Abstract
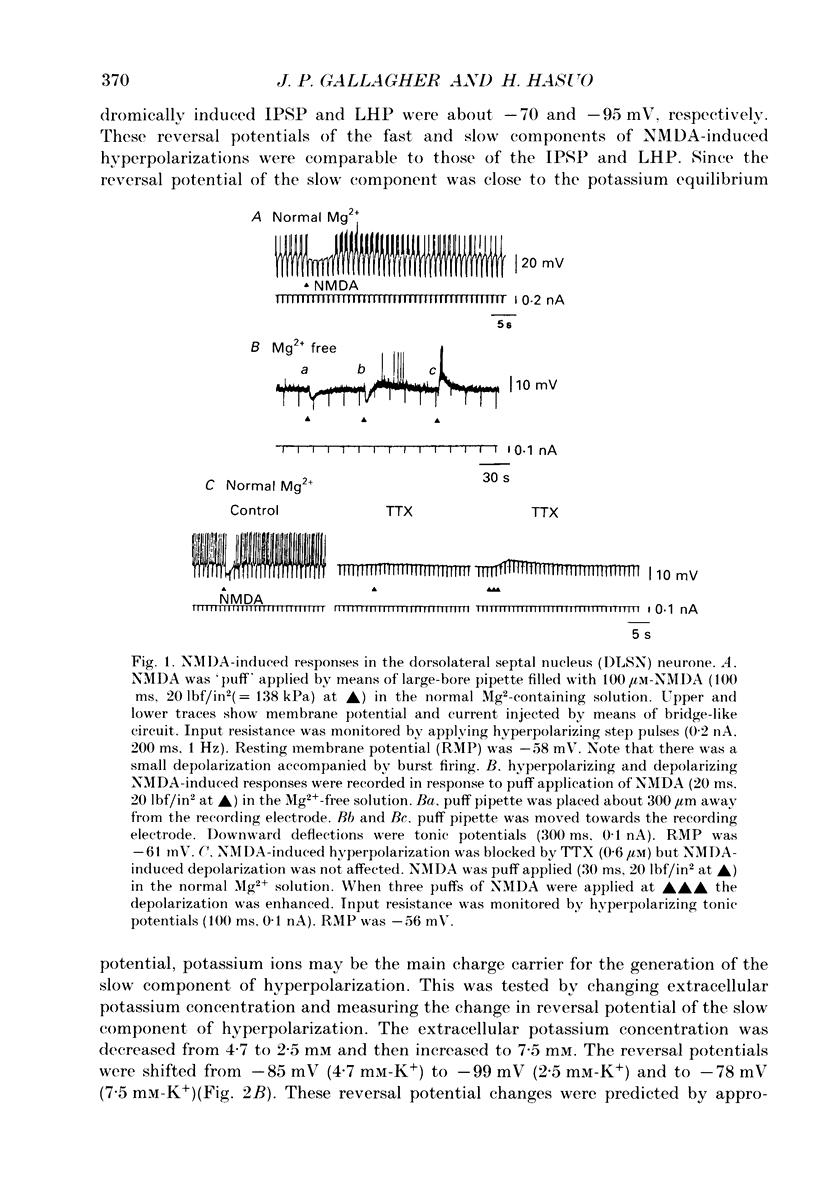
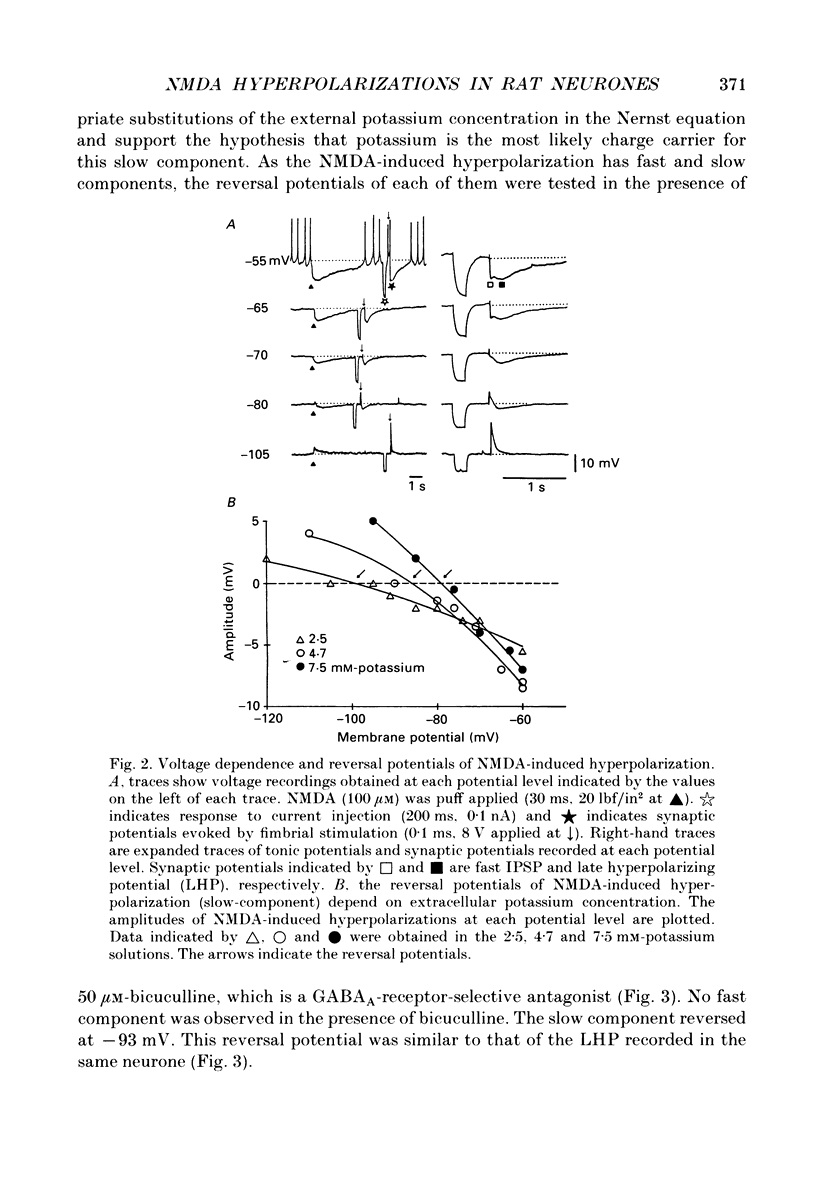
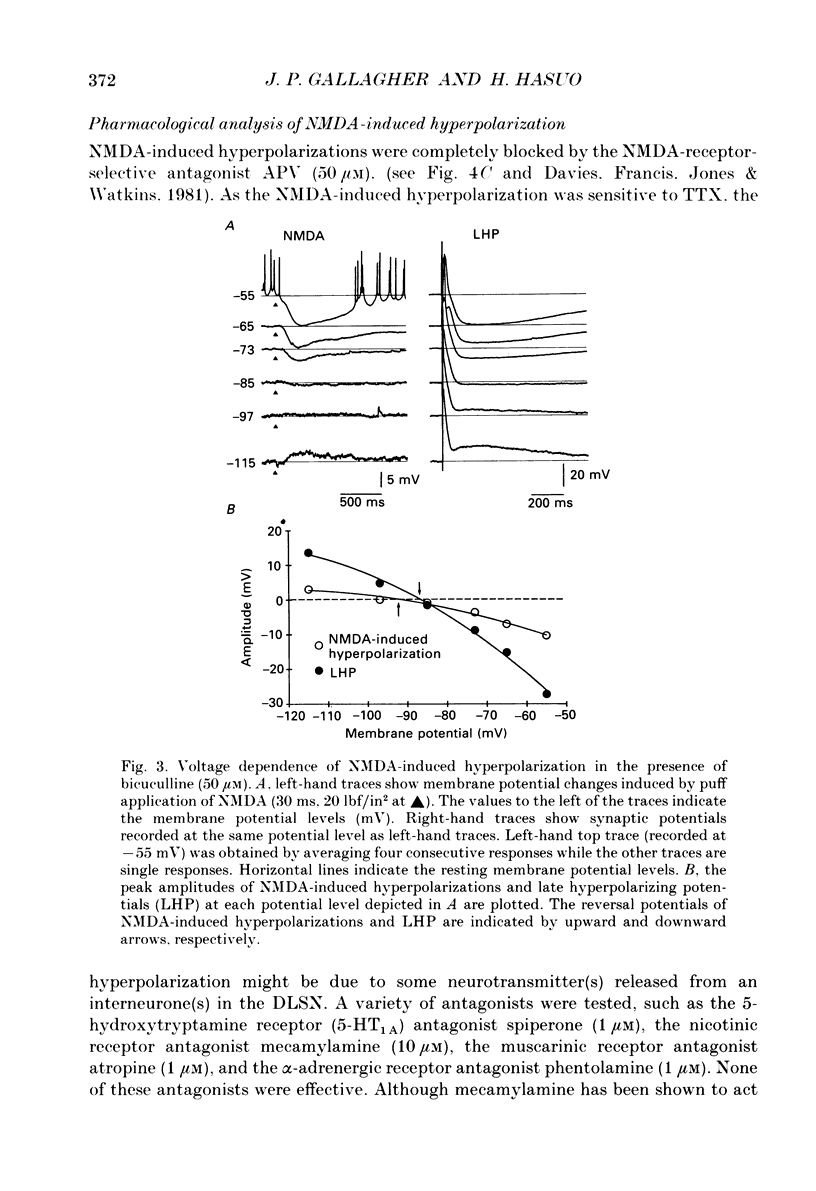
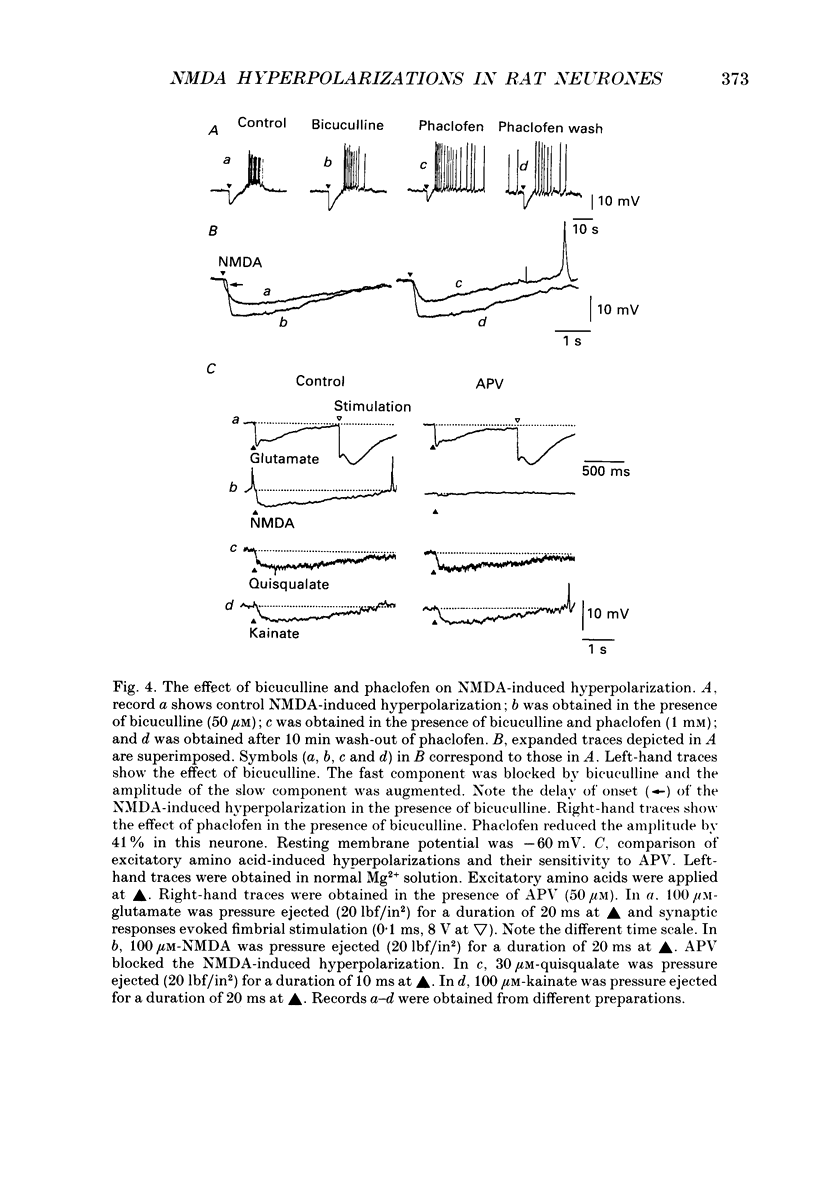
1. Intracellular recordings were made from rat dorsolateral septal nucleus (DLSN) neurones in vitro. Pressure application (puff) of N-methyl-D-aspartate (NMDA) usually produced a hyperpolarization followed by a depolarization in normal Mg2+ solution. 2. A hyperpolarizing response and a depolarizing response could be evoked separately by appropriate positioning of the puff pipette. The NMDA-induced hyperpolarization was blocked by a low-Ca2+ solution or a tetrodotoxin (TTX)-containing solution, but under these conditions the NMDA-induced depolarization was spared. The amplitude of NMDA-induced hyperpolarizations was 7.7 +/- 2.7 mV (n = 13) at the resting membrane potential level. 3. An NMDA-induced hyperpolarization had two components. A fast component reversed at about -76 mV and a slow component reversed at -90 mV. The reversal potential of the slow component shifted in the depolarizing direction of hyperpolarizing direction when the slice was bathed in a high or low-K+ solution, respectively. 4. The reversal potentials of NMDA-induced hyperpolarizations were similar to the synaptic potentials evoked by fimbrial stimulation. The reversal potentials of the fast and slow components were close to the IPSP reversal potential (-70 mV) and the late hyperpolarizing potential (LHP) reversal potential (-95 mV), respectively. 5. NMDA-induced hyperpolarizations were blocked by the specific NMDA receptor antagonist 2-amino-5-phosphonopontanoate (APV). The fast component of an NMDA-induced hyperpolarization was blocked by the specific GABAA receptor antagonist, bicuculline, and the slow component was depressed by the specific GABAB receptor antagonist, phaclofen. 6. Glutamate receptor subtype-specific agonists, such as kainate or quisqualate, could induce similar hyperpolarizations which had bicuculline-sensitive and insensitive components. These non-NMDA-type agonist-induced hyperpolarizations were not affected by APV (50 microM) but were blocked by kynurenic acid (1 mM). 7. We conclude that these excitatory amino acid-induced hyperpolarizations observed in the rat DLSN are mediated by GABAergic interneurones which have both non-NMDA-type and NMDA-type receptors.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balcar V. J., Johnston G. A. The structural specificity of the high affinity uptake of L-glutamate and L-aspartate by rat brain slices. J Neurochem. 1972 Nov;19(11):2657–2666. doi: 10.1111/j.1471-4159.1972.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Davies J., Francis A. A., Jones A. W., Watkins J. C. 2-Amino-5-phosphonovalerate (2APV), a potent and selective antagonist of amino acid-induced and synaptic excitation. Neurosci Lett. 1981 Jan 1;21(1):77–81. doi: 10.1016/0304-3940(81)90061-6. [DOI] [PubMed] [Google Scholar]
- DeFrance J. F., Shimono T., Kitai S. T. Hippocampal inputs to the lateral septal nucleus: patterns of facilitation and inhibition. Brain Res. 1972 Feb 25;37(2):333–339. doi: 10.1016/0006-8993(72)90681-6. [DOI] [PubMed] [Google Scholar]
- DeFrance J. F., Yoshihara H., McCrea R. A., Kitai S. T. Pharmacology of the inhibiton in the lateral septal region. Exp Neurol. 1975 Sep;48(3 Pt 1):502–523. doi: 10.1016/0014-4886(75)90009-6. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Hasuo H. Excitatory amino acid-receptor-mediated EPSPs in rat dorsolateral septal nucleus neurones in vitro. J Physiol. 1989 Nov;418:353–365. doi: 10.1113/jphysiol.1989.sp017845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J. Cellular uptake disguises action of L-glutamate on N-methyl-D-aspartate receptors. With an appendix: diffusion of transported amino acids into brain slices. Br J Pharmacol. 1985 May;85(1):297–307. doi: 10.1111/j.1476-5381.1985.tb08860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Kerr D. I., Ong J., Prager R. H., Gynther B. D., Curtis D. R. Phaclofen: a peripheral and central baclofen antagonist. Brain Res. 1987 Mar 3;405(1):150–154. doi: 10.1016/0006-8993(87)90999-1. [DOI] [PubMed] [Google Scholar]
- Köhler C., Chan-Palay V. Distribution of gamma aminobutyric acid containing neurons and terminals in the septal area. An immunohistochemical study using antibodies to glutamic acid decarboxylase in the rat brain. Anat Embryol (Berl) 1983;167(1):53–65. doi: 10.1007/BF00304600. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- McLennan H., Miller J. J. The hippocampal control of neuronal discharges in the septum of the rat. J Physiol. 1974 Mar;237(3):607–624. doi: 10.1113/jphysiol.1974.sp010500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olverman H. J., Jones A. W., Watkins J. C. L-glutamate has higher affinity than other amino acids for [3H]-D-AP5 binding sites in rat brain membranes. Nature. 1984 Feb 2;307(5950):460–462. doi: 10.1038/307460a0. [DOI] [PubMed] [Google Scholar]
- Onteniente B., Tago H., Kimura H., Maeda T. Distribution of gamma-aminobutyric acid-immunoreactive neurons in the septal region of the rat brain. J Comp Neurol. 1986 Jun 15;248(3):422–430. doi: 10.1002/cne.902480310. [DOI] [PubMed] [Google Scholar]
- Panula P., Revuelta A. V., Cheney D. L., Wu J. Y., Costa E. An immunohistochemical study on the location of GABAergic neurons in rat septum. J Comp Neurol. 1984 Jan 1;222(1):69–80. doi: 10.1002/cne.902220107. [DOI] [PubMed] [Google Scholar]
- Phelan K. D., Hasuo H., Twery M. J., Gallagher J. P. Projection neurons in the rat dorsolateral septal nucleus possess recurrent axon collaterals. Neurosci Lett. 1989 Feb 27;97(3):259–265. doi: 10.1016/0304-3940(89)90607-1. [DOI] [PubMed] [Google Scholar]
- Quinlan J. E., Davies J. Excitatory and inhibitory responses of Purkinje cells, in the rat cerebellum in vivo, induced by excitatory amino acids. Neurosci Lett. 1985 Sep 16;60(1):39–46. doi: 10.1016/0304-3940(85)90378-7. [DOI] [PubMed] [Google Scholar]
- Stevens D. R., Gallagher J. P., Shinnick-Gallagher P. Further studies on the action of baclofen on neurons of the dorsolateral septal nucleus of the rat, in vitro. Brain Res. 1985 Dec 9;358(1-2):360–363. doi: 10.1016/0006-8993(85)90984-9. [DOI] [PubMed] [Google Scholar]
- Stevens D. R., Gallagher J. P., Shinnick-Gallagher P. In vitro studies of the role of gamma-aminobutyric acid in inhibition in the lateral septum of the rat. Synapse. 1987;1(2):184–190. doi: 10.1002/syn.890010206. [DOI] [PubMed] [Google Scholar]
- Stevens D. R., Gallagher J. P., Shinnick-Gallagher P. Intracellular recordings from rat dorsolateral septal neurons, in vitro. Brain Res. 1984 Jul 9;305(2):353–356. doi: 10.1016/0006-8993(84)90441-4. [DOI] [PubMed] [Google Scholar]
- Taube J. S., Schwartzkroin P. A. Hyperpolarizing responses to application of glutamate in hippocampal CA1 pyramidal neurons. Neurosci Lett. 1987 Jul 9;78(1):85–90. doi: 10.1016/0304-3940(87)90566-0. [DOI] [PubMed] [Google Scholar]


