Abstract
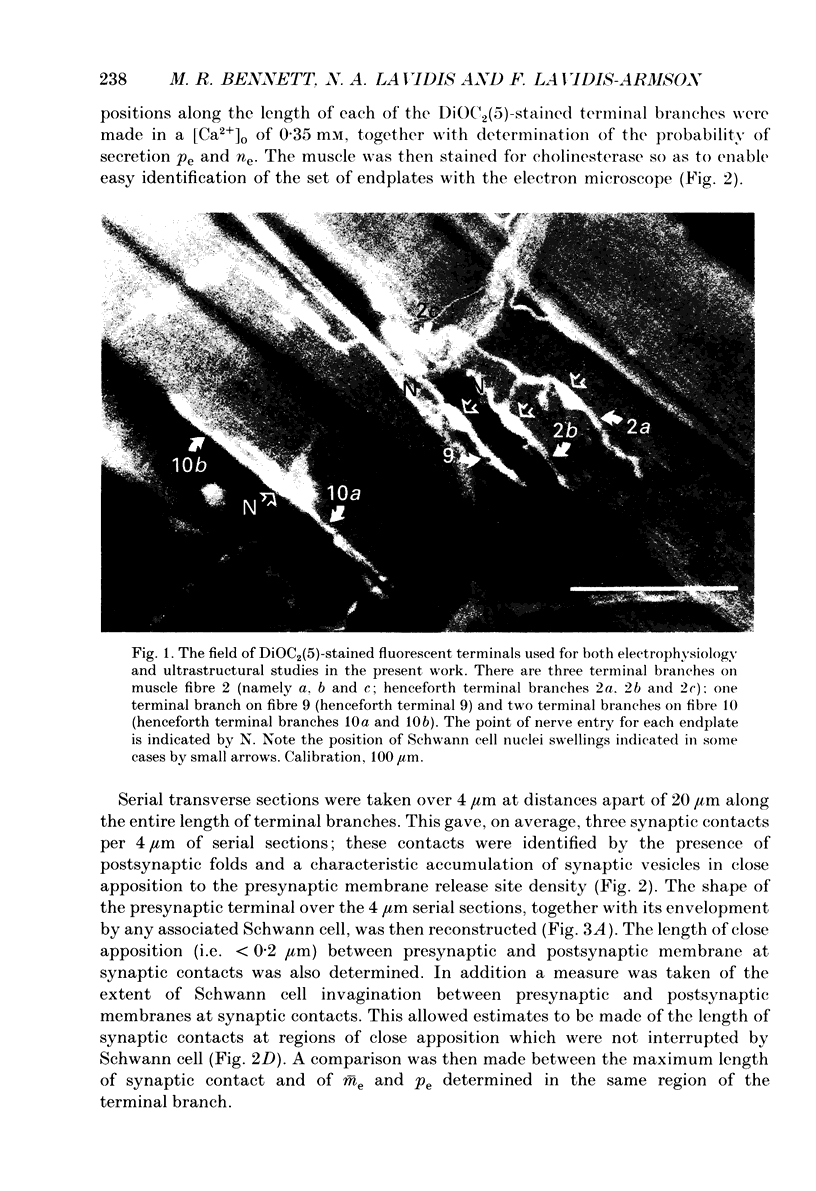
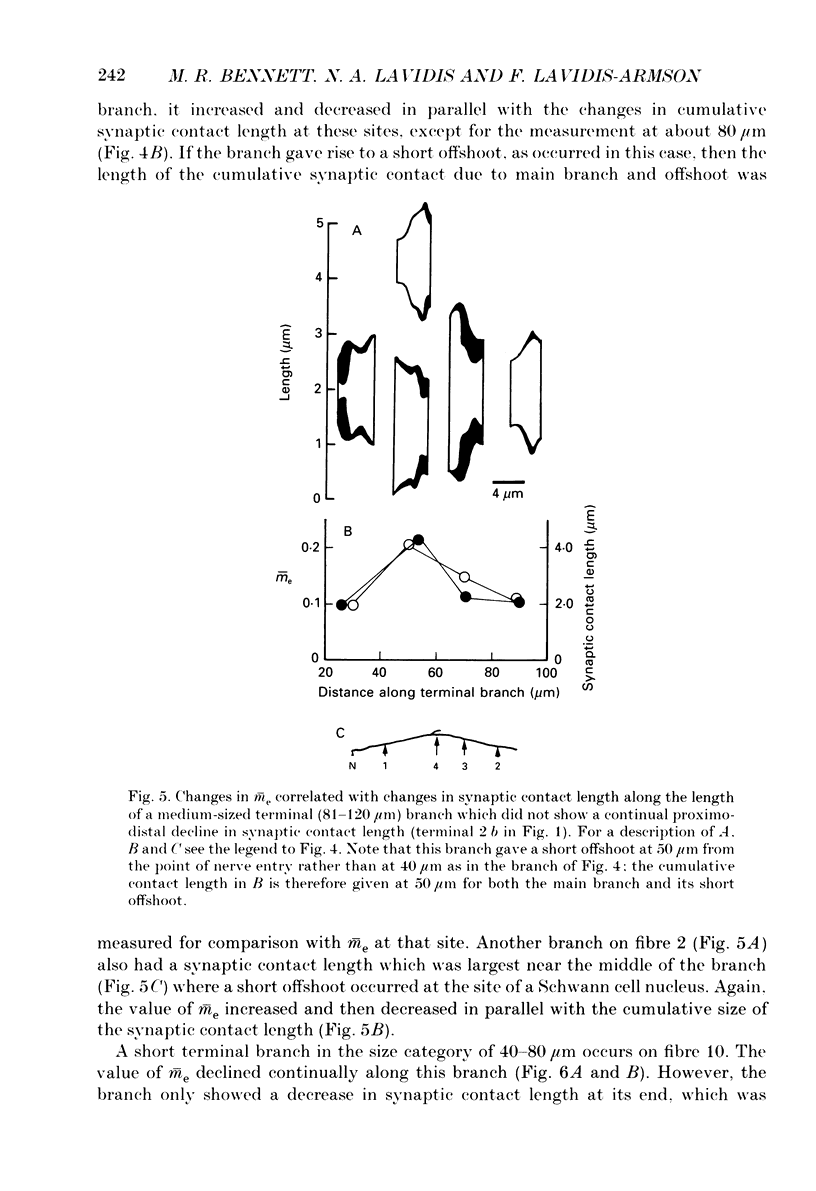
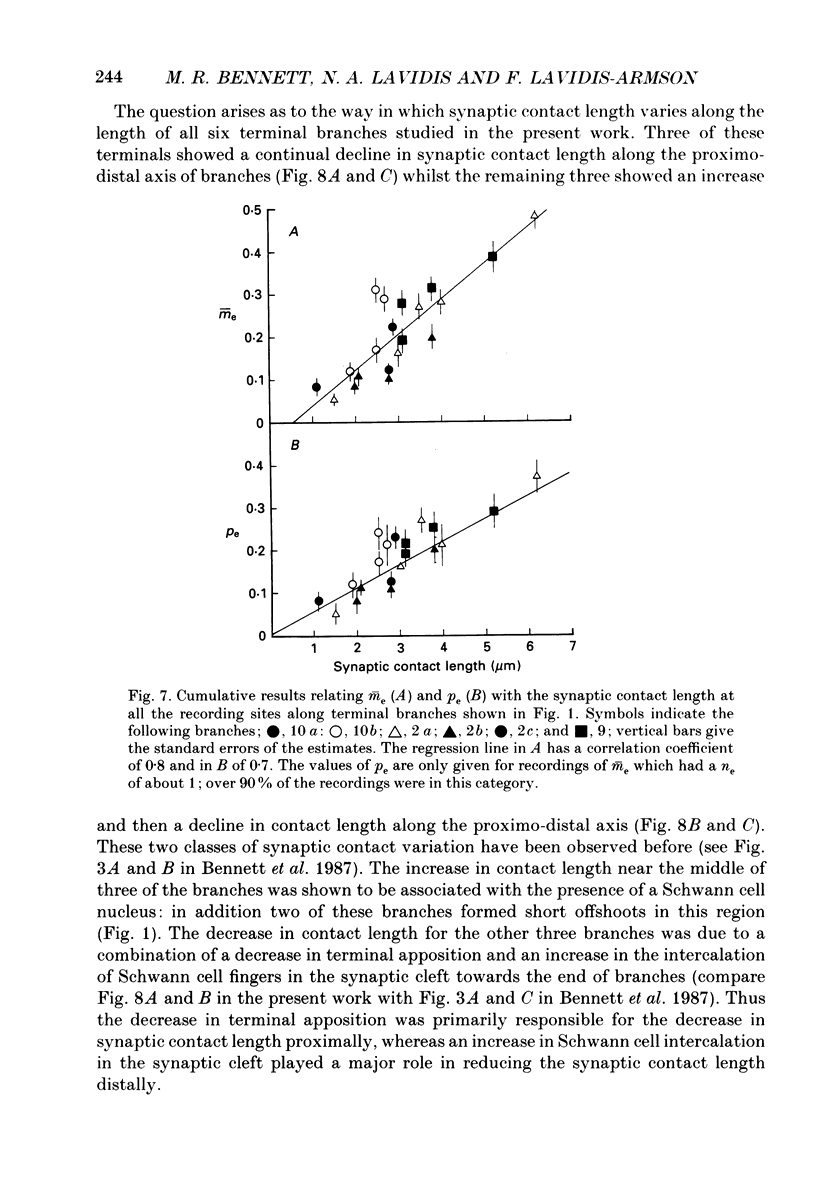
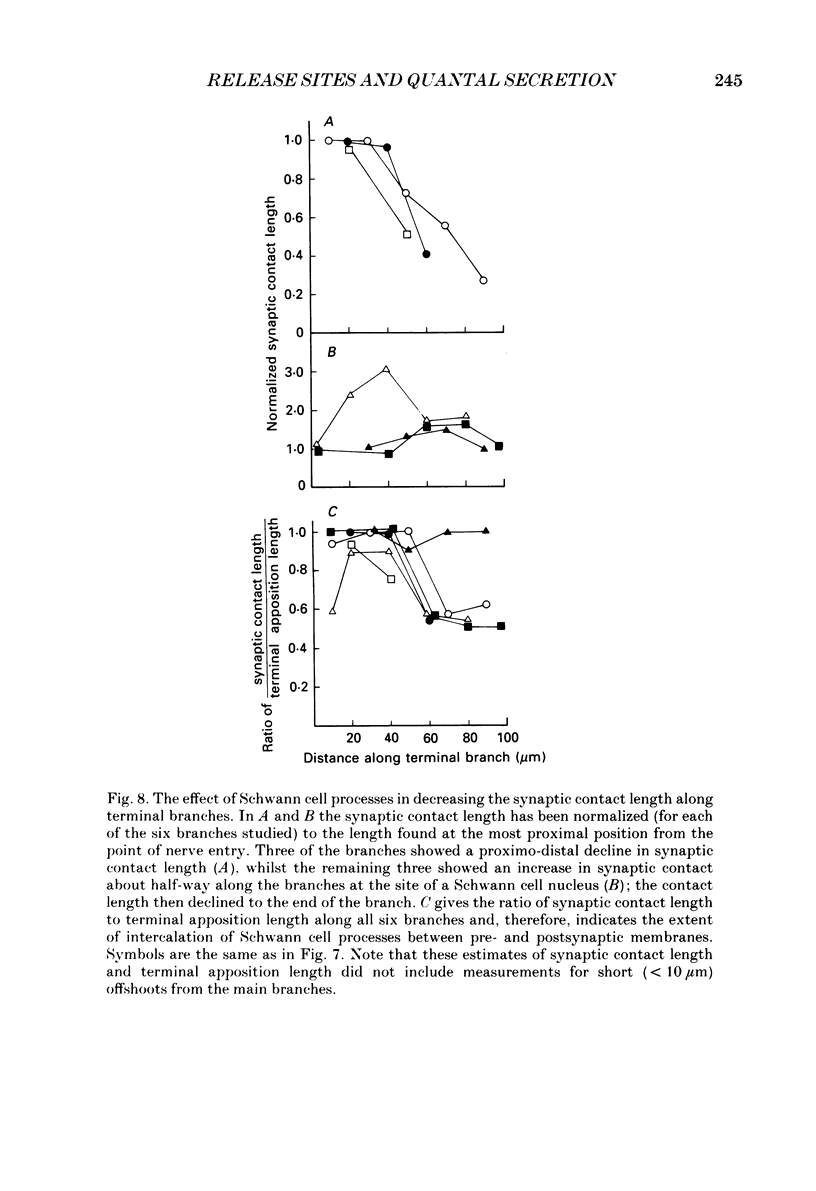
1. The evoked quantal secretion recorded with an extracellular microelectrode (me) at selected sites along motor terminal branches visualized with the fluorescent dye 3-3 diethyloxardicarbocyanine iodide (DiOC2(5)) was compared with the maximum length of the synaptic contact at these release sites reconstructed from serial sections examined with the electron microscope. In addition the relationship between the binomial probability of secretion at release sites (pe) and the length of the synaptic contact was determined in an extracellular calcium. [Ca2+]o, of 0.35 mM. 2. Three of the six terminal branches studied in this way showed a decline in synaptic contact length of release sites from near the point of nerve entry (proximal) to the end of the branch (distal). The remaining three branches showed an increase in synaptic contact length near their middle, and in each case this was associated with a Schwann cell nucleus: contact length then declined to the end of the branches. 3. Both me and pe increased linearly with an increase in the maximum length of the synaptic contact over a range from 0.4 to 4.0 microns. This occurred independently of how the synaptic contact length varied along the length of terminal branches. The value of pe increased by about 0.05 for each 1 micron increase in synaptic contact length in a [Ca2+]o of 0.35 mM. 4. The decrease in synaptic contact length along the proximal parts of terminal branches, in which this occurs, is mostly due to a decrease in the length of close opposition (less than 0.2 micron) between the nerve terminal membrane and the postsynaptic membrane: the decrease in more distal parts of branches is due to the progressive encroachment of Schwann cell processes between the presynaptic and postsynaptic membranes as well as a decrease in synaptic contact length.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angaut-Petit D., Mallart A. Dual innervation of end-plate sites and its consequences for neuromuscular transmission in muscles of adult Xenopus laevis. J Physiol. 1979 Apr;289:203–218. doi: 10.1113/jphysiol.1979.sp012733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Jones P., Lavidis N. A. The probability of quantal secretion along visualized terminal branches at amphibian (Bufo marinus) neuromuscular synapses. J Physiol. 1986 Oct;379:257–274. doi: 10.1113/jphysiol.1986.sp016252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A. The effect of calcium ions on the secretion of quanta evoked by an impulse at nerve terminal release sites. J Gen Physiol. 1979 Oct;74(4):429–456. doi: 10.1085/jgp.74.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A. The probability of quantal secretion at release sites in different calcium concentrations in toad (Bufo marinus) muscle. J Physiol. 1989 Nov;418:219–233. doi: 10.1113/jphysiol.1989.sp017836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Raftos J. The formation and regression of synapses during the re-innervation of axolotl striated muscles. J Physiol. 1977 Feb;265(2):261–295. doi: 10.1113/jphysiol.1977.sp011716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couteaux R., Pécot-Dechavassine M. Vésicules synaptiques et poches au niveau des "zones actives" de la jonction neuromusculaire. C R Acad Sci Hebd Seances Acad Sci D. 1970 Dec 21;271(25):2346–2349. [PubMed] [Google Scholar]
- D'Alonzo A. J., Grinnell A. D. Profiles of evoked release along the length of frog motor nerve terminals. J Physiol. 1985 Feb;359:235–258. doi: 10.1113/jphysiol.1985.sp015583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey D. F., Bennett M. R. Variation in the size of synaptic contacts along developing and mature motor terminal branches. Brain Res. 1982 Sep;281(1):11–22. doi: 10.1016/0165-3806(82)90108-0. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K., Akert K., Sandri C., Moor H. Ultrastructure of the "active zone" in the frog neuromuscular junction. Brain Res. 1973 Nov 23;62(2):373–380. doi: 10.1016/0006-8993(73)90699-9. [DOI] [PubMed] [Google Scholar]
- Grinnell A. D., Herrera A. A. Physiological regulation of synaptic effectiveness at frog neuromuscular junctions. J Physiol. 1980 Oct;307:301–317. doi: 10.1113/jphysiol.1980.sp013436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Ribchester R. R. The relationship between end-plate size and transmitter release in normal and dystrophic muscles of the mouse. J Physiol. 1979 Nov;296:245–265. doi: 10.1113/jphysiol.1979.sp013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A. A., Grinnell A. D., Wolowske B. Ultrastructural correlates of experimentally altered transmitter release efficacy in frog motor nerve terminals. Neuroscience. 1985 Nov;16(3):491–500. doi: 10.1016/0306-4522(85)90187-3. [DOI] [PubMed] [Google Scholar]
- Herrera A. A., Grinnell A. D., Wolowske B. Ultrastructural correlates of naturally occurring differences in transmitter release efficacy in frog motor nerve terminals. J Neurocytol. 1985 Apr;14(2):193–202. doi: 10.1007/BF01258447. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. J. THE LOCALIZATION OF CHOLINESTERASE ACTIVITY IN RAT CARDIAC MUSCLE BY ELECTRON MICROSCOPY. J Cell Biol. 1964 Nov;23:217–232. doi: 10.1083/jcb.23.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C. P. Formation of the active zone at developing neuromuscular junctions in larval and adult bullfrogs. J Neurocytol. 1985 Jun;14(3):487–512. doi: 10.1007/BF01217757. [DOI] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. M., Heuser J. E. Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction. J Cell Biol. 1984 Feb;98(2):685–698. doi: 10.1083/jcb.98.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudell B. M., Grinnell A. D. Inverse relationship between transmitter release and terminal length in synapses on frog muscle fibers of uniform input resistance. J Neurosci. 1982 Feb;2(2):216–224. doi: 10.1523/JNEUROSCI.02-02-00216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudell B. M., Grinnell A. D. Regulation of synaptic position, size, and strength in anuran skeletal muscle. J Neurosci. 1983 Jan;3(1):161–176. doi: 10.1523/JNEUROSCI.03-01-00161.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom R. R., Ko C. P. Disruption of active zones in frog neuromuscular junctions following treatment with proteolytic enzymes. J Neurocytol. 1988 Feb;17(1):63–71. doi: 10.1007/BF01735378. [DOI] [PubMed] [Google Scholar]
- Peper K., Dreyer F., Sandri C., Akert K., Moor H. Structure and ultrastructure of the frog motor endplate. A freeze-etching study. Cell Tissue Res. 1974 Jun 24;149(4):437–455. doi: 10.1007/BF00223024. [DOI] [PubMed] [Google Scholar]
- Propst J. W., Herrera A. A., Ko C. P. A comparison of active zone structure in frog neuromuscular junctions from two fast muscles with different synaptic efficacy. J Neurocytol. 1986 Aug;15(4):525–534. doi: 10.1007/BF01611734. [DOI] [PubMed] [Google Scholar]
- Propst J. W., Ko C. P. Correlations between active zone ultrastructure and synaptic function studied with freeze-fracture of physiologically identified neuromuscular junctions. J Neurosci. 1987 Nov;7(11):3654–3664. doi: 10.1523/JNEUROSCI.07-11-03654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin D. W. Normal variations in presynaptic active zones of frog neuromuscular junctions. J Neurocytol. 1983 Apr;12(2):317–323. doi: 10.1007/BF01148467. [DOI] [PubMed] [Google Scholar]
- Pumplin D. W., Reese T. S., Llinás R. Are the presynaptic membrane particles the calcium channels? Proc Natl Acad Sci U S A. 1981 Nov;78(11):7210–7213. doi: 10.1073/pnas.78.11.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Nakajima Y., Hirosawa K., Nakajima S., Onodera K. Structure and physiology of developing neuromuscular synapses in culture. J Neurosci. 1987 Feb;7(2):473–481. doi: 10.1523/JNEUROSCI.07-02-00473.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrum L. E., Gray E. G. New observations on the substructure of the active zone of brain synapses and motor endplates. Proc R Soc Lond B Biol Sci. 1986 Oct 22;229(1254):29–38. doi: 10.1098/rspb.1986.0072. [DOI] [PubMed] [Google Scholar]
- Zefirov A. L. Sekretsiia mediatora v proksimal'nykh i distal'nykh uchastkakh nervnogo okonchaniia portniazhnoi myshtsy liagushki. Neirofiziologiia. 1983;15(4):362–369. [PubMed] [Google Scholar]
- Zucker R. S., Fogelson A. L. Relationship between transmitter release and presynaptic calcium influx when calcium enters through discrete channels. Proc Natl Acad Sci U S A. 1986 May;83(9):3032–3036. doi: 10.1073/pnas.83.9.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]




