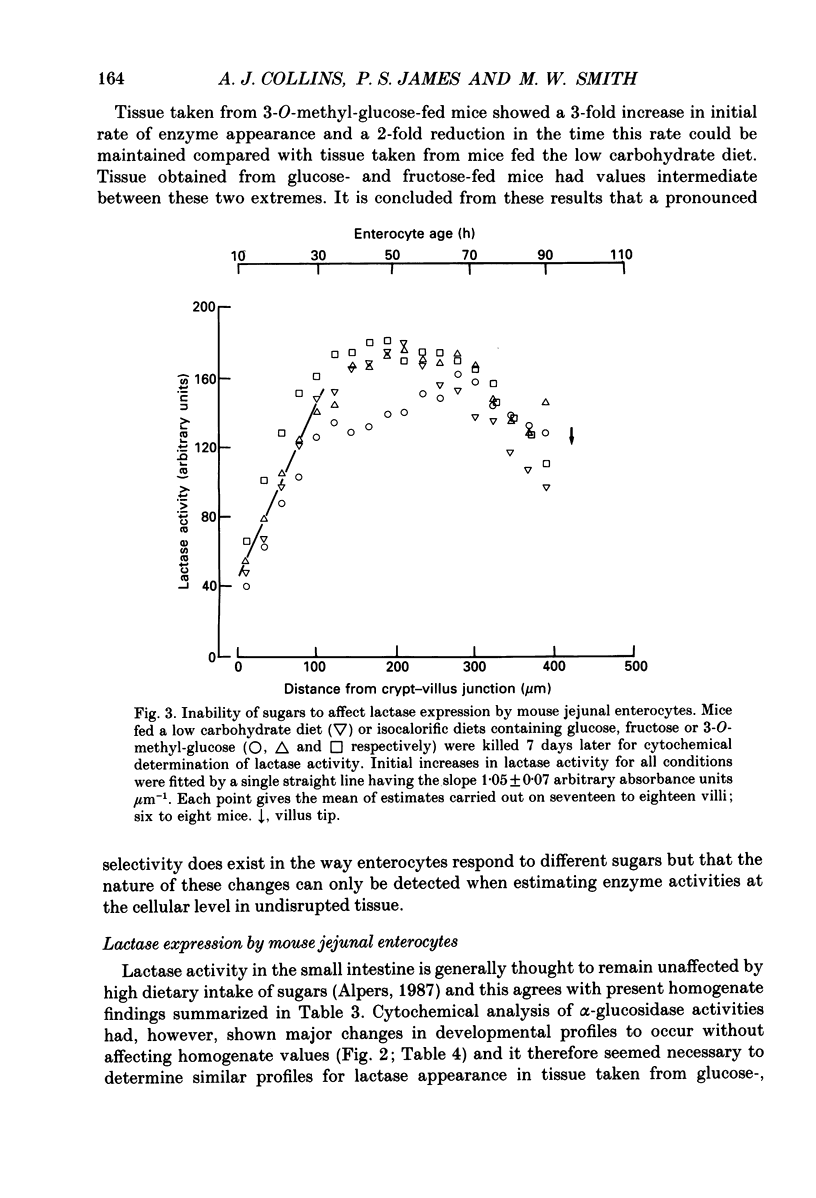
Abstract
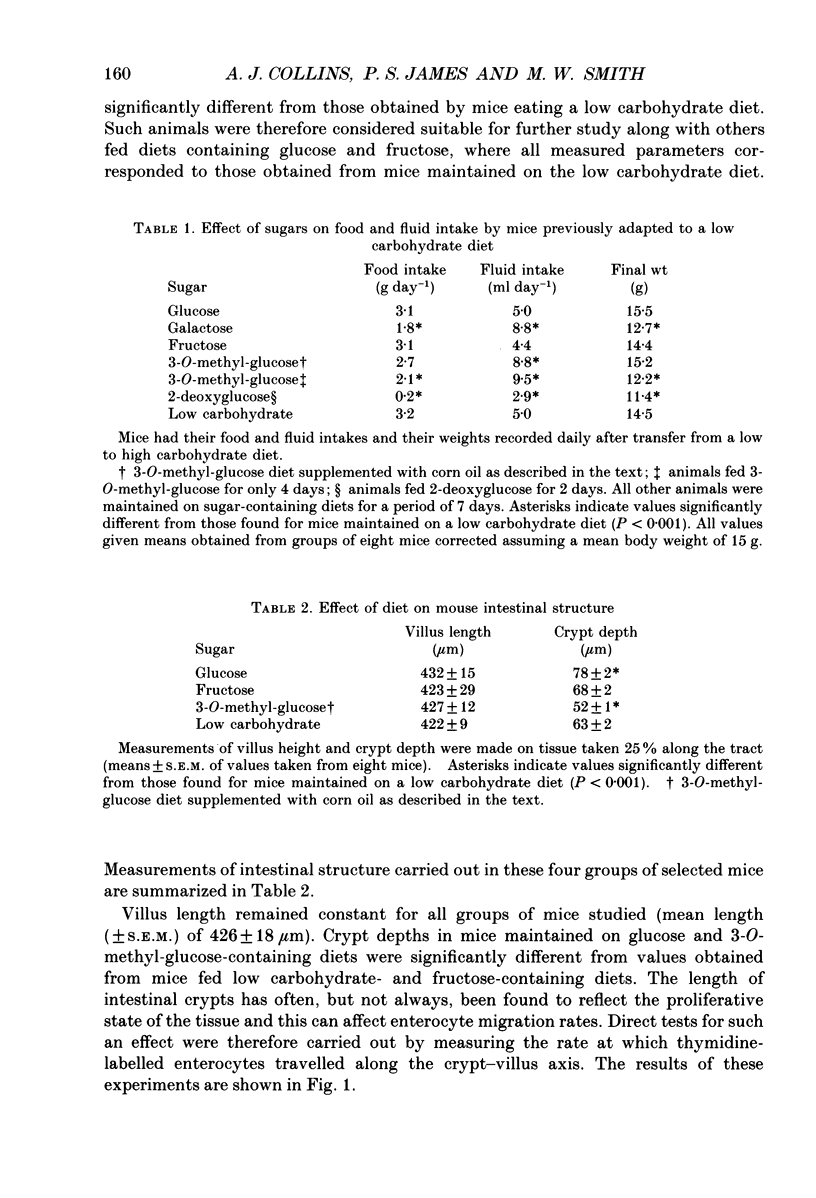
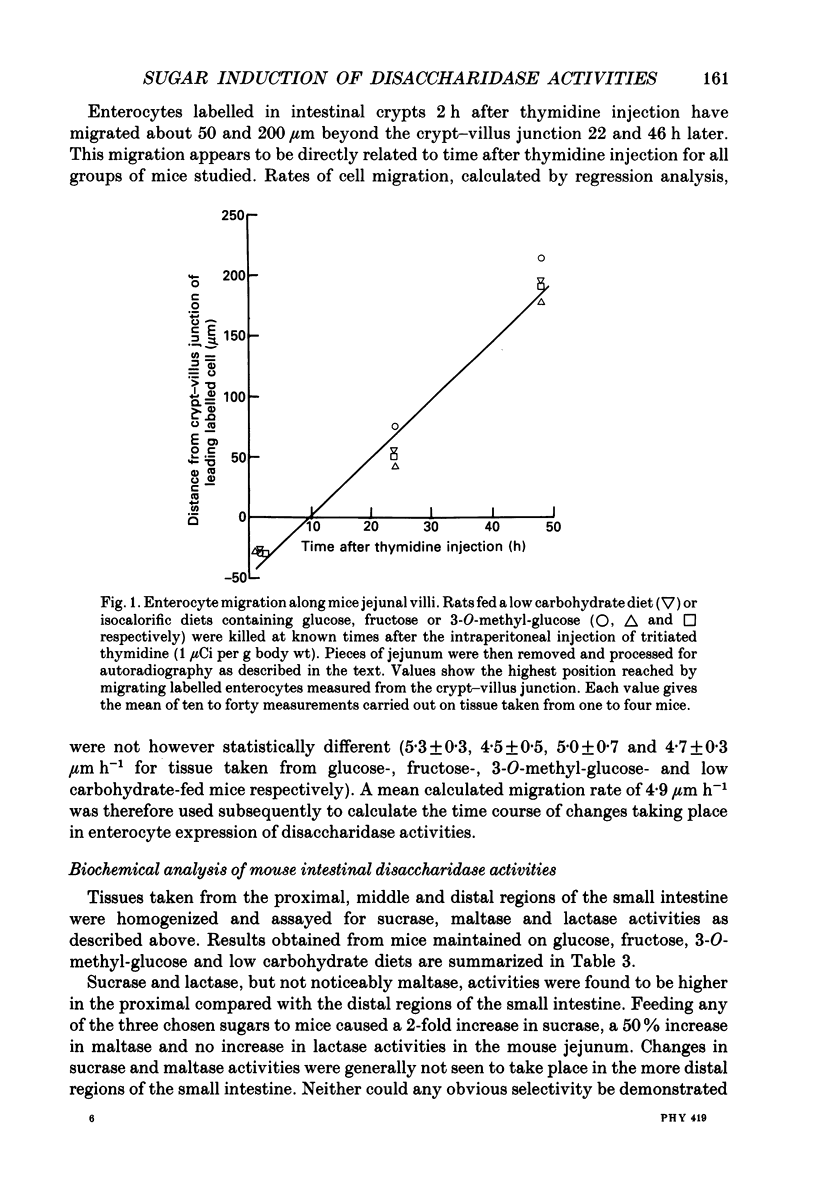
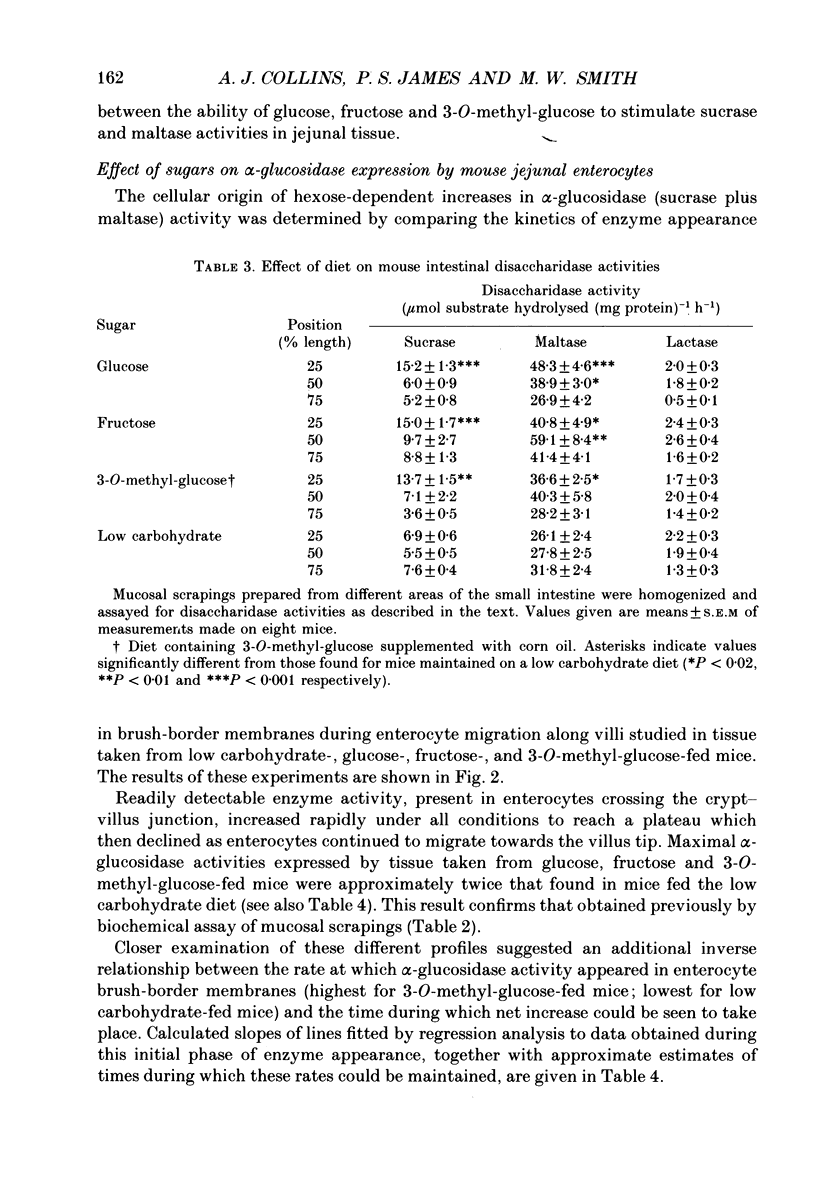
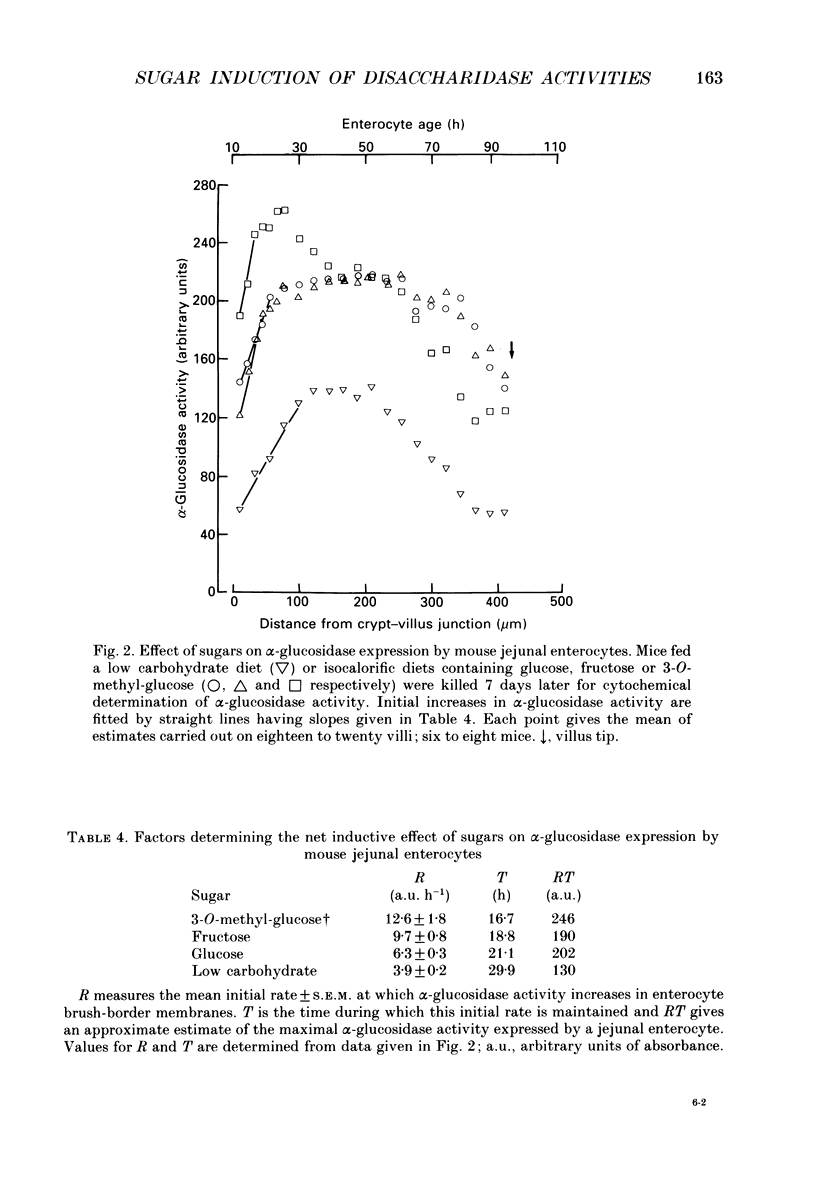
1. Sugar-containing diets chosen not to affect intestinal structure or enterocyte turnover have been fed to mice previously maintained on a low carbohydrate diet in order to determine their ability to induce disaccharidase enzymes in the small intestine. 2. Glucose-, fructose- and 3-O-methyl-glucose-containing diets increased sucrase and maltase but not lactase activities in mouse jejunal homogenates. These effects were either absent or negligible in more distal regions of the small intestine. 3. Placing mice on glucose-, fructose- or 3-O-methyl-glucose-containing diets was further shown, by quantitative cytochemistry, to cause a 1.6-, 2.6- and 3.2-fold increase in the initial rate at which alpha-glucosidase activity (sucrase + maltase) appeared in the brush-border membrane of developing enterocytes. 4. The time during which alpha-glucosidase activity increased in enterocyte brush-border membranes fell from 30 h for low carbohydrate fed mice to 21, 19 and 17 h in mice fed glucose, fructose and 3-O-methyl-glucose respectively. Change of diet had no effect on the kinetics of lactase expression by developing enterocytes. 5. Maximal alpha-glucosidase activity detected in enterocyte brush-border membranes is equal to RT, where R is the initial rate of enzyme appearance and T is the time during which this rate operates. The ability of sugars to increase R selectively, but only at the expense of T, defines unexpected limits to the capacity of enterocytes to adapt to changes in luminal nutrition. 6. The above results are discussed in relation to other aspects of enterocyte differentiation recently subjected to quantitative analysis. The need to standardize other aspects of intestinal physiology and redefine the energy content of diets containing non-metabolizable substrates in this type of work is also emphasized.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLAIR D. G., YAKIMETS W., TUBA J. Rat intestinal sucrase. II. The effects of rat age and sex and of diet on sucrase activity. Can J Biochem Physiol. 1963 Apr;41:917–929. [PubMed] [Google Scholar]
- Bustamante S., Gasparo M., Kendall K., Coates P., Brown S., Somawane B., Koldovsky O. Increased activity of rat intestinal lactase due to increased intake of alpha-saccharides (starch, sucrose) in isocaloric diets. J Nutr. 1981 Jun;111(6):943–953. doi: 10.1093/jn/111.6.943. [DOI] [PubMed] [Google Scholar]
- Chantret I., Barbat A., Dussaulx E., Brattain M. G., Zweibaum A. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res. 1988 Apr 1;48(7):1936–1942. [PubMed] [Google Scholar]
- Chaves M., Smith M. W., Williamson R. C. Increased activity of digestive enzymes in ileal enterocytes adapting to proximal small bowel resection. Gut. 1987 Aug;28(8):981–987. doi: 10.1136/gut.28.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cézard J. P., Broyart J. P., Cuisinier-Gleizes P., Mathieu H. Sucrase-isomaltase regulation by dietary sucrose in the rat. Gastroenterology. 1983 Jan;84(1):18–25. [PubMed] [Google Scholar]
- Deren J. J., Broitman S. A., Zamcheck N. Effect of diet upon intestinal disaccharidases and disaccharide absorption. J Clin Invest. 1967 Feb;46(2):186–195. doi: 10.1172/JCI105521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris R. P., Diamond J. M. Use of phlorizin binding to demonstrate induction of intestinal glucose transporters. J Membr Biol. 1986;94(1):77–82. doi: 10.1007/BF01901015. [DOI] [PubMed] [Google Scholar]
- Gutschmidt S., Emde C. Early changes in brush border disaccharidase kinetics in rat jejunum following subcutaneous administration of tetraiodothyronine: a quantitative histochemical study on villi revealing normal morphology. Histochemistry. 1981;73(1):151–160. doi: 10.1007/BF00493141. [DOI] [PubMed] [Google Scholar]
- Gutschmidt S., Kaul W., Riecken E. O. A quantitative histochemical technique for the characterisation of alpha-glucosidases in the brush-border membrane of rat jejunum. Histochemistry. 1979 Sep;63(1):81–101. doi: 10.1007/BF00508014. [DOI] [PubMed] [Google Scholar]
- James P. S., Smith M. W., Tivey D. R., Wilson T. J. Epidermal growth factor selectively increases maltase and sucrase activities in neonatal piglet intestine. J Physiol. 1987 Dec;393:583–594. doi: 10.1113/jphysiol.1987.sp016842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King I. S., Paterson J. Y., Peacock M. A., Smith M. W., Syme G. Effect of diet upon enterocyte differentiation in the rat jejunum. J Physiol. 1983 Nov;344:465–481. doi: 10.1113/jphysiol.1983.sp014952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine G. M., Deren J. J., Steiger E., Zinno R. Role of oral intake in maintenance of gut mass and disaccharide activity. Gastroenterology. 1974 Nov;67(5):975–982. [PubMed] [Google Scholar]
- Lund E. K., Smith M. W. Rat jejunal disaccharidase activity increases biphasically during early post-natal development. J Physiol. 1987 Oct;391:487–497. doi: 10.1113/jphysiol.1987.sp016751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Rosenweig N. S., Herman R. H. Time response of jejunal sucrase and maltase activity to a high sucrose diet in normal man. Gastroenterology. 1969 Mar;56(3):500–505. [PubMed] [Google Scholar]
- SOLS A. The hexokinase activity of the intestinal mucosa. Biochim Biophys Acta. 1956 Jan;19(1):144–152. doi: 10.1016/0006-3002(56)90396-1. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Jarvis L. G., King I. S. Cell proliferation in follicle-associated epithelium of mouse Peyer's patch. Am J Anat. 1980 Oct;159(2):157–166. doi: 10.1002/aja.1001590204. [DOI] [PubMed] [Google Scholar]
- Syme G. The effect of protein-deficient isoenergetic diets on the growth of rat jejunal mucosa. Br J Nutr. 1982 Jul;48(1):25–36. doi: 10.1079/bjn19820084. [DOI] [PubMed] [Google Scholar]


