Abstract
Objective
To present, organize, and assess the methodological quality of the current research related to tDCS on motor function after a stroke and to identify gaps and clinical implications using an evidence mapping approach.
Methods
Six electronic databases (PubMed, Embase, Cochrane Library, Web of Science, CINAHL, PEDro), gray literature, and reference lists of articles were searched from inception until October 2023. The Assessment of Multiple Systematic Reviews 2 (AMSTAR-2) checklist and PEDro scale were used to assess the methodology quality of systematic reviews (SRs) and randomized controlled trials (RCTs).
Results
A total of 172 articles met the inclusion criteria from 5759 records, including 46 SRs and 126 RCTs. Related studies came from 29 countries around the world, and China has performed the most, with 12 SRs and 21 RCTs. More than half of SRs (65.22%) were evaluated with low or critically low quality, while 78.58% of RCTs have shown excellent or good quality. A total of 26 SRs and 93 RCTs have reported outcomes on upper limb motor function with kinds of tDCS, and 15 SRs and 44 RCTs have focused on lower extremity function. Studies with safety concerns have reported no or mild adverse events.
Conclusions
This study systematically identified gaps and indicated that tDCS is a kind of potential and safe intervention. Given potential concerns on the clinical application, more high-quality research with large sample size and kinds of objectives is needed in the future.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13643-025-02795-2.
Keywords: Transcranial direct current stimulation, Stroke, Evidence mapping, Methodology quality, Rehabilitation, Motor function
Introduction
Stroke is the second leading cause of death and the third of death and disability all over the world [1–3]. Most stroke patients would continue to have different degrees of sequelae in the subacute or chronic phase [4]. Motor dysfunction is the most common impairment among poststroke sequelae, such as weakness, paralysis, spasticity, and coordination difficulties. Approximately, 80% of stroke survivors experience varying degrees of motor impairment, typically affecting movement control on one side of the body [5]. Lower extremity function impacts the ability to stand, keep balance, and walk. These deficits always have significant impacts on an individual’s life in activities of daily living, social participation, mental health, and quality of life [6].
Transcranial direct current stimulation (tDCS) is one of the most used noninvasive brain stimulation techniques. It is usually delivered by saline-soaked sponge electrodes connected to a direct current stimulator of low intensity [7]. There are three types of stimulation programs based on the placement of electrodes: anodal tDCS (A-tDCS), cathodal tDCS (C-tDCS), and dual tDCS. Studies have proven that stimulation can modulate cortical excitability, with anodal stimulation resulting in increased cortical excitability and cathodal stimulation decreasing excitability [7, 8]. tDCS is widely used in depression, chronic pain, poststroke motor dysfunction, Alzheimer’s disease, and others [9].
Evidence mapping (EM) is a kind of comprehensive method to clarify the research status and recognize knowledge gaps by comprehensively collecting, assessing, and presenting existing evidence on a specific topic. Many clinical studies and reviews have explored the effectiveness as well as the safety of using tDCS for motor rehabilitation among stroke patients in recent decades [10–12]. However, the current evidence cannot support any recommendations for any type of tDCS on motor function caused by a stroke [9]. Nevertheless, a systematic mapping study that presents, assesses, and analyzes the existing RCTs and SRs of the application of tDCS is lacking. Thus, the primary objective of this study was to develop an evidence matrix mapping based on current evidence relevant to tDCS in stroke patient management of motor dysfunction. A secondary objective was to identify evidence gaps and implications for future research.
Methods
Data sources and searches
Six electronic databases (PubMed, Embase, Cochrane Library, Web of Science, CINAHL, PEDro) were searched for studies published from inception to October 13, 2023. In addition, gray literature and reference lists of articles were also searched. The search strategy was developed on Medical Subject Headings (MeSH) terms and relevant free terms. The full strategies were shown in Supplementary Materials Table S1.
Inclusion and exclusion criteria
The inclusion and exclusion criteria were in line with the PRISMA protocol [13]. All types of SRs and RCTs that evaluated the efficacy of tDCS on upper and lower extremity motor function among people with stroke were eligible for inclusion. The inclusion criteria were developed according to PICOS principles: (1) population: people affected by one or more strokes; (2) intervention: all kinds of tDCS; (3) comparison: no restrictions; (4) outcome: outcomes related to motor function; and (5) study design: SRs and RCTs.
The following will be excluded: (a) duplicate reports, (b) studies with insufficient information, (c) studies not published in peer-reviewed journals, (d) only the newest and largest population publication was included for the same trial, and (e) not published in English or Chinese.
Study selection and data extraction
The results of the search were independently screened, extracted, and cross-checked by two researchers. Any disagreements were resolved by discussion with the third researcher. Missing data were retrieved by contacting the relevant authors. Duplicate articles were removed using EndNote X9 as well as manually. The titles and abstracts of the remaining articles were screened. After removing irrelevant studies, the full texts were downloaded and further read for inclusion.
Data was extracted independently by two authors. The following data were extracted: publication year, first author, country, study design, sample size/number of RCTs, population (age, gender, stroke phases, etc.), setting, intervention (stimulation type, position, dose, sessions, etc.), control, outcome, p-values, adverse effect, and funding information.
Quality assessment
Two authors independently conducted the quality assessment, and any conflicts were discussed with the third researcher. A Measurement Tool to Assess Systematic Review 2 (AMSTAR-2) was used to assess the methodological quality of SRs [14]. AMSTAR-2 consists of 16 items evaluated as “yes,” “partial yes,” or “no.” The comprehensive quality assessment process was performed online (https://amstar.ca/Amstar_Checklist.php) to generate the overall quality (“very low quality,” “low quality,” “moderate quality,” and “high quality”). The PEDro scale was used to assess the quality of the included RCTs [15]. A total of 11 criteria were included, and each criterion was classified as “yes” or “no.” Each criterion except the first is counted for 1 point, for a total of 10 points, with studies scoring 1–3 as poor quality, 4–5 as fair quality, 6–8 as good quality, and 9–10 as excellent quality.
Data synthesis and analysis
This study was based on the methodology of Global Evidence Mapping and Campbell evidence and gap map [16, 17]. The Microsoft Excel 365 (Microsoft Corporation, Redmond, WA, USA) was used for data management and analysis. Descriptive analysis was used for basic information of included studies. Bubble charts were used to present the key features of the evidence and were designed in four dimensions: (a) each bubble represented a single pairwise comparison (with multiple-arm studies separated into individual pairwise comparisons) with the colors representing different types, (b) the size of the bubble represented the sample size or the number of studies related to the comparisons, (c) the outcome measurements reported in more than two studies were plotted on the horizontal axis, and (d) the methodology quality of studies and effectiveness were presented on the vertical axis. For SRs, effectiveness was categorized based on effect sizes, where “effect” indicates p < 0.05 or 95% CI does not contain 1, “no effect” indicates p > 0.05 or 95% CI contains 1, and “unclear” represents cases where effect size was not reported. For RCTs, effectiveness was graded based on both within-group and between-group differences, where “more effective” indicates significant differences in both comparisons, “less effective” indicates no significant differences in either comparison, and “uncertain” represents inconsistent findings between comparisons.
Results
Study selection
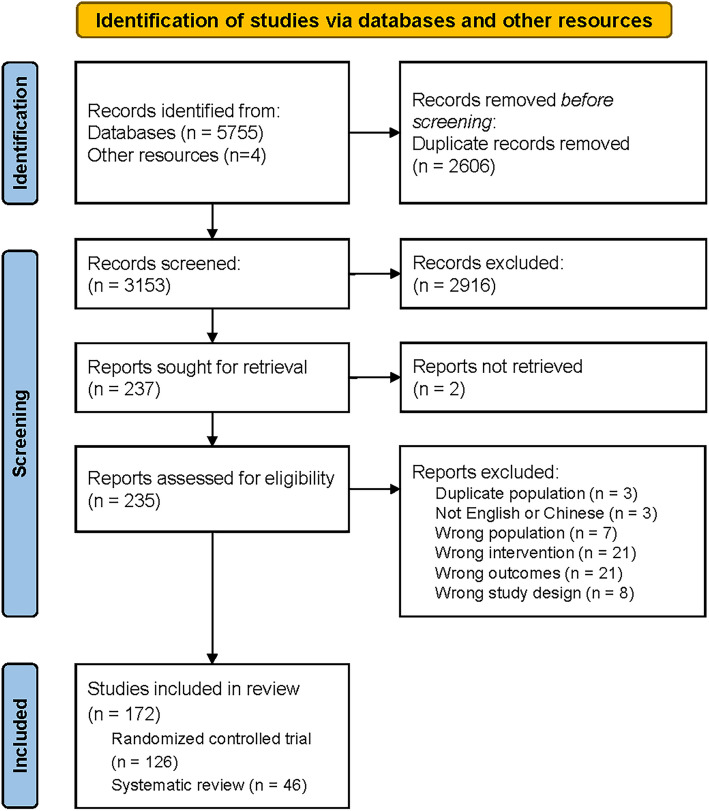
As shown in Figure 1, a total of 5755 studies were searched from electronic databases and 4 from other resources. Removing duplicates and irrelevant studies by titles and abstracts, the full texts of 235 studies were screened, which excluded 63 studies because of detailed reasons (Supplemental Materials Table S2). Finally, a total of 172 studies were included in this study, including 126 RCTs and 46 SRs.
Fig. 1.
PRISMA flowchart for the study
Study characteristics
Since the first study was published in 2005, there is an increasing trend in the number of articles over time, particularly in 2021 (Supplemental Materials Fig. S1). One-fourth of included SRs were conducted by Chinese researchers (12, 26.09%). Others were from 15 countries/regions, including Brazil, Germany, Italy, and Spain. Only two of the included SRs focused on chronic stroke patients, and the others had no restrictions on phase or not reported. Thirty studies (65.22%) did not restrict the type of tDCS. Among the other 16 SRs, 7 studies reported the outcomes of different types of tDCS separately. A total of 11 studies (23.91%) have reported the outcomes related to motor function improved by A-tDCS. Sham or placebo intervention was the most used method of comparison. Among 46 studies, more than half of them examined the effect on the upper extremity (25, 54.35%) while nearly one-third on the lower extremity (15, 32.61%). One study has reported outcomes not only for the upper extremity but also lower extremity. The detailed characteristics of included SRs were shown in Table 1 and Supplemental Material Table S3.
Table 1.
The essential characteristics of the included SRs
| Category | Characteristics | Number | Percentage n = 46 |
|---|---|---|---|
| First author’s country | China | 12 | 26.09 |
| Brazil | 4 | 8.70 | |
| Germany | 4 | 8.70 | |
| Italy | 4 | 8.70 | |
| Spain | 4 | 8.70 | |
| Belgium | 3 | 6.52 | |
| India | 3 | 6.52 | |
| Republic of Korea | 3 | 6.52 | |
| Turkey | 2 | 4.35 | |
| Australia | 1 | 2.17 | |
| Canada | 1 | 2.17 | |
| Georgia | 1 | 2.17 | |
| Japan | 1 | 2.17 | |
| Jordan | 1 | 2.17 | |
| UK | 1 | 2.17 | |
| United States | 1 | 2.17 | |
| Type of study design | Systematic review | 11 | 23.91 |
| Meta-analysis | 28 | 60.87 | |
| Network meta-analysis | 7 | 15.22 | |
| Type of population | Chronic stroke (> 6 months) | 2 | 4.35 |
| Not specific phase | 38 | 82.61 | |
| Not reported | 6 | 13.04 | |
| Type of intervention (including overlap) | A-tDCS | 11 | 23.91 |
| Dual tDCS | 5 | 10.87 | |
| C-tDCS | 7 | 15.22 | |
| Not specific | 30 | 65.22 | |
| Type of comparison | Sham tDCS | 20 | 43.48 |
| Placebo control | 1 | 2.17 | |
| Sham tDCS or placebo | 15 | 32.61 | |
| Sham tDCS or active control | 3 | 6.52 | |
| Network meta-analysis | 7 | 15.22 | |
| Upper or lower extremity | Upper extremity | 26 | 56.52 |
| Lower extremity | 15 | 32.61 | |
| Not specific | 5 | 10.87 | |
| Source of funding | Government (national, provincial, etc.) | 10 | 21.74 |
| University | 7 | 15.22 | |
| Organization or institution | 3 | 6.52 | |
| No funding | 15 | 32.61 | |
| Not reported | 11 | 23.91 |
Researchers from 27 countries/regions have published relevant articles. China, the Republic of Korea, and the United States have the most publications. More than half of the included RCTs focused on chronic stroke patients (65, 51.59%), and some others concerned acute or subacute patients (43, 34.13%). There are 69, 49, and 36 studies that have reported motor function outcomes improved by A-tDCS, dual tDCS, and C-tDCS, respectively. Only one research focused on high-definition tDCS (HD-tDCS), which is an advanced method to control the stimulation current. Most studies use sham or placebo intervention as the comparison, and other 12.07% of studies compared the effect among different kinds of tDCS. More studies examined the effect on the upper extremity (82, 65.08%) than that on the lower extremity (33, 26.19%). The detailed characteristics of included RCTs were shown in Table 2 and Supplemental Material Table S4.
Table 2.
The essential characteristics of the included RCTs
| Category | Characteristics | Number | Percentage n = 126 |
|---|---|---|---|
| First author’s country | China | 21 | 16.67% |
| Republic of Korea | 14 | 11.11% | |
| United States | 14 | 11.11% | |
| Brazil | 11 | 8.73% | |
| Italy | 11 | 8.73% | |
| Thailand | 7 | 5.56% | |
| Singapore | 5 | 3.97% | |
| UK | 5 | 3.97% | |
| Belgium | 4 | 3.17% | |
| Iran | 4 | 3.17% | |
| Japan | 4 | 3.17% | |
| France | 3 | 2.38% | |
| Turkey | 3 | 2.38% | |
| Australia | 2 | 1.59% | |
| Denmark | 2 | 1.59% | |
| India | 2 | 1.59% | |
| Saudi Arabia | 2 | 1.59% | |
| Spain | 2 | 1.59% | |
| Sweden | 2 | 1.59% | |
| Canada | 1 | 0.79% | |
| Chile | 1 | 0.79% | |
| Egypt | 1 | 0.79% | |
| Germany | 1 | 0.79% | |
| Netherlands | 1 | 0.79% | |
| Nigeria | 1 | 0.79% | |
| Serbia | 1 | 0.79% | |
| Switzerland | 1 | 0.79% | |
| Type of study design | Parallel RCT | 97 | 76.98% |
| Crossover RCT | 29 | 23.02% | |
| Type of population | Chronic stroke | 65 | 51.59% |
| Subacute stroke | 28 | 22.22% | |
| Subacute or chronic stroke | 16 | 12.70% | |
| Acute stroke | 11 | 8.73% | |
| Acute or subacute stroke | 4 | 3.17% | |
| Not reported | 2 | 1.59% | |
| Type of intervention | A-tDCS | 69 | 54.76% |
| Dual-tDCS | 49 | 38.89% | |
| C-tDCS | 36 | 28.57% | |
| HD-tDCS | 1 | 0.79% | |
| Not reported specific type | 1 | 0.79% | |
| Type of comparison | Sham tDCS or placebo | 122 | 96.83% |
| Different types of tDCS | 16 | 12.70% | |
| Other active intervention | 7 | 5.56% | |
| Upper or lower extremity | Upper extremity | 82 | 65.08% |
| Lower extremity | 33 | 26.19% | |
| Upper and lower extremity | 11 | 8.73% | |
| Source of funding | Government (national, provincial, etc.) | 51 | 440.48% |
| University or hospital | 18 | 14.29% | |
| Organization or institution | 17 | 13.49% | |
| No funding | 5 | 3.97% | |
| Not reported | 35 | 27.78% |
tDCS parameters
The detailed stimulation parameters were summarized based on included RCTs. Among 93 studies on the upper extremity, nearly half of studies (45, 48.39%) applied A-tDCS with the anodal electrodes placed on the primary motor cortex (M1, C3, or C4) or premotor cortex (PMC) and the cathodal over the contralateral supraorbital ridge or contralateral shoulder. One-third of studies (37, 39.78%) that used dual tDCS with the anode and cathode was placed over the ipsilesional M1 and the contralesional M1. The other 49 studies (31.18%) applied C-tDCS in which the cathodal electrode was placed over the contralesional M1 and the reference electrode over the contralateral supraorbital ridge or shoulder. Stimulation intensity was usually set from 0.5 to 2 mA. Most studies have set the stimulation intensity as 2.0 mA (37, 39.78%) or 1.0 mA (54, 58.06%). Stimulation duration ranged from 10 to 48 min. A total of 86.02% of studies set the duration at 20 min. The number of stimulation sessions ranged from 1 session to 30 sessions, including 23 studies that have set a single-session design.
A total of 44 RCTs have reported outcomes on the lower extremity, with 30 studies that applied A-tDCS with the anodal electrodes placed on ipsilesional M1 or over the region around Cz. One-third studies (17, 38.64%) used dual tDCS, and other 10 studies applied C-tDCS using similar placements with upper extremity. Stimulation intensity was set and ranged 1.0 to 2.0 mA, and more than half of them (28, 63.64%) were applied tDCS with 2.0 mA. Stimulation duration was ranged from 7 to 30 min, and 29 studies have set the duration as 20 min (65.91%). The total number of stimulation sessions ranged from 1 session to 40 sessions, including 9 studies with single-session design.
Quality assessment
There are 28 SRs (60.87%) which were assessed as “critically low,” 12 (26.09%) as “low,” and only 3 (6.52%) as “moderate” and “high” separately. Only four items have more than 75% responsiveness of “yes” as follows: constructing research question and inclusion criteria followed PICO, reporting sources of conflict of interest, providing detailed characteristics of included studies, and using appropriate methods for statistics. On the other hand, three items had significantly worse rates of “yes” lower than 20% (explain the reason for selection of the study designs, provide a list of excluded studies with justifications, and report funding for the included studies). Typically, only one SR published as a Cochrane systematic review has reported relevant information on funding for included studies (Figures 2A and S2).
Fig. 2.
Methodological quality assessment for included SRs and RCTs. A Summary of AMSTAR-2 for SRs. B Summary of the PEDro scale for RCTs
The median (IQR) score for included RCTs was 8 (6–9). A total of 35 RCTs were assessed as “excellent quality,” 77 as “fair quality,” 13 as “fair quality,” and 1 as “poor quality.” The detailed results were shown in Figures 2B and S3. Almost every criterion was satisfied with the criteria, except criterion 3 (allocation conceal) and criterion 6 (blinding of therapists) of which the proportion of “yes” were below 50%. Criterion 6 has the worst responsiveness that only 27 studies conducted excellent therapists blinding. Most included studies have been conducted double blind that set blinding to subjects (criterion 5, 93) and assessors (criterion 7, 89). One-hundred and twelve included studies have reported that they obtained responsiveness from subjects larger than 85% (criterion 8). Nearly all have reported the difference between groups, except seven studies (5.56%).
Mapping
Bubble charts were used to visualize the SRs and RCTs focused on upper or lower-extremity motor function improved by tDCS after a stroke according to the different reported comparisons, the sample size in RCTs/number of RCTs, the outcome measurements, methodological quality, and related p-values.
Upper extremity
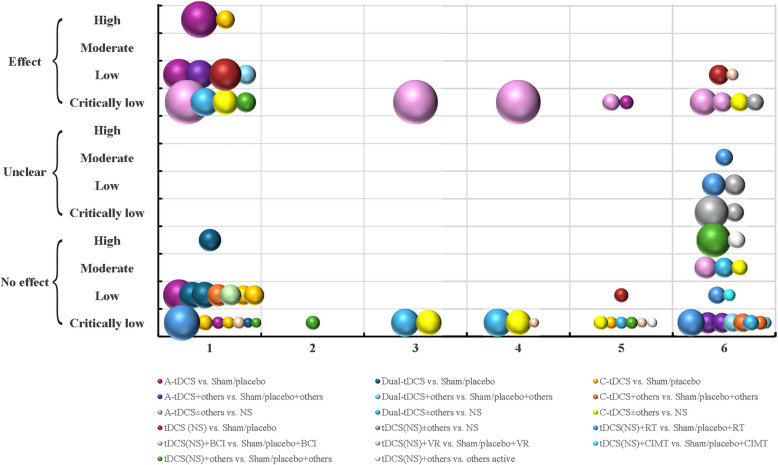
Twenty-nine SRs focused on improving upper extremity hemiplegia among stroke patients using tDCS. A total of 69 bubbles were shown in Figure 3, concerning 17 kinds of comparisons and 6 kinds of outcome measurements. There were 15, 13, and 15 studies restricted to A-tDCS, dual tDCS, and C-tDCS, respectively. In addition, many included SRs did not restrict the types of tDCS among their inclusion criteria and did not conduct subgroup analyses by different tDCS types (26, 37.68%). Results from nearly one-third of these bubbles (20, 28.99%) showed that tDCS has a definite effect on upper limb motor function among stroke patients. However, 44 bubbles (63.77%) represented that there was no more effect compared to tDCS treatment groups than sham or placebo groups. Most bubbles were considered as “critically low quality” (42, 60.87%).
Fig. 3.
Evidence mapping of tDCS on upper extremity among stroke patients in SRs. Outcome measurements codes: 1, FMA-UE; 2, WMFT; 3, ARAT; 4, BBT; 5, MAS; 6, NR/NA. Abbreviations: ARAT, Action Research Arm Test; BBT, Box and Block Test; FMA-UE, Fugl-Meyer Assessment-Upper Extremity; MAS, Modified Ashworth Scale; NR/NA, not reported/not applicable; WMFT, Wolf Motor Function Test
A total of 93 RCTs have reported outcomes related to upper extremity motor function shown in Figure 4, concerning 41 kinds of comparisons and 15 different outcomes. There were 42, 33, and 25 studies which have focused on the effect of A-tDCS, dual-tDCS, and C-tDCS compared with sham or placebo intervention, respectively. About one-third of these bubbles have shown that tDCS is a kind of effective intervention for stroke patients (87, 34.25%). More than half bubbles only reported uncertain results (n = 133, 52.36%). There are 27 (29.03%), 57 (61.29%), and 8 articles (8.60%) that were assessed as “excellent quality,” “good quality,” or “fair quality,” respectively, while only one was as “poor quality.”
Fig. 4.
Evidence mapping of tDCS on upper extremity among stroke patients in RCTs. Outcome measurements codes: 1, FMA-UE; 2, WMFT; 3, ARAT; 4, JTT/mJTT; 5, BBT; 6, 9HPT; 7, grip strength; 8, muscle strength; 9, pinch strength; 10, MAS; 11, ROM; 12, PPT; 13, MI-UE; 14, FNT; 15, kinematics variables. Abbreviations: 9HPT, Nine-Hole Peg Test; ARAT, Action Research Arm Test; BBT, Box and Block Test; FMA-UE, Fugl-Meyer Assessment-Upper Extremity; FNT, finger-to-nose test; JTT/mJTT, Jebsen-Taylor Test/modified Jebsen-Taylor Test; MAS, Modified Ashworth Scale; MI-UE, Motricity Index-Upper Extremity; PPT, Purdue Pegboard Test; ROM, range of motion; WMFT, Wolf Motor Function Test
Lower extremity
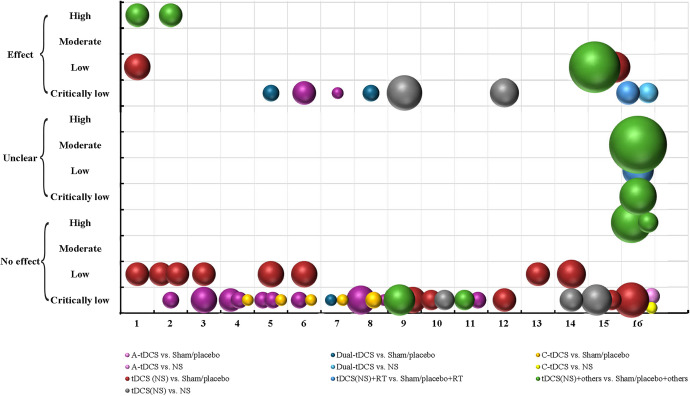
As shown in Figure 5, a total of 18 SRs have concentrated on improving stroke patients’ lower extremity motor function, balance, and gait by tDCS, concerning 10 kinds of comparisons and 16 kinds of measurements. Few studies have focused on the effectiveness of specific types of tDCS, and the others have not restricted the types of tDCS and conducted subgroup analyses (15, 83.33%). Among a total of 55 bubbles, one-fourth bubbles have shown that tDCS has additional effect on lower limb function among stroke patients (13, 23.64%), while others have reported that there was uncertain or no effect. Few SRs have achieved “high quality” (3, 16.67%) or “moderate quality” (1, 5.56%), while nine and five studies were assessed as “low quality” or “critically low quality” respectively.
Fig. 5.
Evidence mapping of tDCS on lower extremity among stroke patients in SRs. Outcome measurements codes: 1, FMA-LE; 2, BBS; 3, 10MWT; 4, 6MWT; 5, TUG; 6, FAC; 7, RMI; 8, Tinetti; 9, walking speed; 10, walking endurance; 11, walking cadence; 12, muscle strength; 13, MAS; 14, motor function (NS); 15, balance (NS); 16, NS/NA. Abbreviations: 6MWT, 6-minute walk test; 10MWT, 10-meter walk test; BBS, Berg Balance Scale; FAC, functional ambulation category; FMA-LE, Fugl-Meyer Assessment-Lower Extremity; MAS, Modified Ashworth Scale; NS, not specified; NA, not applicable; RMI, Rivermead Mobility Index; Tinetti, Tinetti Balance Assessment Tool; TUG, Timed Up and Go test
A total of 44 RCTs have reported the effect of tDCS on improving lower limb function among stroke patients, including 30 kinds of comparisons and 15 kinds of outcome measurements (Figure 6). There were 26, 17, and 10 studies that used A-tDCS, dual tDCS, and C-tDCS compared with sham or placebo intervention. Among all 153 bubbles, one-third have concluded that tDCS can help stroke patients improve their lower extremity function (53, 34.64%). However, other 34 bubbles have reported that no effect was observed. Most studies have excellent or good-quality methodology (39, 88.64%).
Fig. 6.
Evidence mapping of tDCS on lower extremity among stroke patients in RCTs. Outcome measurement codes: 1, FMA-LE; 2, BBS; 3, 10MWT; 4, 6MWT; 5, TUG; 6, gait parameters; 7, FAC; 8, MI-LE; 9, STS/FTSTS; 10, RMI/RMA; 11, muscle strength; 12, AS/MAS; 13, Tinetti POMA; 14, BESTest/miniBESTest; 15, balance (NS). Abbreviations: 6MWT, 6-minute walk test; 10MWT, 10-meter walk test; AS/MAS, Ashworth Scale/Modified Ashworth Scale; BBS, Berg Balance Scale; BESTest/miniBESTest, Balance Evaluation Systems Test/Mini-Balance Evaluation Systems Test; FAC, functional ambulation category; FMA-LE, Fugl-Meyer Assessment-Lower Extremity; MI-LE, Motricity Index-Lower Extremity; NS, not specified; RCTs, randomized controlled trials; RMI/RMA, Rivermead Mobility Index/Rivermead Motor Assessment; STS/FTSTS, sit-to-stand test/five times sit-to-stand test; tDCS, transcranial direct current stimulation; Tinetti POMA, Tinetti Performance-Oriented Mobility Assessment; TUG, Timed Up and Go test
Adverse events
A total of 19 SRs and 78 RCTs provided information on adverse events related to tDCS. Nearly half of these studies (11 SRs and 30 RCTs) reported some minimal or mild adverse events. The subjects reported adverse events including tingling, itching, burning sensation, headache, skin redness, sleepiness, neck pain, trouble concentrating, fatigue, nausea, dizziness, light flashes, acute mood change, and insomnia. Of these, temporary slight tingling and itching at the location of electrodes and headache were the most reported adverse reactions by subjects. Four SRs and 48 RCTs declaimed that there were no adverse events in all subjects, with percentages of 21.05% and 61.54%, respectively.
Discussion
Summary of findings
In this evidence-mapping study, we systematically searched and screened published RCTs and SRs that evaluated the effectiveness of tDCS on motor function among stroke patients. A total of 46 SRs and 126 RCTs from 29 countries/regions were included in the study. There has been an increasing trend of publications over the years, especially for the past 5 years. Many studies have proven the effectiveness of using tDCS for motor rehabilitation among people with stroke [18–20]. All included studies covered all four kinds of tDCS, including A-tDCS, dual DCS, C-tDCS, and HD-tDCS. Studies that focused on the effect of the combination of tDCS and other interventions were certainly included, including conventional therapy, task-oriented training, constraint-induced movement training, robotic therapy, rTMS, virtual reality, and treadmill training.
The overall quality of included SRs was concerning with more than 90% assessed as “critically low” or “low.” Three assessment items had a “yes” response rate lower than 20%. Only 8 of 46 SRs have reported the rationale for the inclusion criteria on study designs. This poor reporting appears to stem from researchers’ assumption that including only RCTs automatically ensures high-quality evidence, leading them to overlook other crucial methodological details. The reporting of excluded studies was also inadequate, with only five included SRs providing a list of excluded studies with justifications. While some studies provided simple reasons, the detailed number in the screening flowchart and references for the excluded studies, however, this limited information makes it difficult to replicate the results by others. Moreover, only one study has reported relevant information on funding for included studies [21]. It is known that studies that received funding from special origin or had potential conflicts of interest may affect the reliability of results. So, it is crucial for SRs to report not only their funding and conflicts of interest but also the studies that were included. Another methodological concern was that many SRs did not restrict stimulation parameters in their inclusion criteria. It will lead to high heterogeneity between included studies and decrease the total methodology quality of the review. This makes it challenging for clinicians to determine optimal tDCS parameters and may lead to inconsistent treatment outcomes. Future reviews should either conduct proper subgroup analyses or set specific intervention criteria to improve review quality and clinical applicability.
The PEDro scale is a kind of commonly used tool in clinical trials [22]. There were 27.78% and 61.11% of included studies which were considered “excellent quality” or “good quality,” and the median total score is 8, which means that included RCTs have achieved acceptable methodology quality. However, less than half of the included studies carried out excellent allocation concealment using opaque sealed envelopes or another independent researcher. Concealing the group allocation is as equally important as generating the random list for different groups, although it is always ignored during RCT conducting [23]. Blinding during research can help to decrease the performance bias, which means systematic differences between groups besides the interventions of interest. More than 70% included RCTs that were blinding the subjects and assessors [24]. Forty of the included RCTs did not achieve full responsiveness from all subjects; however, only 12 of them used ITT to manage these missing data. The advantages of ITT analysis are to avoid the bias from dropouts related to outcomes or group assignments and to preserve the original balance of random assignment [25]. The lack of ITT analysis may destroy the baseline homogeneity and lead to the unreliability of results. RCTs that have any dropouts need to analyze their data by the ITT method.
A notable finding in the mapping study is the marked discrepancy between the methodological quality of SRs and RCTs. While 78.58% of RCTs demonstrated good or excellent quality, the majority of SRs were assessed as low quality. This contrast likely stems from several factors. First, RCTs benefit from well-established CONSORT guidelines and mandatory trial registration requirements, which have standardized trial reporting and conduct. Second, the poor quality of SRs primarily reflects inadequate reporting of methodology and results synthesis rather than the quality of included evidence. Many SRs failed to report crucial elements such as excluded studies, funding sources, and heterogeneity analyses, despite including high-quality RCTs. This suggests that while primary evidence quality is strong, the synthesis and reporting of this evidence need substantial improvement.
The present study demonstrated that the effect of tDCS on improving upper limb function has obtained more attention than lower extremity from researchers. This may be caused by some specific reasons. One of the most important reason is related to the projection control areas of the brain for upper and lower limb movements. The primary motor cortex, also known as M1 or Brodmann area 4, is somatotopically organized which means there are specific areas corresponding to control movements in different parts of the body [26]. The neurons responsible for controlling the muscles of the upper limbs are located in the lateral part of the primary motor cortex while those that control the lower limbs are in the medial aspect, closer to the midline of the brain and deep in the sulcus. The representation of the lower limb in the motor cortex is smaller compared to the upper limb, which correlates with the less intricate movements performed by the lower limbs. Upper limb movements are primarily controlled by a larger and more superficially located region that can be more precise and easily reached and stimulated by tDCS than the lower extremity. On the other hand, the neural pathway organization differs significantly. Upper extremity movements rely more heavily on direct corticospinal projections, which are more susceptible to modulation by tDCS. In contrast, lower extremity function involves more complex polysynaptic pathways and bilateral control mechanisms, particularly for gait and balance. These were reflected in the mapped studies where studies targeting upper limb motor recovery [27–29] demonstrated more consistent improvements in fine motor control compared to studies focusing on lower limb function [11, 30, 31]. Secondly, the motor function of the upper extremity is critically related to an individual’s ability, such as taking care of oneself and participating in daily activities. It is one of the most concerning and important things we can do for stroke rehabilitation so many researchers focused on it [32]. Thirdly, compared to the lower extremity, upper extremity motor function is kind of easier to evaluate and exercise. Lower extremity function often involves balance and coordination as well and is more sophisticated. Finally, based on the results of bubble charts, a greater proportion of studies with effective results are found among studies on the upper extremity studies than those on the lower extremity. Eliminating the factor of validity, the potential existing publication bias in the currently published studies may also cause the difference between the upper and lower extremity.
Safety is an important and non-negligible factor in determining whether an intervention can be applied in clinical, especially for tDCS as a kind of brain stimulation. Nearly half of these studies (11 SRs and 30 RCTs) reported some minimal or mild adverse events, including tingling, itching, burning sensation, and skin redness that were similar to other electrical stimulation and always disappeared after the stimulation. All these did not lead to any serious consequences or large dropouts. It confirmed that tDCS is safe to use among stroke patients.
Strengths and limitations
The present study systematically searched published SRs and RCTs related to tDCS improving motor function among stroke patients. The evidence maps can clearly represent the current research comparison design, results, and quality. Remarkably, the mapping conduction was independently completed by two authors that ensured the rigorous process to help our findings reliable and credible. Moreover, evidence mapping can help identify the knowledge gaps that provide a reference for researchers and clinicians on stroke patient management.
However, this study had some limitations. First, considering the hierarchy of evidence, this study has restricted the study design to SRs and RCTs. Other types of studies were not included so not all current evidence has been shown. Secondly, the study focused on presenting the evidence rather than using quantitative statistics to calculate the combined effect size and make statistical results, as well as to evaluate the potential publication bias. Lastly, we included articles that were published in English and Chinese because of the language restriction. Several publications using other languages were excluded.
Evidence gaps and implications for further research
The present study identified that many studies have an effective conclusion on tDCS applied among stroke patients to improve motor function. Moreover, it also indicates some evidence gaps based on current studies that can provide implications for further research. First, there is an urgent need for high-quality SRs on the effect of tDCS, particularly those focusing on specific tDCS protocols. Current SRs often suffer from methodological limitations and broad inclusion criteria, making it difficult to draw precise conclusions about optimal stimulation parameters. SRs with excellent methodology can be considered the highest-grade evidence [33]. Future SRs should restrict their inclusion criteria to reduce heterogeneity and improve the methodological quality. Secondly, the mapping revealed significant gaps in understanding the timing of tDCS intervention because the phase of stroke patients was not restricted in a number of SRs. The results of SRs even RCTs are strongly related to the safety of early application and the appropriate time to start the intervention. Future studies should systematically investigate the optimal intervention window post-stroke, particularly focusing on safety and efficacy in different stroke phases. This temporal optimization is crucial for developing evidence-based clinical protocols. Moreover, there is a marked imbalance between upper and lower extremity research that more studies concentrated on the upper limb than the lower limb. Improving lower limb function means not only restoring the muscles’ function but also improving balance, coordination, and walking ability. While upper limb studies predominate, more research is needed on lower extremity applications. These are both important to help stroke patients back to the normal activity of living as well as social participation. Furthermore, more than 80% of RCTs have a sample size of less than 50 which highlights the need for larger, well-powered trials. Future studies should prioritize larger sample sizes to enhance statistical power and reduce bias. Multicenter trials could help achieve this while also improving generalizability. Finally, while current research largely examines tDCS in isolation, future studies should explore combination therapies more systematically. Investigation of different combinations with conventional rehabilitation techniques could optimize treatment protocols and provide more comprehensive rehabilitation strategies.
The evidence mapping has several important implications for clinical practice. First, while tDCS shows promise for stroke rehabilitation, clinicians should carefully consider the quality of available evidence. The predominance of low-quality systematic reviews suggests that treatment decisions should not rely solely on these reviews but should also consider findings from high-quality RCTs. In addition, the differential effects observed between the upper and lower extremities suggest that clinicians should adjust their expectations and treatment approaches accordingly. For upper extremity rehabilitation, there is stronger evidence supporting tDCS application, while lower extremity applications may require more careful monitoring and assessment of outcomes. Thirdly, given the heterogeneity in stimulation protocols, the timing of intervention appears crucial. Clinicians should consider that while early intervention may be beneficial, the optimal window for tDCS application remains unclear. This uncertainty necessitates careful patient monitoring and regular assessment of treatment responses, particularly in the early poststroke phase.
Conclusions
This mapping study included 46 relevant SRs and 126 RCTs on the effect of tDCS on upper and lower extremity motor function. It is a potential and safe strategy for managing stroke patients with motor dysfunction, although the number and quality of studies are limited. However, there are unclear and negative results from some studies, which require further confirmation by high-quality research. Furthermore, it remains to pay more attention to the effectiveness of improving lower extremity motor function, balance, and gait using tDCS. It is still needed for those trials with a larger sample size and more effective combination strategies.
Supplementary Information
Authors’ contributions
YQ, conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing — original draft, and writing — review and editing. JX, data curation, formal analysis, investigation, methodology, and writing — original draft. SSMN, conceptualization, data curation, funding acquisition, project administration, supervision, validation, and writing—review and editing.
Funding
The work described in this paper was partially supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (Project No. PolyU15101023).
Data Availability
All relevant data are included in the manuscript and supplementary materials. Additional information can be requested from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. World Health Organization. ICD-11 for Mortality and Morbidity Statistics. 08 Diseases of the Nervous System. 2024 [cited 2024 April 8]. https://icd.who.int/browse/2024-01/mms/en#843843448.
- 2.Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feigin VL, et al. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int J Stroke. 2022;17(1):18–29. [DOI] [PubMed] [Google Scholar]
- 4.NICE, g.N. Stroke rehabilitation in adults. 2023. https://www.nice.org.uk/guidance/NG236. [PubMed]
- 5.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8(8):741–54. [DOI] [PubMed] [Google Scholar]
- 6.Cramer SC, et al. Stroke recovery and rehabilitation research: issues, opportunities, and the National Institutes of Health StrokeNet. Stroke. 2017;48(3):813–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527 Pt 3(Pt 3):633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floel A, Cohen LG. Recovery of function in humans: cortical stimulation and pharmacological treatments after stroke. Neurobiol Dis. 2010;37(2):243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefaucheur JP, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology. 2017;128(1):56–92. [DOI] [PubMed] [Google Scholar]
- 10.Ojardias E, et al. The effects of anodal transcranial direct current stimulation on the walking performance of chronic hemiplegic patients. Neuromodulation. 2020;23(3):373–9. [DOI] [PubMed] [Google Scholar]
- 11.Mitsutake T, et al. The effects of combining transcranial direct current stimulation and gait training with functional electrical stimulation on trunk acceleration during walking in patients with subacute stroke. J Stroke Cerebrovasc Dis. 2021;30(4):105635. [DOI] [PubMed]
- 12.Mitsutake T, et al. Effects of combining online anodal transcranial direct current stimulation and gait training in stroke patients: a systematic review and meta-analysis. Front Hum Neurosci. 2021;15:782305. [DOI] [PMC free article] [PubMed]
- 13.Shamseer L, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 14.Shea BJ, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maher CG, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–21. [PubMed] [Google Scholar]
- 16.Bragge P, et al. The Global Evidence Mapping Initiative: scoping research in broad topic areas. BMC Med Res Methodol. 2011;11:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White H, et al. Guidance for producing a Campbell evidence and gap map. Campbell Syst Rev. 2020;16(4):e1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geroin C, et al. Combined transcranial direct current stimulation and robot-assisted gait training in patients with chronic stroke: a preliminary comparison. Clinical rehabilitation. 2011;25(6):537–48. [DOI] [PubMed] [Google Scholar]
- 19.Park SD, Kim JY, Song HS. Effect of application of transcranial direct current stimulation during task-related training on gait ability of patients with stroke. J Phys Ther Sci. 2015;27(3):623–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang XH, et al. The effect of transcranial direct current stimulation and functional electrical stimulation on the lower limb function of stroke patients. Front Neurosci. 2021;15:685931. [DOI] [PMC free article] [PubMed]
- 21.Elsner B, et al. Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database Sys Rev. 2020;2020(11). https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009645.pub4/full. [DOI] [PMC free article] [PubMed]
- 22.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–33. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moseley AM, et al. Using research to guide practice: the Physiotherapy Evidence Database (PEDro). Braz J Phys Ther. 2020;24(5):384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tripepi G, et al. Intention to treat and per protocol analysis in clinical trials. Nephrology (Carlton). 2020;25(7):513–7. [DOI] [PubMed] [Google Scholar]
- 26.Zilles K, Amunts K. Centenary of Brodmann’s map–conception and fate. Nat Rev Neurosci. 2010;11(2):139–45. [DOI] [PubMed] [Google Scholar]
- 27.Van Hoornweder S, et al. The effects of transcranial direct current stimulation on upper-limb function post-stroke: a meta-analysis of multiple-session studies. Clinical Neurophysiology. 2021;132(8):1897–918. [DOI] [PubMed] [Google Scholar]
- 28.Adeagbo CA, Olawale OA, Gbiri CAO. Transcranial direct current stimulation and repetitive functional task-oriented programme for upper limb functional rehabilitation in stroke survivors. Physical therapy reviews. 2021;26(6):420–7. [Google Scholar]
- 29.Wu D, et al. Effects on decreasing upper-limb poststroke muscle tone using transcranial direct current stimulation: a randomized sham-controlled study. Archives of Physical Medicine and Rehabilitation. 2013;94(1):1–8. [DOI] [PubMed] [Google Scholar]
- 30.Lima E, de Souza Neto JMR, Andrade SM. Andrade, Effects of transcranial direct current stimulation on lower limb function, balance and quality of life after stroke: a systematic review and meta-analysis. Neurol Res. 2023;45(9):843–53. [DOI] [PubMed] [Google Scholar]
- 31.Wong PL, et al. Comparing different montages of transcranial direct current stimulation on dual-task walking and cortical activity in chronic stroke: double-blinded randomized controlled trial. BMC Neurol. 2022;22(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prabhakaran S, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22(1):64–71. [DOI] [PubMed] [Google Scholar]
- 33.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are included in the manuscript and supplementary materials. Additional information can be requested from the corresponding author on reasonable request.