Abstract
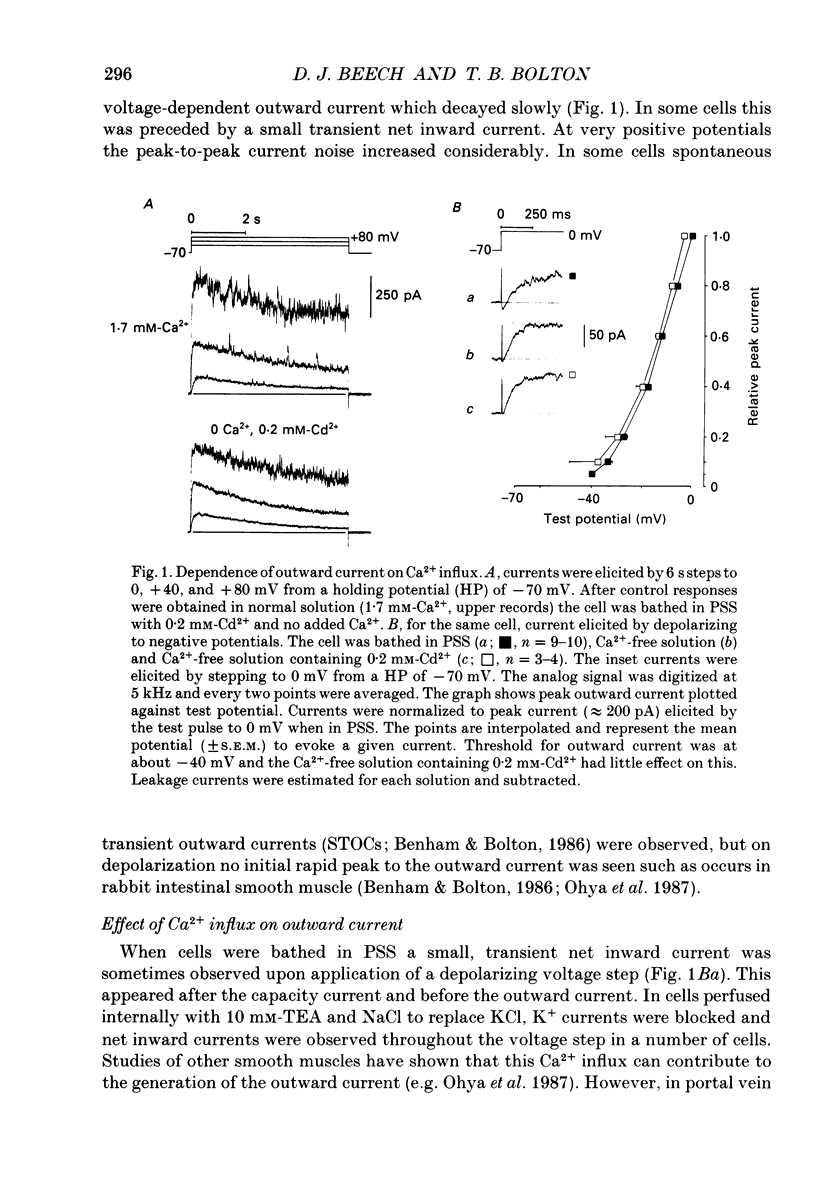
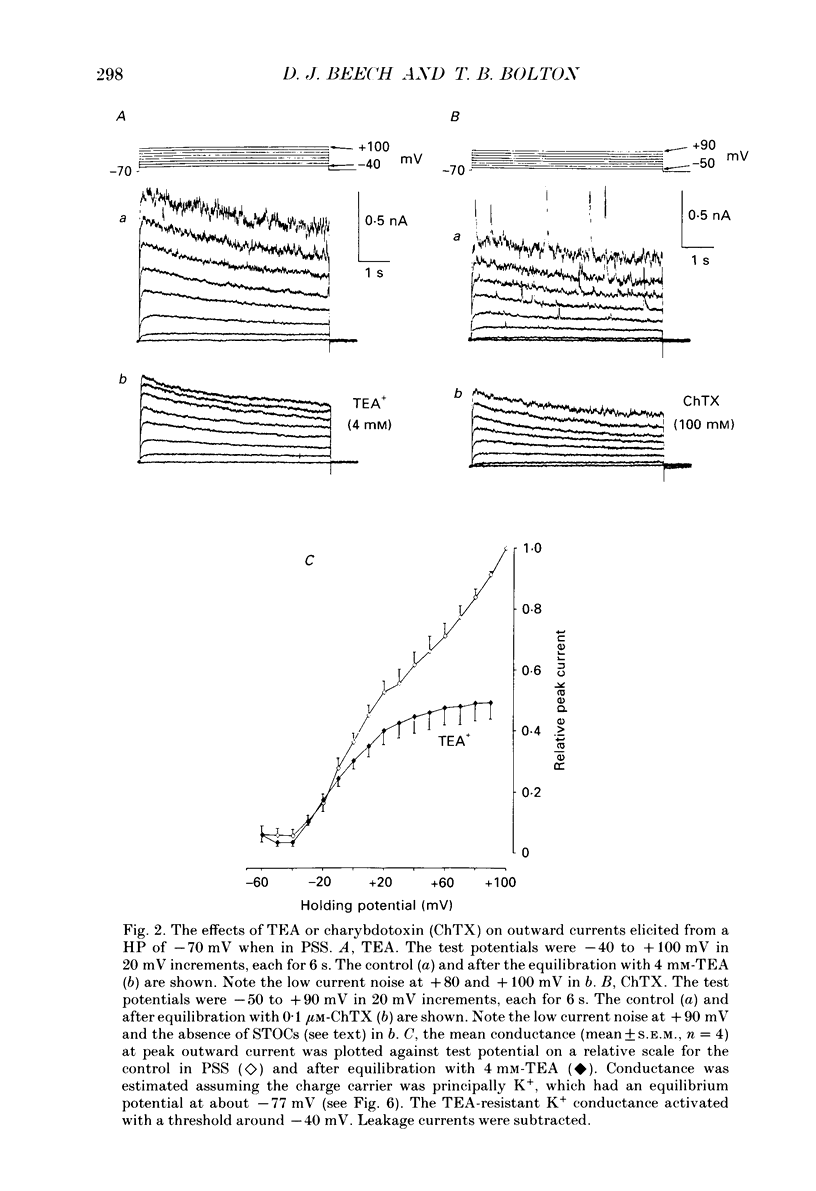
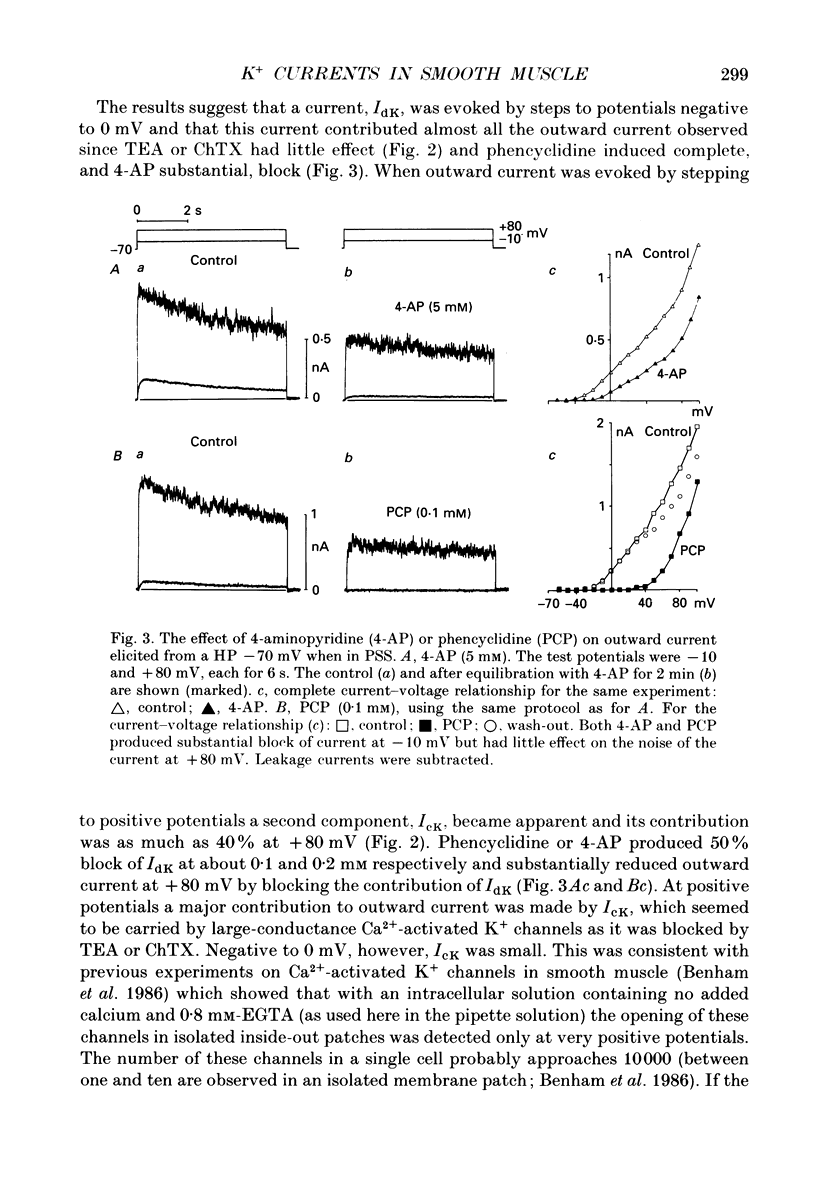
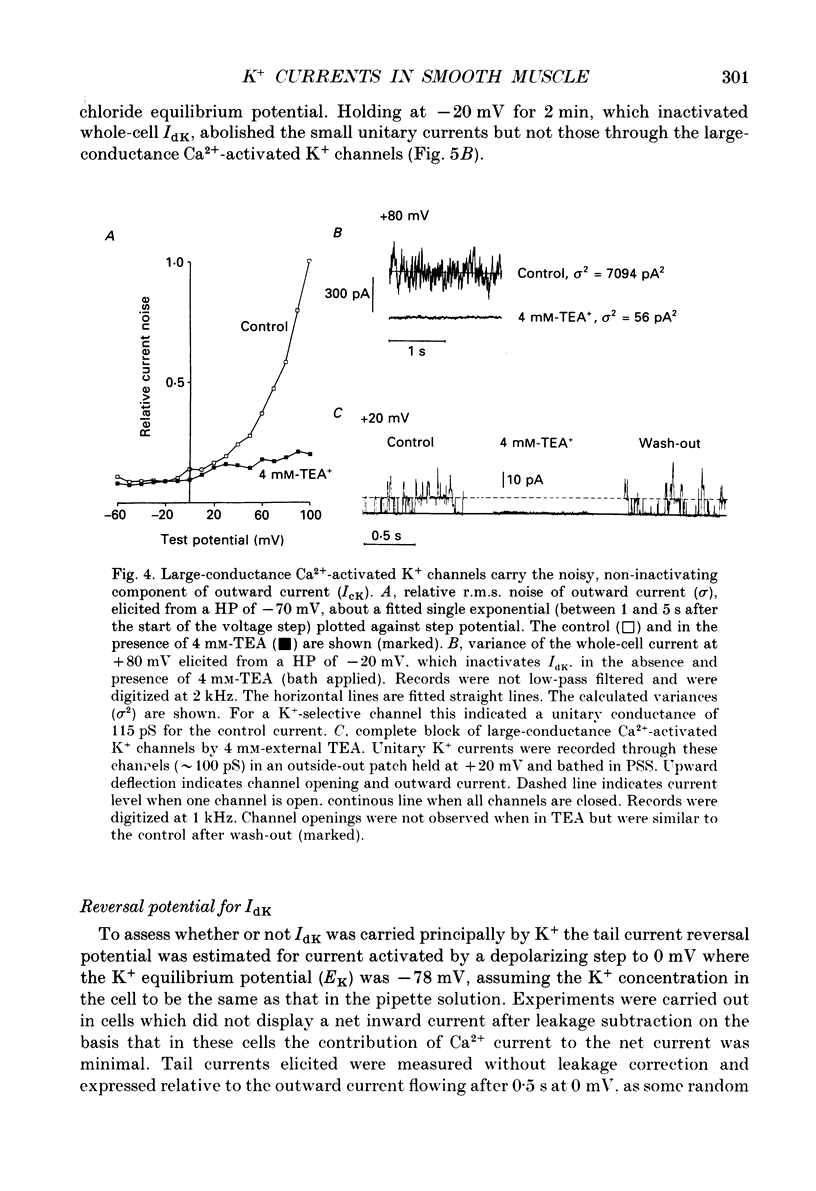
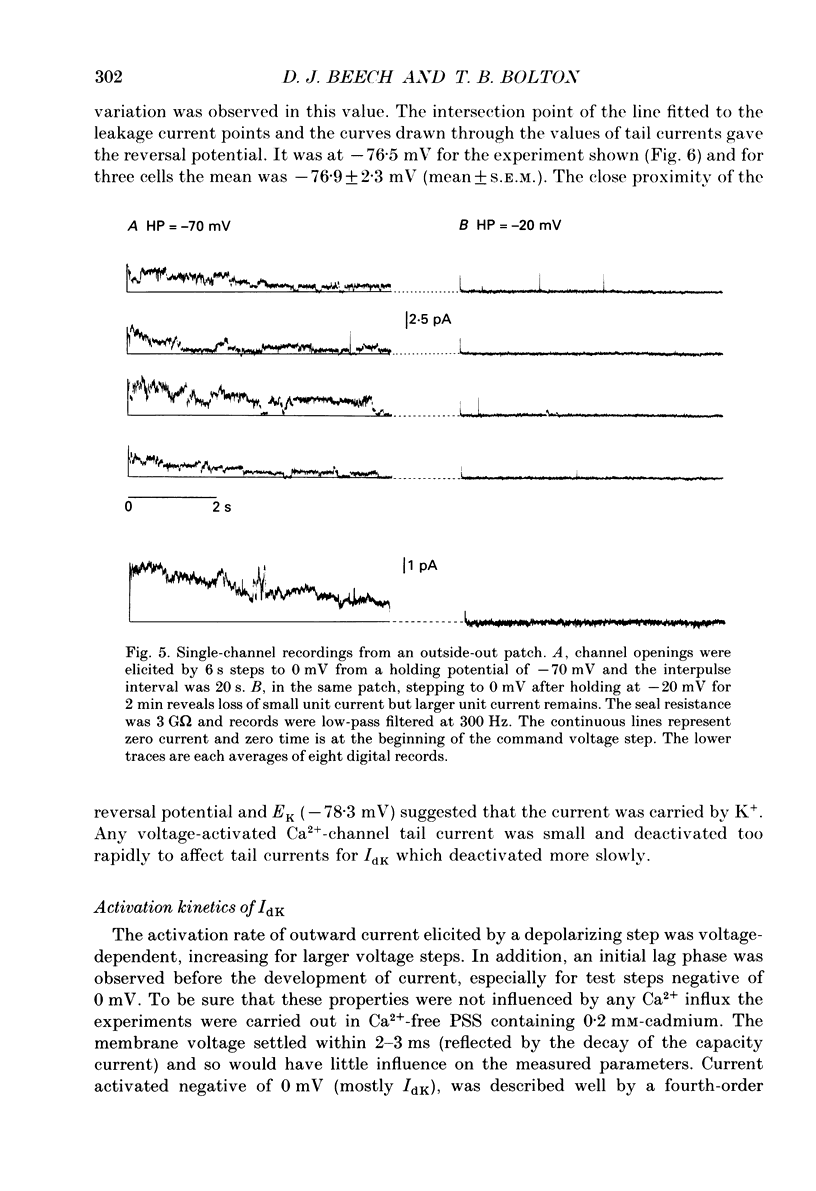
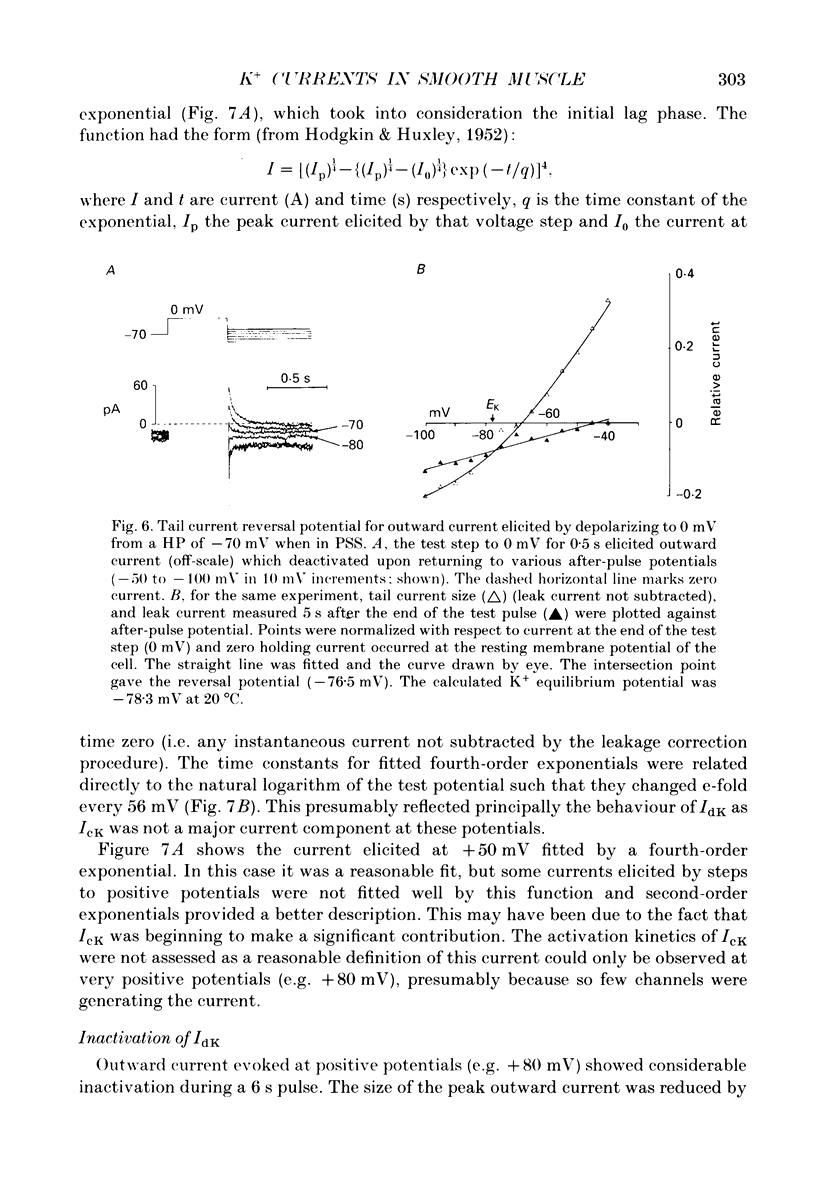
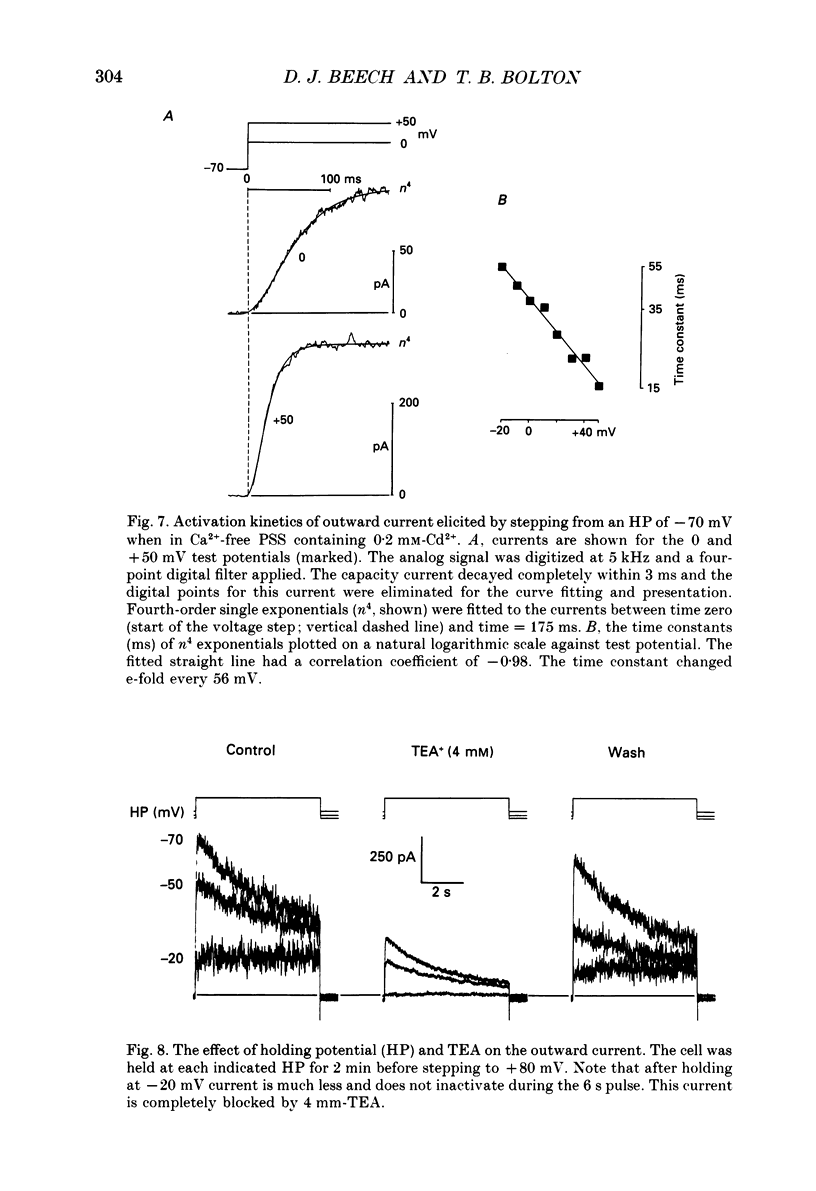
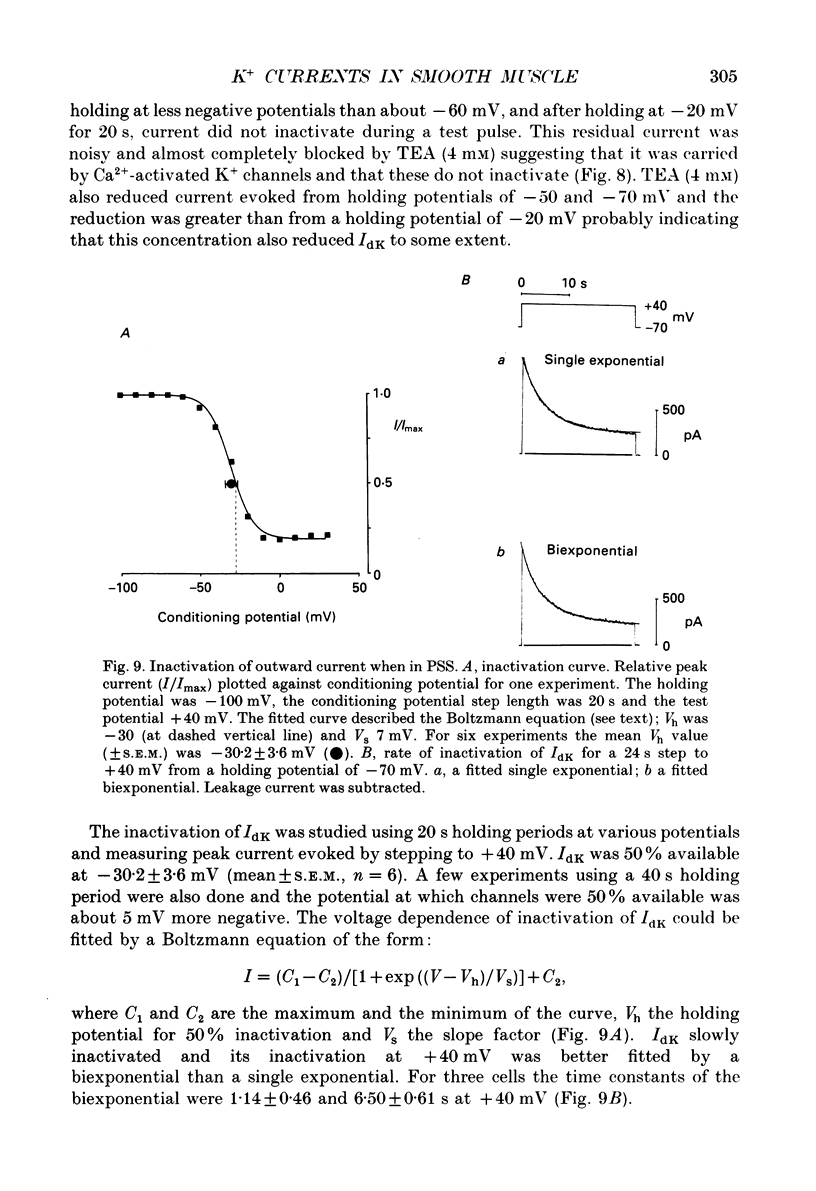
1. Using the patch-clamp technique at 20-23 degrees C membrane currents were recorded from single smooth muscle cells enzymatically isolated from the rabbit portal vein. Single-channel currents were observed in outside-out patches excised from these. 2. Outward current elicited upon depolarization from -70 mV was not activated as a result of Ca2+ influx. It could be divided into two components: an inactivating, 4-aminopyridine- and phencyclidine-sensitive low-noise current (IdK), and a non-inactivating, tetraethylammonium (TEA)- and charybdotoxin-sensitive high-noise current (IcK). 3. IdK activated with a threshold around -40 mV and was carried by K+. It was substantially inhibited by 4-aminopyridine (5 mM) or phencyclidine (0.1 mM) but was insensitive to TEA+ (4 mM), charybdotoxin (0.1 microM) or apamin (0.1 microM). Upon stepping to 0 mV it reached a maximum within about 0.2 s. The time course of its activation could be described by a fourth-order single exponential; the time constants of these exponentials changed e-fold every 56 mV. It inactivated in a time- and voltage-dependent manner with a fast and slow component, and was about 50% available at -30 mV. From single-channel recordings in isolated patches single channels underlying this current have a small unitary conductance (around 5 pS). 4. IcK did not inactivate significantly over 6 s. It activated with a less negative threshold than IdK, usually near 0 mV when the pipette solution contained 0.8 mM-EGTA with no added calcium. It was blocked by TEA (4 mM) or charybdotoxin (0.1 microM), but not by 4-aminopyridine (5 mM), phencyclidine (0.1 mM) or apamin (0.1 microM). Estimates of the single-channel conductance from the noise variance of the whole-cell current IcK indicated a value at +80 mV of 115 pS, very similar to that of the large-conductance Ca2(+)-activated K+ channels studied in these cells using single-channel recording. 5. The results suggest that outward current evoked by depolarization from the resting potential can be carried by 100 pS Ca2(+)-activated K+ channels and by small-conductance delayed-rectifier K+ channels. It is likely that opening of both types of channel contributes to the repolarization phase of the action potential in this smooth muscle.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. A voltage-dependent outward current with fast kinetics in single smooth muscle cells isolated from rabbit portal vein. J Physiol. 1989 May;412:397–414. doi: 10.1113/jphysiol.1989.sp017623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle N. A., Strong P. N. Identification of two toxins from scorpion (Leiurus quinquestriatus) venom which block distinct classes of calcium-activated potassium channel. FEBS Lett. 1986 Dec 1;209(1):117–121. doi: 10.1016/0014-5793(86)81095-x. [DOI] [PubMed] [Google Scholar]
- Conti F., Neher E. Single channel recordings of K+ currents in squid axons. Nature. 1980 May 15;285(5761):140–143. doi: 10.1038/285140a0. [DOI] [PubMed] [Google Scholar]
- Dubois J. M. Evidence for the existence of three types of potassium channels in the frog Ranvier node membrane. J Physiol. 1981 Sep;318:297–316. doi: 10.1113/jphysiol.1981.sp013865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M., Sedlmeir C. Outward currents in voltage-clamped rat sympathetic neurones. J Physiol. 1984 Nov;356:115–133. doi: 10.1113/jphysiol.1984.sp015456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hara Y., Kitamura K., Kuriyama H. Actions of 4-aminopyridine on vascular smooth muscle tissues of the guinea-pig. Br J Pharmacol. 1980 Jan;68(1):99–106. doi: 10.1111/j.1476-5381.1980.tb10704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Kasby C. B., Suthers M. B., Wilson J. A. Some properties of the smooth muscle of rabbit portal vein. J Physiol. 1968 May;196(1):111–132. doi: 10.1113/jphysiol.1968.sp008498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Two Ca-dependent K-channels classified by the application of tetraethylammonium distribute to smooth muscle membranes of the rabbit portal vein. Pflugers Arch. 1985 Oct;405(3):173–179. doi: 10.1007/BF00582557. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflugers Arch. 1985 Dec;405(4):329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Oshima K., Sakamoto Y. The membrane properties of the smooth muscle of the guinea-pig portal vein in isotonic and hypertonic solutions. J Physiol. 1971 Aug;217(1):179–199. doi: 10.1113/jphysiol.1971.sp009565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C., Premont R. T., Brown A. M. A whole-cell and single-channel study of the voltage-dependent outward potassium current in avian hepatocytes. J Gen Physiol. 1988 Feb;91(2):255–274. doi: 10.1085/jgp.91.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Mironneau J., Savineau J. P. Effects of calcium ions on outward membrane currents in rat uterine smooth muscle. J Physiol. 1980 May;302:411–425. doi: 10.1113/jphysiol.1980.sp013253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Morad M. Ca2+ and Ca2+-activated K+ currents in mammalian gastric smooth muscle cells. Science. 1985 Jul 19;229(4710):269–272. doi: 10.1126/science.2409600. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987 Apr;252(4 Pt 1):C401–C410. doi: 10.1152/ajpcell.1987.252.4.C401. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Regulation of calcium current by intracellular calcium in smooth muscle cells of rabbit portal vein. Circ Res. 1988 Feb;62(2):375–383. doi: 10.1161/01.res.62.2.375. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Terada K., Kitamura K., Kuriyama H. Membrane currents recorded from a fragment of rabbit intestinal smooth muscle cell. Am J Physiol. 1986 Sep;251(3 Pt 1):C335–C346. doi: 10.1152/ajpcell.1986.251.3.C335. [DOI] [PubMed] [Google Scholar]
- Okabe K., Kitamura K., Kuriyama H. Features of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflugers Arch. 1987 Aug;409(6):561–568. doi: 10.1007/BF00584654. [DOI] [PubMed] [Google Scholar]
- Osa T. Effects of tetraethylammonium on the electrical activity of pregnant mouse myometrium and the interaction with manganese and cadmium. Jpn J Physiol. 1974 Feb;24(1):119–133. doi: 10.2170/jjphysiol.24.119. [DOI] [PubMed] [Google Scholar]
- Pallotta B. S. Calcium-activated potassium channels in rat muscle inactivate from a short-duration open state. J Physiol. 1985 Jun;363:501–516. doi: 10.1113/jphysiol.1985.sp015724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI T., NISHIYAMA A., INOMATA H. Effect of tetraethyl ammonium ion on the electrical activity of smooth muscle cell. Nature. 1963 Mar 2;197:908–909. doi: 10.1038/197908a0. [DOI] [PubMed] [Google Scholar]
- Schwarz J. R., Vogel W. Potassium inactivation in single myelinated nerve fibres of Xenopus laevis. Pflugers Arch. 1971;330(1):61–73. doi: 10.1007/BF00588735. [DOI] [PubMed] [Google Scholar]
- Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol. 1987 Jun;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Vinall P., Somlyo A. P. Excitation-contraction coupling and electrical events in two types of vascular smooth muscle. Microvasc Res. 1969 Oct;1(4):354–373. doi: 10.1016/0026-2862(69)90014-4. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Inomata H. The ionic mechanism of excitation in intestinal smooth muscle cells. Adv Biophys. 1981;14:239–256. [PubMed] [Google Scholar]
- Terada K., Kitamura K., Kuriyama H. Different inhibitions of the voltage-dependent K+ current by Ca2+ antagonists in the smooth muscle cell membrane of rabbit small intestine. Pflugers Arch. 1987 May;408(6):558–564. doi: 10.1007/BF00581156. [DOI] [PubMed] [Google Scholar]
- Walsh J. V., Jr, Singer J. J. Voltage clamp of single freshly dissociated smooth muscle cells: current-voltage relationships for three currents. Pflugers Arch. 1981 May;390(2):207–210. doi: 10.1007/BF00590209. [DOI] [PubMed] [Google Scholar]
- Weigel R. J., Connor J. A., Prosser C. L. Two roles of calcium during the spike in circular muscle of small intestine in cat. Am J Physiol. 1979 Nov;237(5):C247–C256. doi: 10.1152/ajpcell.1979.237.5.C247. [DOI] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]


