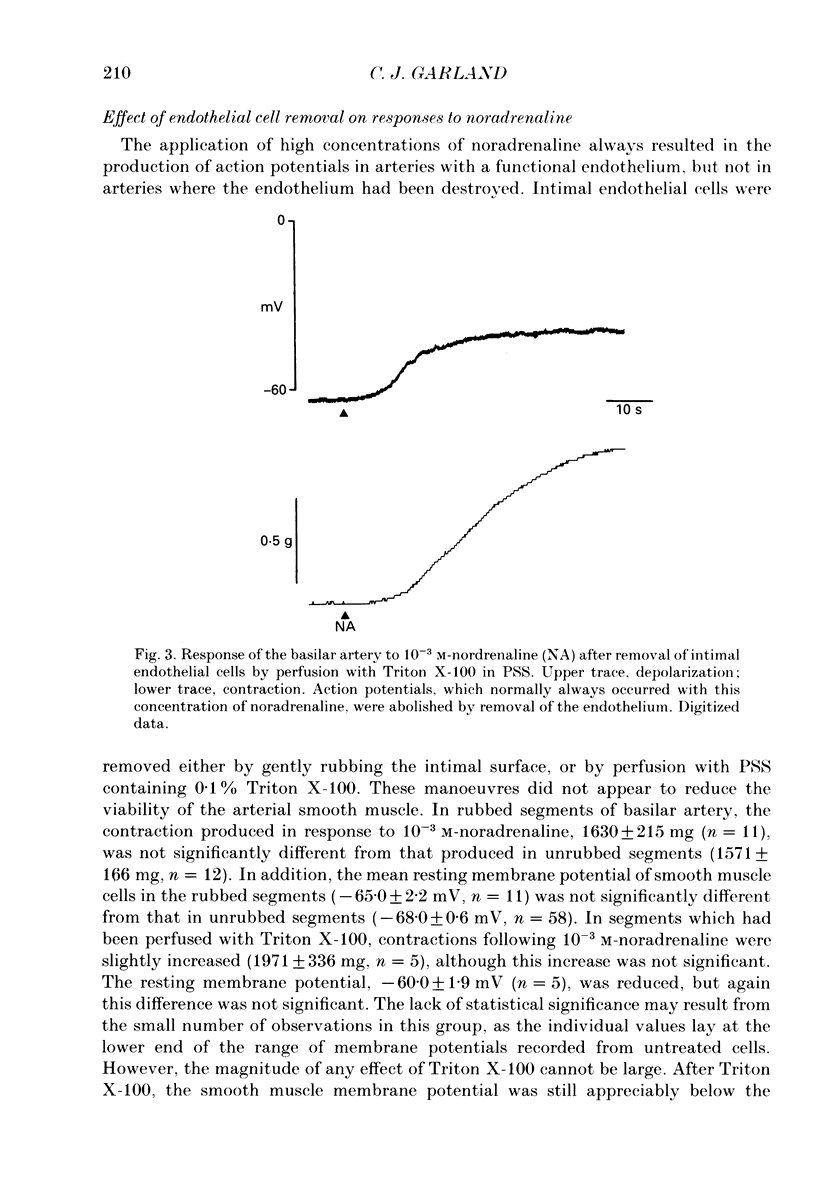
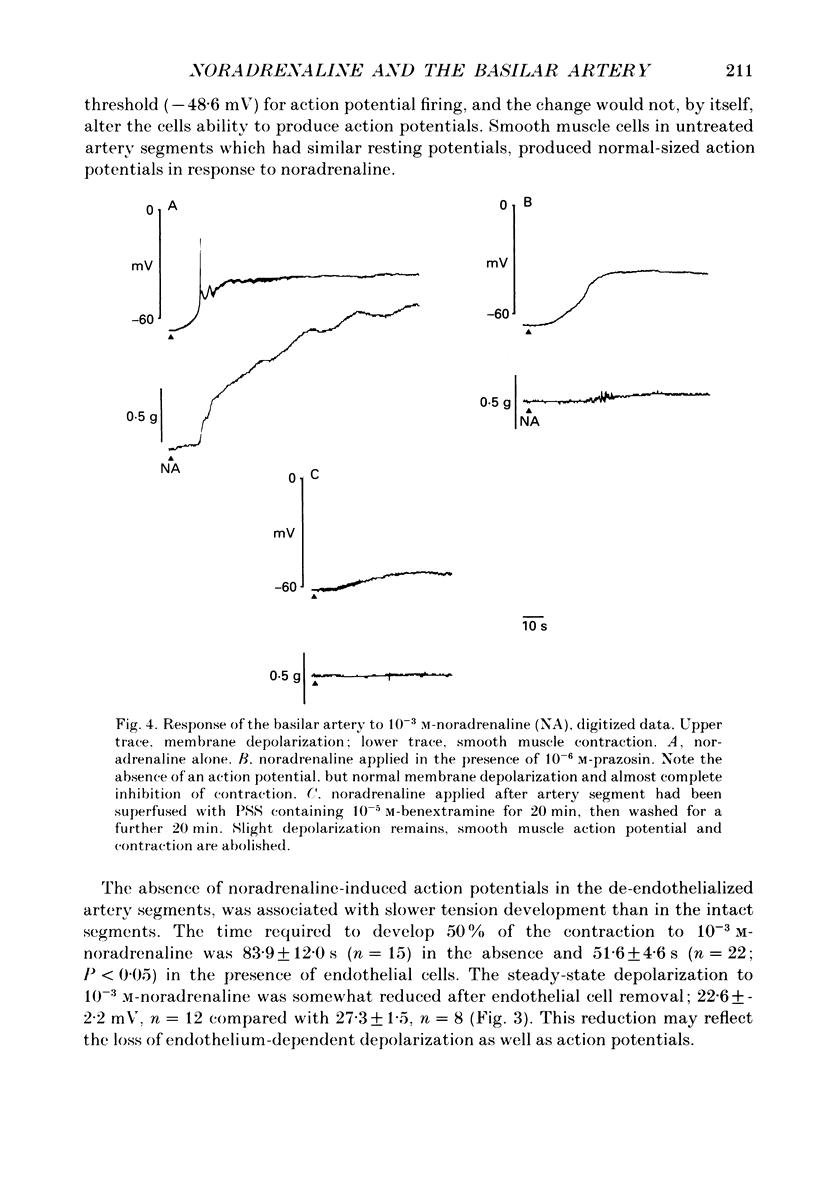
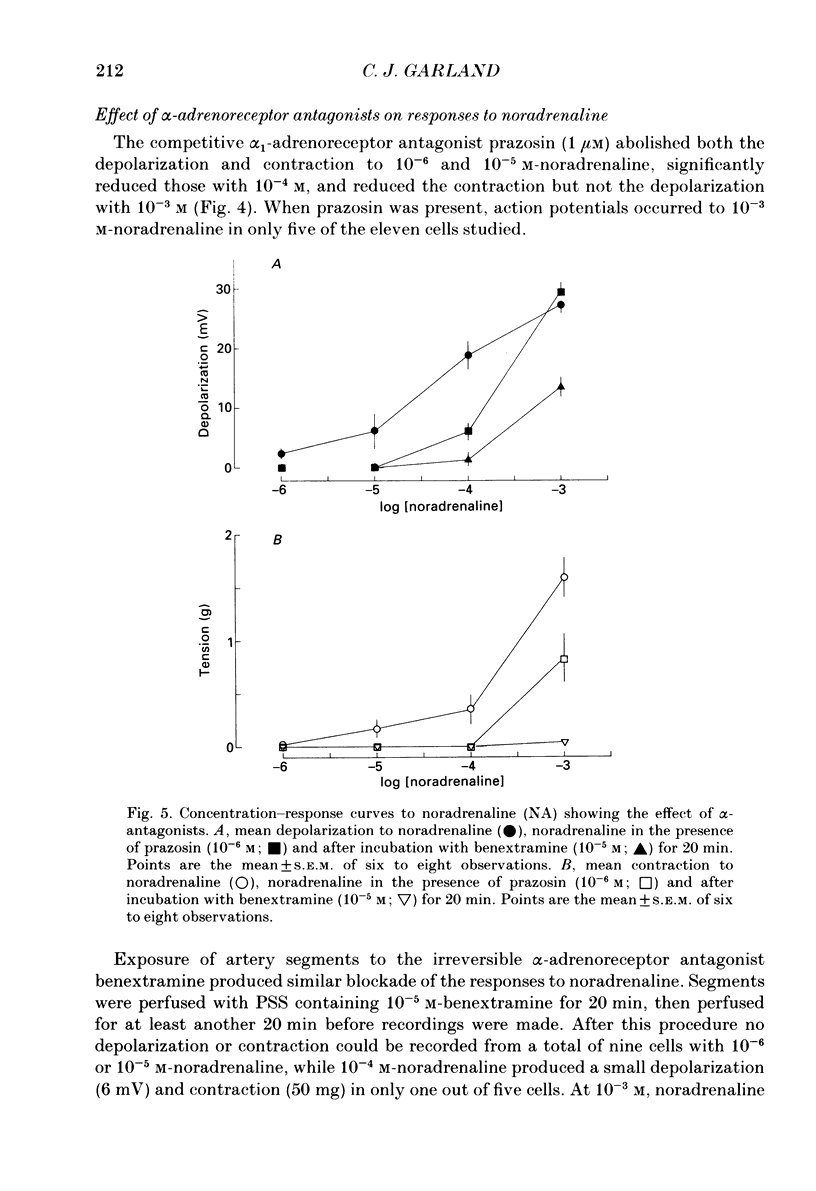
1. Noradrenaline (10(-6)-10(-2) M) produced slow, concentration-dependent depolarization of smooth muscle cells in the rabbit basilar artery, which preceded the onset of contraction by around 8 s (n = 18). 2. With concentrations greater than 10(-4) M, noradrenaline produced action potentials and fast rhythmic depolarizations superimposed on the slow depolarization. Each fast event was followed by a clear increase in the rate of smooth muscle contraction. The selective alpha 1-adrenoreceptor agonist phenylephrine produced very similar membrane and contractile responses. 3. Action potentials were not produced in artery segments where the endothelium had been removed. In these segments, the amplitude of both contraction and slow depolarization to noradrenaline was similar to that observed in segments with an intact endothelium, but the tension increased more slowly; 84 s compared to the 52 s required to produce 50% of total contraction when the endothelium was functional. 4. The selective alpha 1-antagonist prazosin (10(-6) M) either abolished or significantly reduced both the slow depolarization (with concentrations less than 10(-3) M-noradrenaline) and smooth muscle contraction to noradrenaline. When prazosin was present, action potentials with 10(-3) M-noradrenaline were only produced in 50% of the cells studied. 5. Irreversible blockade of alpha-adrenoreceptors with benextramine (10(-5) M for 20 min) abolished action potentials and both the depolarization and contraction produced with all but the highest concentrations of noradrenaline. With 10(-3) M-noradrenaline, depolarization was produced but it was significantly reduced and usually not associated with smooth muscle contraction. 6. The results show that smooth muscle depolarization, contraction and possibly endothelium-dependent action potentials are produced by alpha-adrenoreceptor stimulation. They also show that noradrenaline-induced action potentials produce smooth muscle contraction, and that slow depolarization is an important, but not absolute requirement for contraction. The fact that action potentials were produced in response to high concentrations of noradrenaline in the presence of prazosin, but not after benextramine, suggests that these concentrations of noradrenaline can surmount competitive antagonism with prazosin.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus J. A., Broughton A., Mulvany M. J. Role of alpha-adrenoceptors in constrictor responses of rat, guinea-pig and rabbit small arteries to neural activation. J Physiol. 1988 Sep;403:495–510. doi: 10.1113/jphysiol.1988.sp017260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C., Vogt M. Release of endogenous noradrenaline from an isolated muscular artery. Release of endogenous noradrenaline from an isolated muscular artery. J Physiol. 1971 Jun;215(2):509–520. doi: 10.1113/jphysiol.1971.sp009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate-induced membrane potential oscillations in Xenopus oocytes. J Physiol. 1988 Sep;403:589–599. doi: 10.1113/jphysiol.1988.sp017266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan J. A., Duckworth J., Laher I., Oriowo M. A., McPherson G. A., Bevan R. D. Sympathetic control of cerebral arteries: specialization in receptor type, reserve, affinity, and distribution. FASEB J. 1987 Sep;1(3):193–198. doi: 10.1096/fasebj.1.3.2887477. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984 Jun;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Large W. A. Are junction potentials essential? Dual mechanism of smooth muscle cell activation by transmitter released from autonomic nerves. Q J Exp Physiol. 1986 Jan;71(1):1–28. doi: 10.1113/expphysiol.1986.sp002960. [DOI] [PubMed] [Google Scholar]
- Brazenor R. M., Angus J. A. Ergometrine contracts isolated canine coronary arteries by a serotonergic mechanism: no role for alpha adrenoceptors. J Pharmacol Exp Ther. 1981 Aug;218(2):530–536. [PubMed] [Google Scholar]
- Cheung D. W. An electrophysiological study of alpha-adrenoceptor mediated excitation-contraction coupling in the smooth muscle cells of the rat saphenous vein. Br J Pharmacol. 1985 Jan;84(1):265–271. [PMC free article] [PubMed] [Google Scholar]
- Duckles S. P., Bevan J. A. Pharmacological characterization of adrenergic receptors of a rabbit cerebral artery in vitro. J Pharmacol Exp Ther. 1976 May;197(2):371–378. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garland C. J. The role of membrane depolarization in the contractile response of the rabbit basilar artery to 5-hydroxytryptamine. J Physiol. 1987 Nov;392:333–348. doi: 10.1113/jphysiol.1987.sp016783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie M. N., Owasoyo J. O., McMurtry I. F., O'Brien R. F. Sustained coronary vasoconstriction provoked by a peptidergic substance released from endothelial cells in culture. J Pharmacol Exp Ther. 1986 Feb;236(2):339–343. [PubMed] [Google Scholar]
- Graham J. M., Keatinge W. R. Differences in sensitivity to vasoconstrictor drugs within the wall of the sheep carotid artery. J Physiol. 1972 Mar;221(2):477–492. doi: 10.1113/jphysiol.1972.sp009763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder D. R. Pressure-induced myogenic activation of cat cerebral arteries is dependent on intact endothelium. Circ Res. 1987 Jan;60(1):102–107. doi: 10.1161/01.res.60.1.102. [DOI] [PubMed] [Google Scholar]
- Hickey K. A., Rubanyi G., Paul R. J., Highsmith R. F. Characterization of a coronary vasoconstrictor produced by cultured endothelial cells. Am J Physiol. 1985 May;248(5 Pt 1):C550–C556. doi: 10.1152/ajpcell.1985.248.5.C550. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature. 1980 Feb 21;283(5749):767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O., Silverberg G. D. Noradrenaline receptors on the rat basilar artery. J Physiol. 1982 Jul;328:351–360. doi: 10.1113/jphysiol.1982.sp014268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Silverberg G. D., van Helden D. F. The action potential and underlying ionic currents in proximal rat middle cerebral arterioles. J Physiol. 1986 Feb;371:289–304. doi: 10.1113/jphysiol.1986.sp015975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Laher I., Bevan J. A. Alpha adrenoceptor number limits response of some rabbit arteries to norepinephrine. J Pharmacol Exp Ther. 1985 May;233(2):290–297. [PubMed] [Google Scholar]
- Laher I., Khayal M. A., Bevan J. A. Norepinephrine-sensitive, phenoxybenzamine-resistant receptor sites associated with contraction in rabbit arterial but not venous smooth muscle: possible role in adrenergic neurotransmission. J Pharmacol Exp Ther. 1986 May;237(2):364–368. [PubMed] [Google Scholar]
- Laher I., Nishimura S., Bevan J. A. Prazosin selectively antagonizes norepinephrine contractions at low-affinity, non-alpha adrenoceptor sites (extraceptors) in arterial muscle. J Pharmacol Exp Ther. 1986 Dec;239(3):846–852. [PubMed] [Google Scholar]
- Luff S. E., McLachlan E. M., Hirst G. D. An ultrastructural analysis of the sympathetic neuromuscular junctions on arterioles of the submucosa of the guinea pig ileum. J Comp Neurol. 1987 Mar 22;257(4):578–594. doi: 10.1002/cne.902570407. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Nilsson H., Flatman J. A. Role of membrane potential in the response of rat small mesenteric arteries to exogenous noradrenaline stimulation. J Physiol. 1982 Nov;332:363–373. doi: 10.1113/jphysiol.1982.sp014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild T. O., Kotecha N. Relation between membrane potential and contractile force in smooth muscle of the rat tail artery during stimulation by norepinephrine, 5-hydroxytryptamine, and potassium. Circ Res. 1987 May;60(5):791–795. doi: 10.1161/01.res.60.5.791. [DOI] [PubMed] [Google Scholar]
- Neild T. O., Zelcer E. Noradrenergic neuromuscular transmission with special reference to arterial smooth muscle. Prog Neurobiol. 1982;19(3):141–158. doi: 10.1016/0301-0082(82)90004-1. [DOI] [PubMed] [Google Scholar]
- Taugner R., Kirchheim H., Forssmann W. G. Myoendothelial contacts in glomerular arterioles and in renal interlobular arteries of rat, mouse and Tupaia belangeri. Cell Tissue Res. 1984;235(2):319–325. doi: 10.1007/BF00217856. [DOI] [PubMed] [Google Scholar]
- Usui H., Kurahashi K., Shirahase H., Fukui K., Fujiwara M. Endothelium-dependent vasocontraction in response to noradrenaline in the canine cerebral artery. Jpn J Pharmacol. 1987 Jun;44(2):228–231. doi: 10.1254/jjp.44.228. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- Walmsley J. G., Gore R. W., Dacey R. G., Jr, Damon D. N., Duling B. R. Quantitative morphology of arterioles from the hamster cheek pouch related to mechanical analysis. Microvasc Res. 1982 Nov;24(3):249–271. doi: 10.1016/0026-2862(82)90016-4. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]


