Abstract
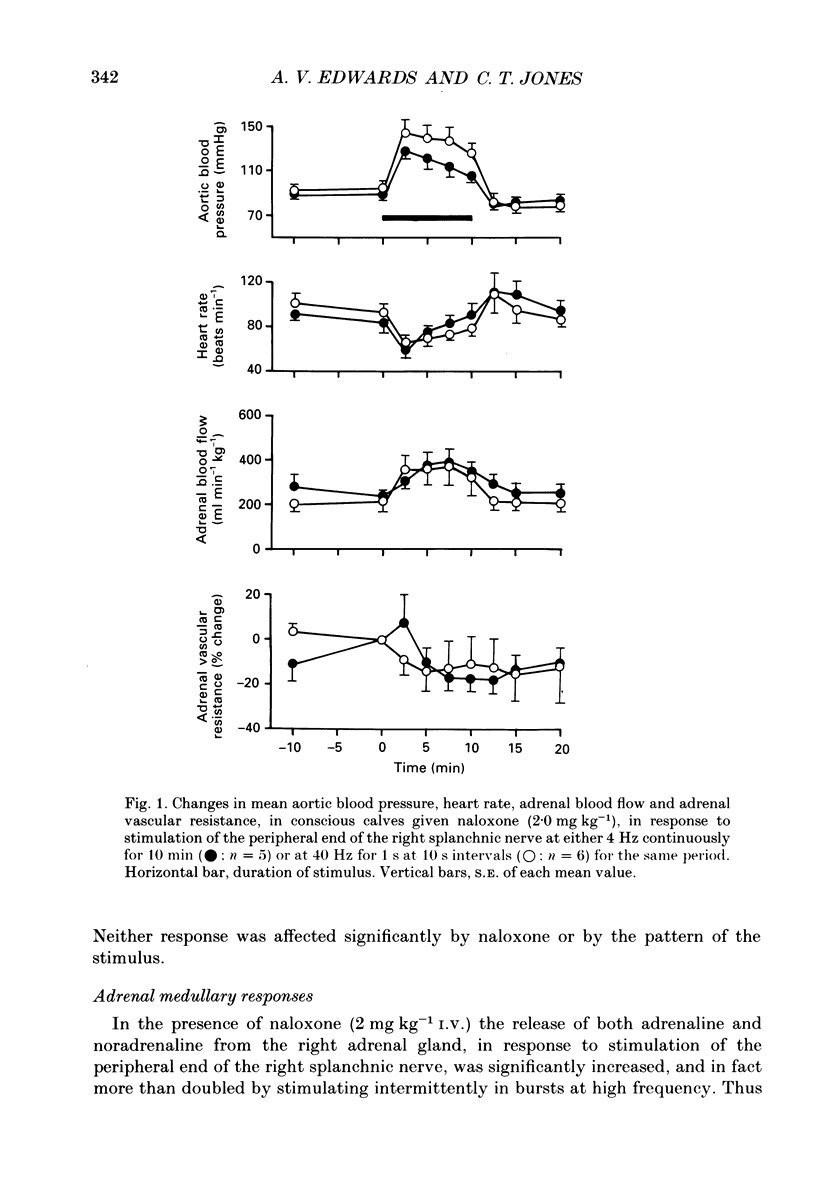
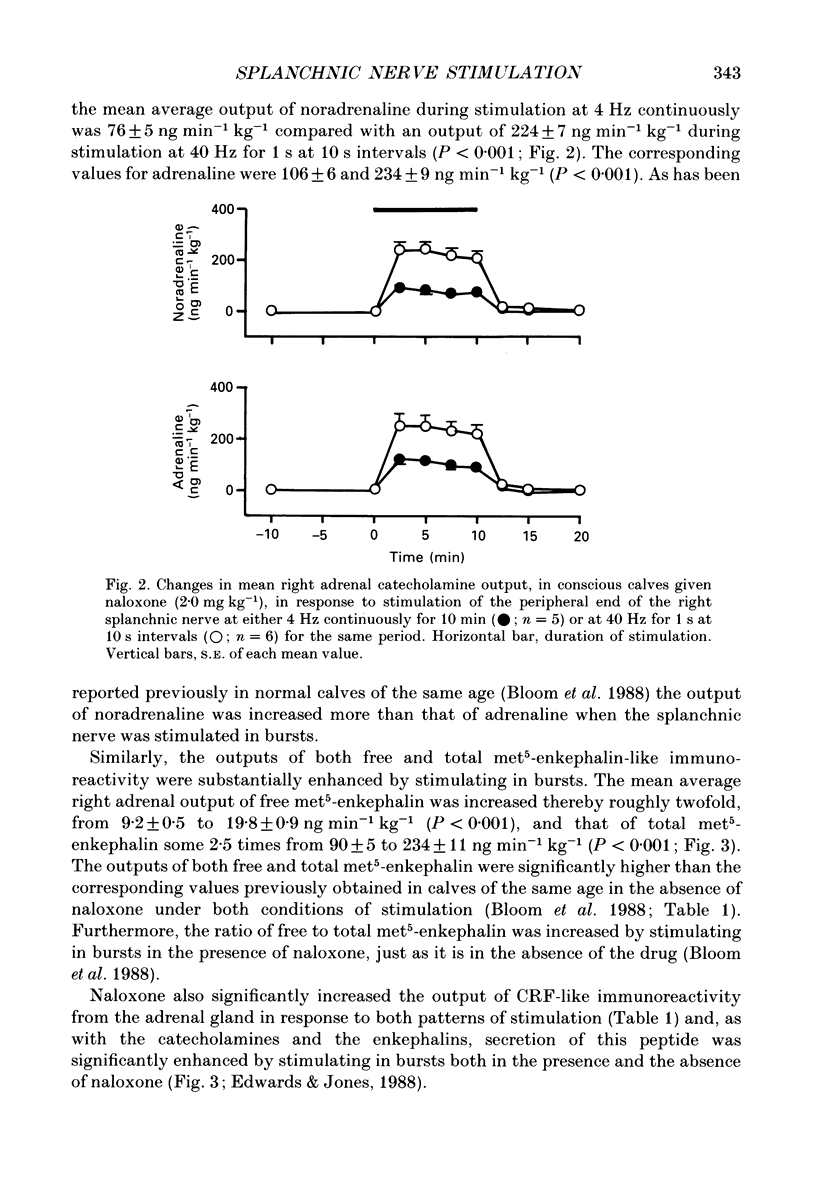
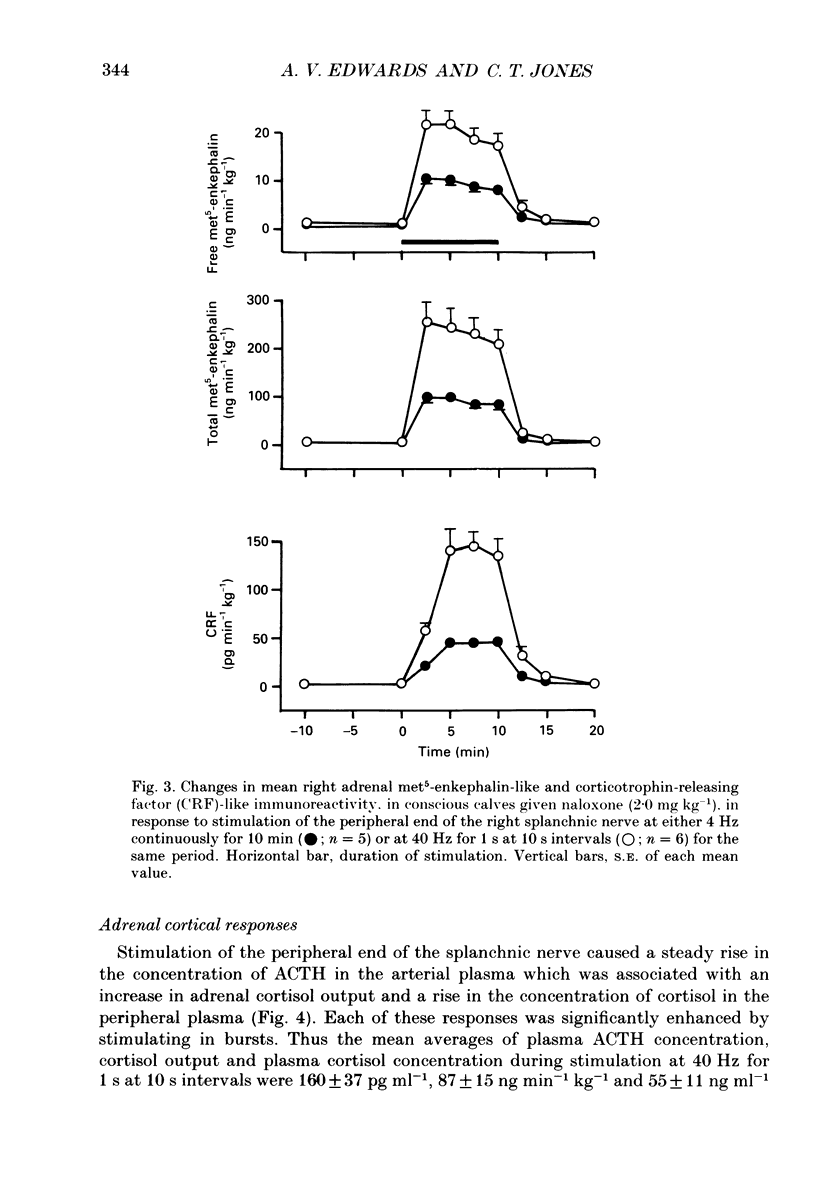
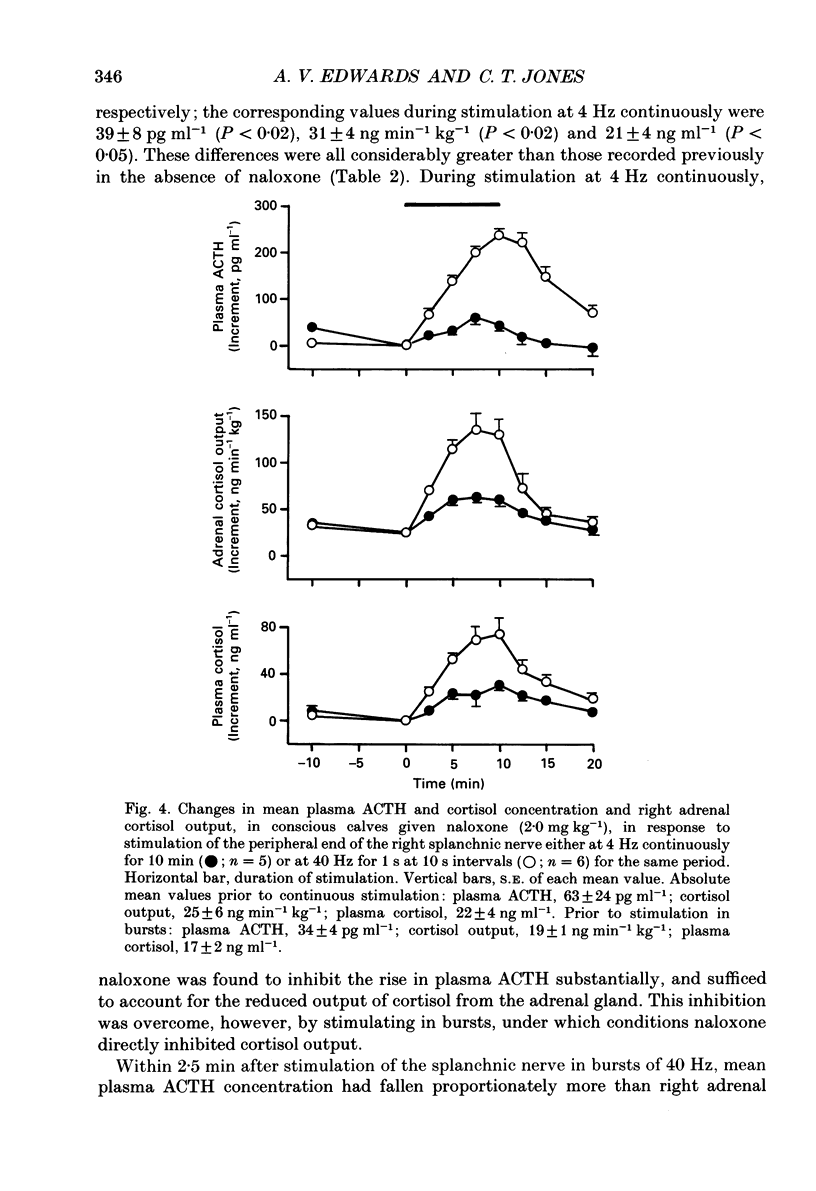
1. The effects of stimulating the peripheral end of the right splanchnic nerve in the presence of naloxone (2 mg kg-1) have been investigated in conscious 3 to 6-week-old calves. 2. Mean aortic blood pressure rose to significantly higher levels during splanchnic stimulation in bursts at 40 Hz for 1 s at 10 s intervals than it did during stimulation at the corresponding continuous frequency (4 Hz). Furthermore, naloxone significantly reduced the fall in mean vascular resistance in response to both patterns of stimulation. 3. The output of catecholamines from the adrenal gland, together with the proportion of noradrenaline released, was significantly enhanced by stimulating the splanchnic nerves in bursts in animals pre-treated with naloxone and the proportion of noradrenaline released also increased. In both cases the output of adrenaline and noradrenaline was within the same range as that reported previously in normal control animals. 4. Naloxone significantly increased the amounts of enkephalin-like immunoreactivity and corticotrophin-releasing factor (CRF)-like immunoreactivity released from the adrenal gland in response to splanchnic nerve stimulation and raised the proportion of total to free met5-enkephalin that was secreted. 5. Naloxone also inhibited the rise in plasma adrenocorticotrophic hormone (ACTH) concentration during continuous stimulation at 4 Hz, but not during stimulation at 40 Hz in bursts. Under these latter conditions the output of cortisol apparently directly from the adrenal gland was inhibited. The finding that splanchnic nerve stimulation can potentiate the output of cortisol in response to ACTH was confirmed. 6. These results provide evidence that release of enkephalins and of CRF from the adrenal is inhibited by activating opioid receptors within the gland itself.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkinstall S. J., Jones C. T. Regional changes in catecholamine content of the pregnant uterus. J Reprod Fertil. 1985 Mar;73(2):547–557. doi: 10.1530/jrf.0.0730547. [DOI] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Jones C. T. The adrenal contribution to the neuroendocrine responses to splanchnic nerve stimulation in conscious calves. J Physiol. 1988 Mar;397:513–526. doi: 10.1113/jphysiol.1988.sp017016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaminade M., Foutz A. S., Rossier J. Co-release of enkephalins and precursors with catecholamines from the perfused cat adrenal gland in situ. J Physiol. 1984 Aug;353:157–169. doi: 10.1113/jphysiol.1984.sp015329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Furness P. N., Helle K. B. Adrenal medullary responses to stimulation of the splanchnic nerve in the conscious calf. J Physiol. 1980 Nov;308:15–27. doi: 10.1113/jphysiol.1980.sp013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Hansell D., Jones C. T. Effects of synthetic adrenocorticotrophin on adrenal medullary responses to splanchnic nerve stimulation in conscious calves. J Physiol. 1986 Oct;379:1–16. doi: 10.1113/jphysiol.1986.sp016237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Hardy R. N., Malinowska K. W. The effects of infusions of synthetic adrenocorticotrophin in the conscious calf. J Physiol. 1974 Jun;239(3):477–498. doi: 10.1113/jphysiol.1974.sp010579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Jones C. T. Secretion of corticotrophin releasing factor from the adrenal during splanchnic nerve stimulation in conscious calves. J Physiol. 1988 Jun;400:89–100. doi: 10.1113/jphysiol.1988.sp017112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Jones C. T. The effect of splanchnic nerve section on the sensitivity of the adrenal cortex to adrenocorticotrophin in the calf. J Physiol. 1987 Sep;390:23–31. doi: 10.1113/jphysiol.1987.sp016683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeland W. C., Bereiter D. F., Gann D. S. Sympathetic control of adrenal secretion of enkephalins after hemorrhage in awake dogs. Am J Physiol. 1986 Aug;251(2 Pt 2):R341–R348. doi: 10.1152/ajpregu.1986.251.2.R341. [DOI] [PubMed] [Google Scholar]
- Fleminger G., Howells R. D., Kilpatrick D. L., Udenfriend S. Intact proenkephalin is the major enkephalin-containing peptide produced in rat adrenal glands after denervation. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7985–7988. doi: 10.1073/pnas.81.24.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni S., Hanbauer I., Hexum T. D., Yang H. Y., Kelly G. D., Costa E. In vivo characterization of the mechanisms that secrete enkephalin-like peptides stored in dog adrenal medulla. Neuropharmacology. 1981 Jul;20(7):639–645. doi: 10.1016/0028-3908(81)90110-6. [DOI] [PubMed] [Google Scholar]
- Hanbauer I., Govoni S., Majane E. A., Yang H. Y., Costa E. In vivo regulation of the release of met-enkephalin-like peptides from dog adrenal medulla. Adv Biochem Psychopharmacol. 1982;33:209–215. [PubMed] [Google Scholar]
- Jones C. T., Boddy K., Robinson J. S., Ratcliffe J. G. Developmental changes in the responses of the adrenal glands of foetal sheep to endogenous adrenocorticotrophin, as indicated by hormone responses to hypoxaemia. J Endocrinol. 1977 Mar;72(3):279–292. doi: 10.1677/joe.0.0720279. [DOI] [PubMed] [Google Scholar]
- Kumakura K., Karoum F., Guidotti A., Costa E. Modulation of nicotinic receptors by opiate receptor agonists in cultured adrenal chromaffin cells. Nature. 1980 Jan 31;283(5746):489–492. doi: 10.1038/283489a0. [DOI] [PubMed] [Google Scholar]
- Lang R. E., Brückner U. B., Hermann K., Kempf B., Rascher W., Sturm V., Unger T., Ganten D. Effect of hemorrhagic shock on the concomitant release of endorphin and enkephalin-like peptides from the pituitary and adrenal gland in the dog. Adv Biochem Psychopharmacol. 1982;33:363–368. [PubMed] [Google Scholar]
- Lewis R. V., Stern A. S., Kimura S., Rossier J., Stein S., Udenfriend S. An about 50,000-dalton protein in adrenal medulla: a common precursor of [Met]- and [Leu]enkephalin. Science. 1980 Jun 27;208(4451):1459–1461. doi: 10.1126/science.7384787. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Dean D. M., Whelan L. G., Udenfriend S., Rossier J. Co-release of enkephalin and catecholamines from cultured adrenal chromaffin cells. Nature. 1981 Jan 22;289(5795):317–319. doi: 10.1038/289317a0. [DOI] [PubMed] [Google Scholar]
- Malhotra R. K., Wakade T. D., Wakade A. R. Vasoactive intestinal polypeptide and muscarine mobilize intracellular Ca2+ through breakdown of phosphoinositides to induce catecholamine secretion. Role of IP3 in exocytosis. J Biol Chem. 1988 Feb 15;263(5):2123–2126. [PubMed] [Google Scholar]
- Schultzberg M., Lundberg J. M., Hökfelt T., Terenius L., Brandt J., Elde R. P., Goldstein M. Enkephalin-like immunoreactivity in gland cells and nerve terminals of the adrenal medulla. Neuroscience. 1978;3(12):1169–1186. doi: 10.1016/0306-4522(78)90137-9. [DOI] [PubMed] [Google Scholar]
- Stern A. S., Lewis R. V., Kimura S., Rossier J., Gerber L. D., Brink L., Stein S., Udenfriend S. Isolation of the opioid heptapeptide Met-enkephalin [Arg6,Phe7] from bovine adrenal medullary granules and striatum. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6680–6683. doi: 10.1073/pnas.76.12.6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros O. H., Diliberto E. J., Jr, Hazum E., Chang K. J. Opiate-like materials in the adrenal medulla: evidence for storage and secretion with catecholamines. Mol Pharmacol. 1979 Nov;16(3):1101–1108. [PubMed] [Google Scholar]
- Wakade A. R. Noncholinergic transmitter(s) maintains secretion of catecholamines from rat adrenal medulla for several hours of continuous stimulation of splanchnic neurons. J Neurochem. 1988 Apr;50(4):1302–1308. doi: 10.1111/j.1471-4159.1988.tb10608.x. [DOI] [PubMed] [Google Scholar]


