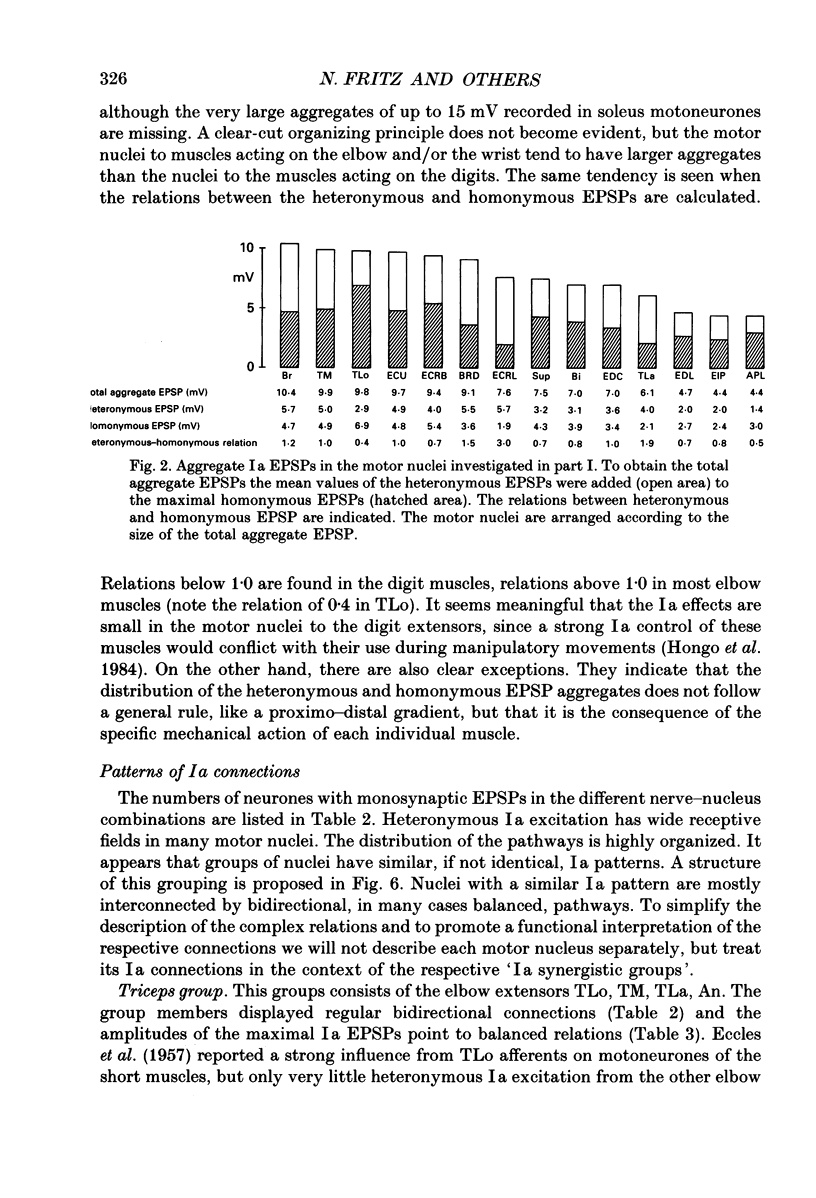
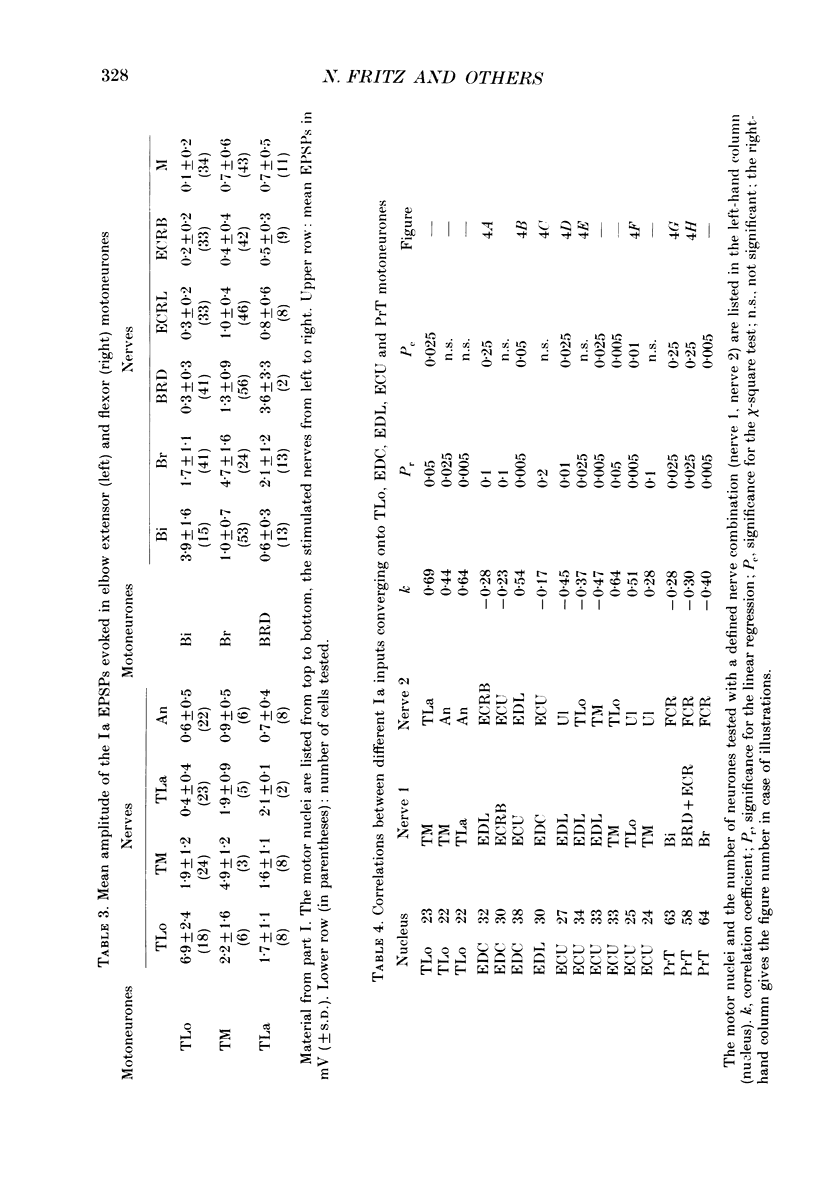
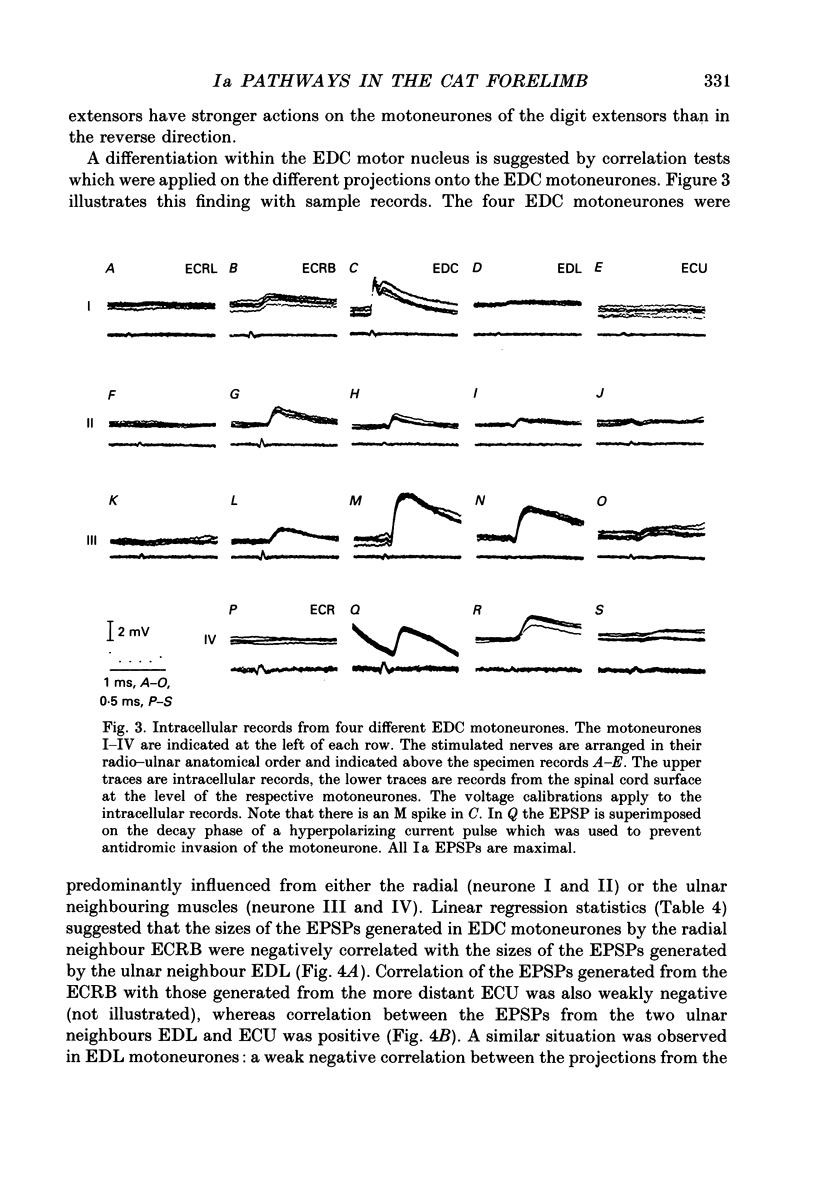
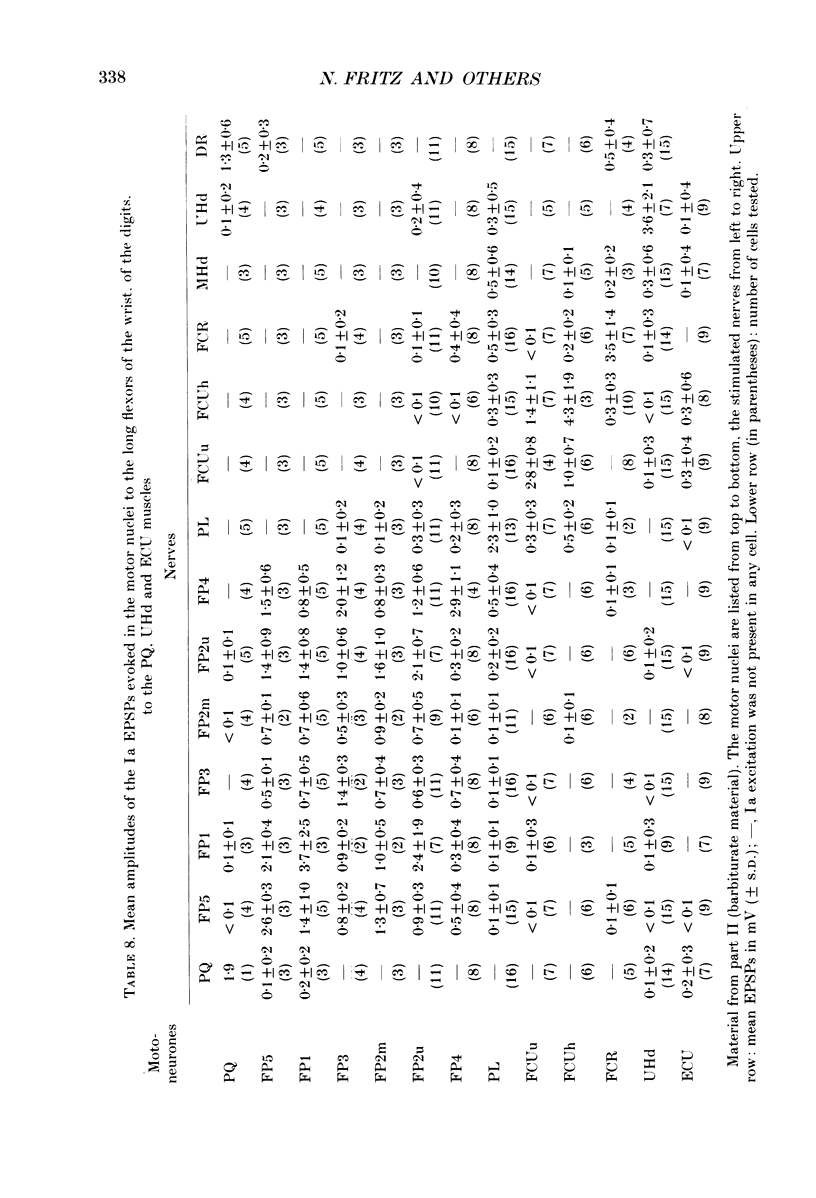
Abstract
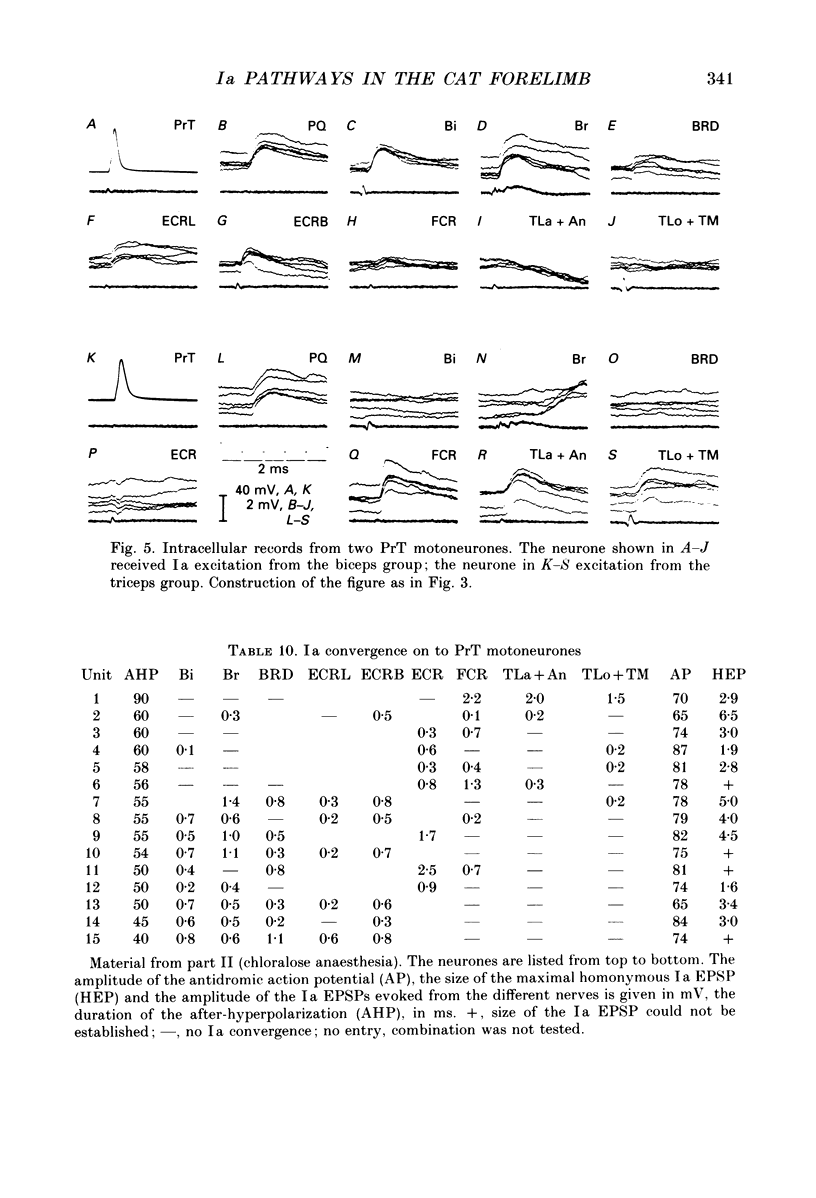
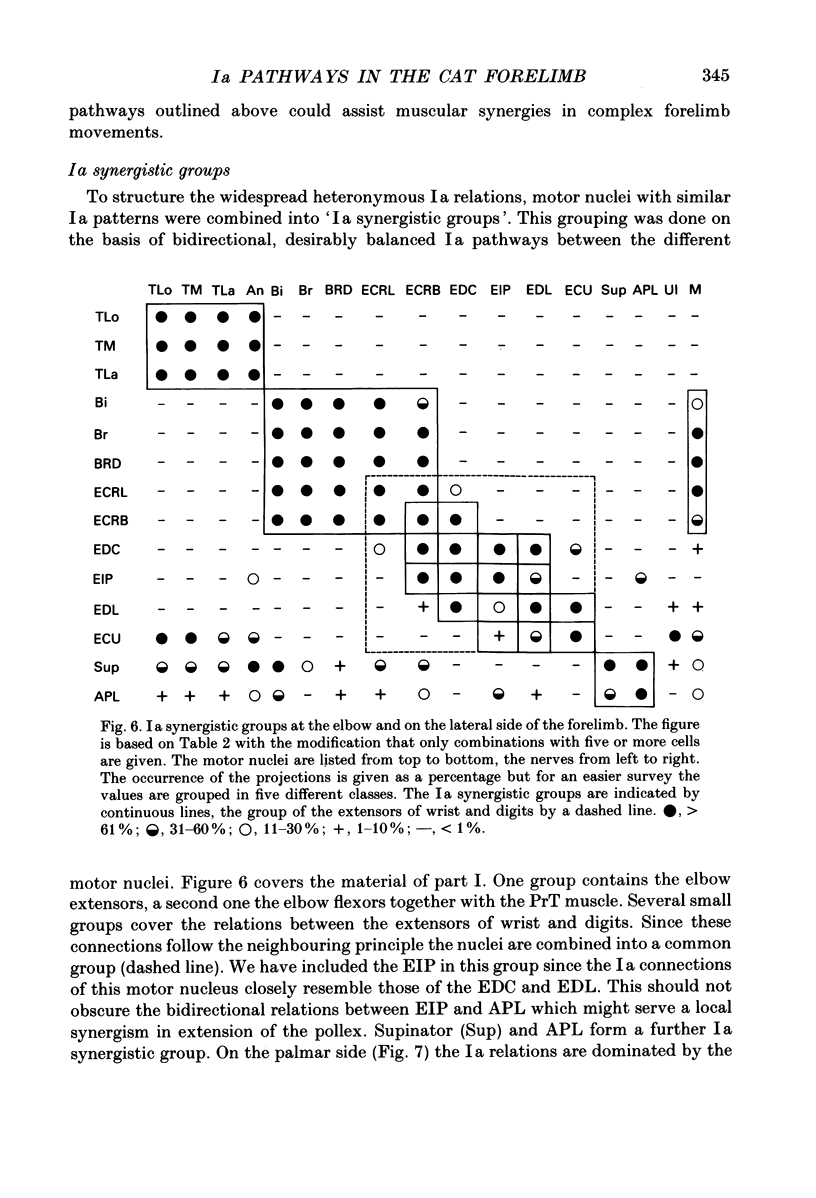
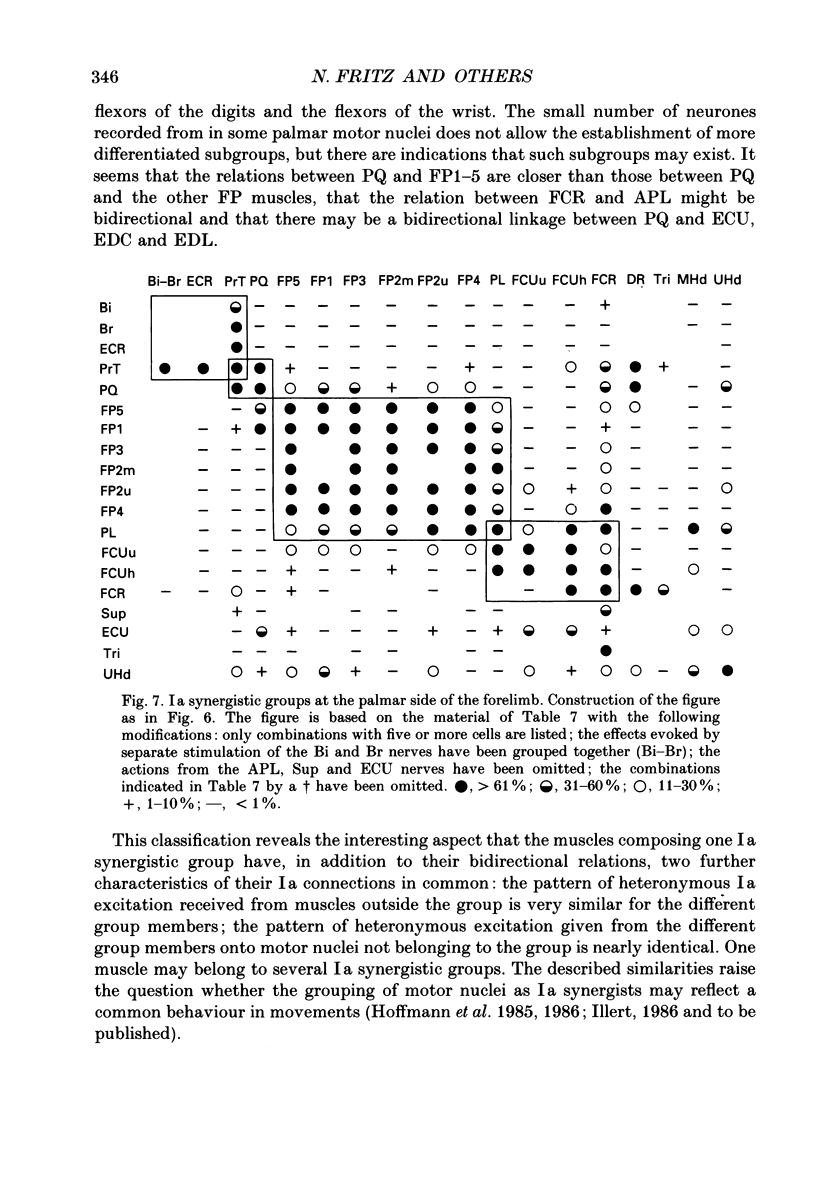
1. In anaesthetized cats intracellular records were obtained from antidromically identified motoneurones. The motor nuclei to the elbow extensor and flexor muscles and to the muscles innervated by the deep radial, ulnar and median nerves were investigated. The maximum Ia EPSPs from electrical stimulation of various peripheral nerves were measured. The characteristic convergence and projection patterns to each motor nucleus were established from pooled data. 2. The total aggregates of the Ia EPSPs between the different motor nuclei ranged from 3.5 to 11.7 mV. The smallest aggregates were found in the nuclei to the digit muscles. The ratio of the heteronymous versus homonymous EPSP amplitudes varied between 3.9 and 0.5. A general rule which would govern the distribution of the EPSP aggregates, such as a proximo-distal gradient, was not observed. 3. The Ia connections followed a complex but highly organized pattern. Bidirectional and unidirectional pathways were present. In many cases the convergence pattern of a motor nucleus included muscles acting at different joints. The connections of one nucleus were not necessarily restricted to one side of the limb, but could cross the radio-ulnar plane. 4. Muscles with similar actions onto the same joint were interconnected with bidirectional, rather balanced Ia pathways. Such relations were also present between close functional synergists and then often extended across several joints. The relations between the anatomical extensors of wrist and digits were graded according to the neighbourhood of these muscles. It is suggested that this reflects the graded mechanical synergism in the wrist action of these muscles. 5. A large number of unidirectional or strongly skewed bidirectional Ia pathways project from proximal to distal muscles. It is suggested that they may serve a readjustment of distal joints during changes in the position of proximal ones (e.g. stabilization of the position of the radio-ulnar plane during elbow extension in case of the undirectional projections onto supinator and abductor pollicis longus motoneurones). 6. The motor nuclei to some multifunctional muscles display a negative correlation between different heteronymous Ia inputs: motoneurones with a large input from one muscle show a significant tendency to receive a smaller input from another muscle and vice versa. This organization leads to subpopulations of neurones with different convergence patterns within the same motor nucleus. 7. Motor nuclei with bidirectional Ia relations between each other displayed similar convergence and projection patterns. They were combined into 'Ia synergistic groups.' One motor nucleus may belong to several groups.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E. Segmental reflex inputs to motoneurons innervating dorsal neck musculature in the cat. Exp Brain Res. 1977 May 23;28(1-2):175–187. doi: 10.1007/BF00237095. [DOI] [PubMed] [Google Scholar]
- BROCK L. G., COOMBS J. S., ECCLES J. C. Intracellular recording from antidromically activated motoneurones. J Physiol. 1953 Dec 29;122(3):429–461. doi: 10.1113/jphysiol.1953.sp005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botterman B. R., Hamm T. M., Reinking R. M., Stuart D. G. Localization of monosynaptic Ia excitatory post-synaptic potentials in the motor nucleus of the cat biceps femoris muscle. J Physiol. 1983 May;338:355–377. doi: 10.1113/jphysiol.1983.sp014677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink E. E., Jinnai K., Wilson V. J. Pattern of segmental monosynaptic input to cat dorsal neck motoneurons. J Neurophysiol. 1981 Sep;46(3):496–505. doi: 10.1152/jn.1981.46.3.496. [DOI] [PubMed] [Google Scholar]
- Burke R. E. Group Ia synaptic input to fast and slow twitch motor units of cat triceps surae. J Physiol. 1968 Jun;196(3):605–630. doi: 10.1113/jphysiol.1968.sp008526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough J. F., Kernell D., Phillips C. G. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurones of the baboon's hand and forearm. J Physiol. 1968 Sep;198(1):145–166. doi: 10.1113/jphysiol.1968.sp008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958 Dec 4;144(2):271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Lundberg A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiol Scand. 1969 Apr;75(4):614–630. doi: 10.1111/j.1748-1716.1969.tb04415.x. [DOI] [PubMed] [Google Scholar]
- English A. W. An electromyographic analysis of forelimb muscles during overground stepping in the cat. J Exp Biol. 1978 Oct;76:105–122. doi: 10.1242/jeb.76.1.105. [DOI] [PubMed] [Google Scholar]
- Fritz N., Illert M., Reeh P. Location of motoneurones projecting to the cat distal forelimb. II. Median and ulnar motornuclei. J Comp Neurol. 1986 Feb 15;244(3):302–312. doi: 10.1002/cne.902440304. [DOI] [PubMed] [Google Scholar]
- Fritz N., Illert M., Saggau P. Location of motoneurones projecting to the cat distal forelimb. I. Deep radial motornuclei. J Comp Neurol. 1986 Feb 15;244(3):286–301. doi: 10.1002/cne.902440303. [DOI] [PubMed] [Google Scholar]
- Gonyea W. J., Marushia S. A., Dixon J. A. Morphological organization and contractile properties of the wrist flexor muscles in the cat. Anat Rec. 1981 Mar;199(3):321–339. doi: 10.1002/ar.1091990303. [DOI] [PubMed] [Google Scholar]
- Hahne M., Illert M., Wietelmann D. Recurrent inhibition in the cat distal forelimb. Brain Res. 1988 Jul 19;456(1):188–192. doi: 10.1016/0006-8993(88)90362-9. [DOI] [PubMed] [Google Scholar]
- Hoffer J. A., Loeb G. E., Sugano N., Marks W. B., O'Donovan M. J., Pratt C. A. Cat hindlimb motoneurons during locomotion. III. Functional segregation in sartorius. J Neurophysiol. 1987 Feb;57(2):554–562. doi: 10.1152/jn.1987.57.2.554. [DOI] [PubMed] [Google Scholar]
- Hongo T., Lundberg A., Phillips C. G., Thompson R. F. The pattern of monosynaptic Ia-connections to hindlimb motor nuclei in the baboon: a comparison with the cat. Proc R Soc Lond B Biol Sci. 1984 May 22;221(1224):261–289. doi: 10.1098/rspb.1984.0034. [DOI] [PubMed] [Google Scholar]
- Hulliger M. The mammalian muscle spindle and its central control. Rev Physiol Biochem Pharmacol. 1984;101:1–110. doi: 10.1007/BFb0027694. [DOI] [PubMed] [Google Scholar]
- Illert M., Lundberg A., Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 2. Convergence on neurones mediating disynaptic cortico-motoneuronal excitation. Exp Brain Res. 1976 Dec 22;26(5):521–540. doi: 10.1007/BF00238825. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Padel Y., Zarzecki P. Crossed disynaptic inhibition of sacral motoneurones. J Physiol. 1978 Dec;285:425–444. doi: 10.1113/jphysiol.1978.sp012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAPORTE Y., LLOYD D. P. C. Nature and significance of the reflex connections established by large afferent fibers of muscular origin. Am J Physiol. 1952 Jun;169(3):609–621. doi: 10.1152/ajplegacy.1952.169.3.609. [DOI] [PubMed] [Google Scholar]
- Miller S., Van Der Meché F. G. Movements of the forelimbs of the cat during stepping on a treadmill. Brain Res. 1975 Jun 27;91(2):255–269. doi: 10.1016/0006-8993(75)90546-6. [DOI] [PubMed] [Google Scholar]
- Rapoport S. Reflex connexions of motoneurones of muscles involved in head movement in the cat. J Physiol. 1979 Apr;289:311–327. doi: 10.1113/jphysiol.1979.sp012739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT R. F., WILLIS W. D. Intracellular recording from motoneurons of the cervical spinal cord of the cat. J Neurophysiol. 1963 Jan;26:28–43. doi: 10.1152/jn.1963.26.1.28. [DOI] [PubMed] [Google Scholar]
- Thomas J. S., Schmidt E. M., Hambrecht F. T. Facility of motor unit control during tasks defined directly in terms of unit behaviors. Exp Neurol. 1978 May 1;59(3):384–397. doi: 10.1016/0014-4886(78)90230-3. [DOI] [PubMed] [Google Scholar]
- Vanden Noven S., Hamm T. M., Stuart D. G. Partitioning of monosynaptic Ia excitatory postsynaptic potentials in the motor nucleus of the cat lateral gastrocnemius muscle. J Neurophysiol. 1986 Mar;55(3):569–586. doi: 10.1152/jn.1986.55.3.569. [DOI] [PubMed] [Google Scholar]
- Willis W. D., Tate G. W., Ashworth R. D., Willis J. C. Monosynaptic excitation of motoneurons of individual forelimb muscles. J Neurophysiol. 1966 May;29(3):410–424. doi: 10.1152/jn.1966.29.3.410. [DOI] [PubMed] [Google Scholar]
- ter Haar Romeny B. M., Denier van der Gon J. J., Gielen C. C. Changes in recruitment order of motor units in the human biceps muscle. Exp Neurol. 1982 Nov;78(2):360–368. doi: 10.1016/0014-4886(82)90054-1. [DOI] [PubMed] [Google Scholar]
- ter Haar Romeny B. M., van der Gon J. J., Gielen C. C. Relation between location of a motor unit in the human biceps brachii and its critical firing levels for different tasks. Exp Neurol. 1984 Sep;85(3):631–650. doi: 10.1016/0014-4886(84)90036-0. [DOI] [PubMed] [Google Scholar]




