Abstract
1. Transducer currents were recorded in turtle cochlear hair cells during mechanical stimulation of the hair bundle. The currents were measured under whole-cell voltage clamp in isolated cells that were firmly stuck to the floor of the recording chamber. 2. Stimuli were calibrated by projecting the image of the hair bundle onto a rapidly scanned 128 photodiode array. This technique showed that, while the cell body was immobilized, the tip of the bundle would follow faithfully the motion of an attached glass probe up to frequencies of more than 1 kHz. 3. The relationship between inward transducer current and bundle displacement was sigmoidal. Maximum currents of 200-400 pA were observed for deflections of the tip of the bundle of 0.5 microns, equivalent to rotating the bundle by about 5 deg. 4. In response to a step deflection of the bundle, the current developed with a time constant (about 0.4 ms for small stimuli) that decreased with the size of displacement. This suggests that the onset of the current was limited by the gating kinetics of the transduction channel. The onset time course was slowed about fourfold for a 20 degrees C drop in temperature. 5. For small maintained displacements, the current relaxed to about a quarter of the peak level with a time constant of 3-5 ms. This adaptation was associated with a shift of the current-displacement relationship in the direction of the stimulus. The rate and extent of adaptation were decreased by lowering external Ca2+. 6. Adaptation was strongly voltage sensitive, and was abolished at holding potentials positive to the reversal potential of the transducer current of about 0 mV. It was also diminished by loading cells with 10 mM of the Ca2+ chelator BAPTA. These observations suggest that adaptation may be partly controlled by influx of Ca2+ through the transducer channels. 7. Removal of adaptation produced asymmetric responses, with fast onsets but slow decays following return of the bundle to its resting position; the offset time course depended on both the magnitude and duration of the prior displacement. 8. In some experiments, hair bundles were deflected with a flexible glass fibre whose motion was monitored using a dual photodiode arrangement. Positive holding potentials abolished adaptation of the transducer currents, but had no influence on the time course of motion of the fibre. We have no evidence therefore that adaptation is caused by a mechanical reorganization within the bundle.
Full text
PDF















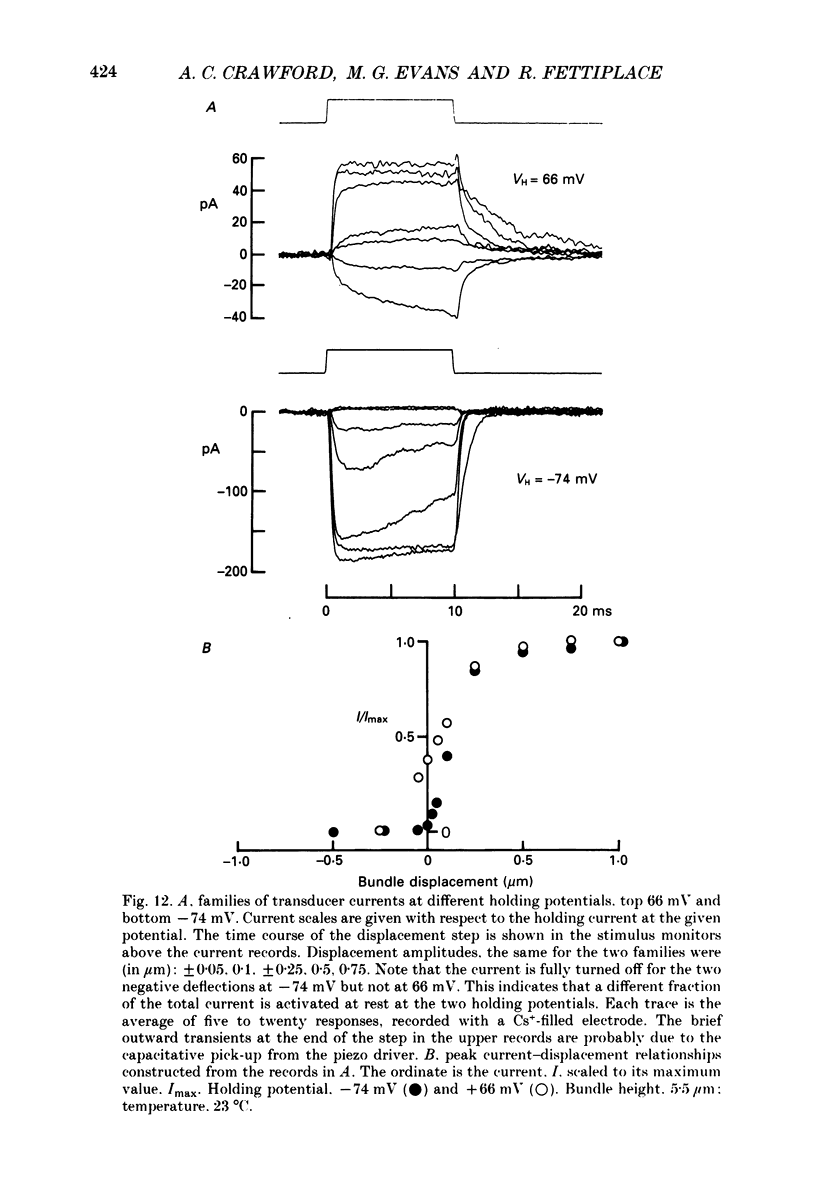

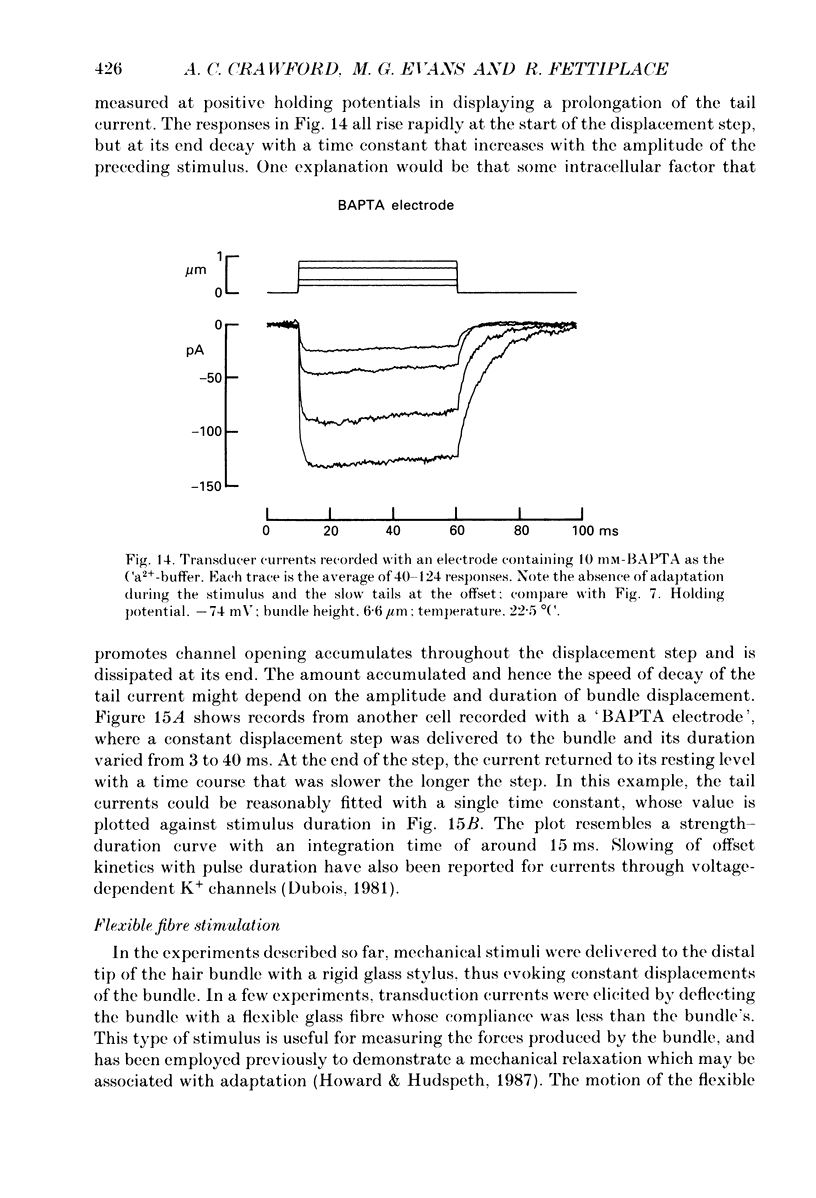








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Art J. J., Fettiplace R. Variation of membrane properties in hair cells isolated from the turtle cochlea. J Physiol. 1987 Apr;385:207–242. doi: 10.1113/jphysiol.1987.sp016492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad J. A., Hacohen N., Corey D. P. Voltage dependence of adaptation and active bundle movement in bullfrog saccular hair cells. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2918–2922. doi: 10.1073/pnas.86.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher S. K., Warren R. L. Very low calcium content of cochlear endolymph, an extracellular fluid. Nature. 1978 Jun 1;273(5661):377–378. doi: 10.1038/273377a0. [DOI] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Analysis of the microphonic potential of the bullfrog's sacculus. J Neurosci. 1983 May;3(5):942–961. doi: 10.1523/JNEUROSCI.03-05-00942.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979 Oct 25;281(5733):675–677. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci. 1983 May;3(5):962–976. doi: 10.1523/JNEUROSCI.03-05-00962.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Mechanical stimulation and micromanipulation with piezoelectric bimorph elements. J Neurosci Methods. 1980 Dec;3(2):183–202. doi: 10.1016/0165-0270(80)90025-4. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. Non-linearities in the responses of turtle hair cells. J Physiol. 1981 Jun;315:317–338. doi: 10.1113/jphysiol.1981.sp013750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol. 1985 Jul;364:359–379. doi: 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock R. A., Corey D. P., Hudspeth A. J. Adaptation of mechanoelectrical transduction in hair cells of the bullfrog's sacculus. J Neurosci. 1987 Sep;7(9):2821–2836. doi: 10.1523/JNEUROSCI.07-09-02821.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness D. N., Hackney C. M. Cross-links between stereocilia in the guinea pig cochlea. Hear Res. 1985 May;18(2):177–188. doi: 10.1016/0378-5955(85)90010-3. [DOI] [PubMed] [Google Scholar]
- Holton T., Hudspeth A. J. The transduction channel of hair cells from the bull-frog characterized by noise analysis. J Physiol. 1986 Jun;375:195–227. doi: 10.1113/jphysiol.1986.sp016113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J., Hudspeth A. J. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog's saccular hair cell. Neuron. 1988 May;1(3):189–199. doi: 10.1016/0896-6273(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Howard J., Hudspeth A. J. Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog's saccular hair cell. Proc Natl Acad Sci U S A. 1987 May;84(9):3064–3068. doi: 10.1073/pnas.84.9.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J., Roberts W. M., Hudspeth A. J. Mechanoelectrical transduction by hair cells. Annu Rev Biophys Biophys Chem. 1988;17:99–124. doi: 10.1146/annurev.bb.17.060188.000531. [DOI] [PubMed] [Google Scholar]
- Koch K. W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988 Jul 7;334(6177):64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Gating properties of the mechano-electrical transducer channel in the dissociated vestibular hair cell of the chick. J Physiol. 1987 Jun;387:589–609. doi: 10.1113/jphysiol.1987.sp016590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol. 1985 Feb;359:189–217. doi: 10.1113/jphysiol.1985.sp015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles J. O., Comis S. D., Osborne M. P. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res. 1984 Aug;15(2):103–112. doi: 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Torre V., Matthews H. R., Lamb T. D. Role of calcium in regulating the cyclic GMP cascade of phototransduction in retinal rods. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7109–7113. doi: 10.1073/pnas.83.18.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]