Abstract
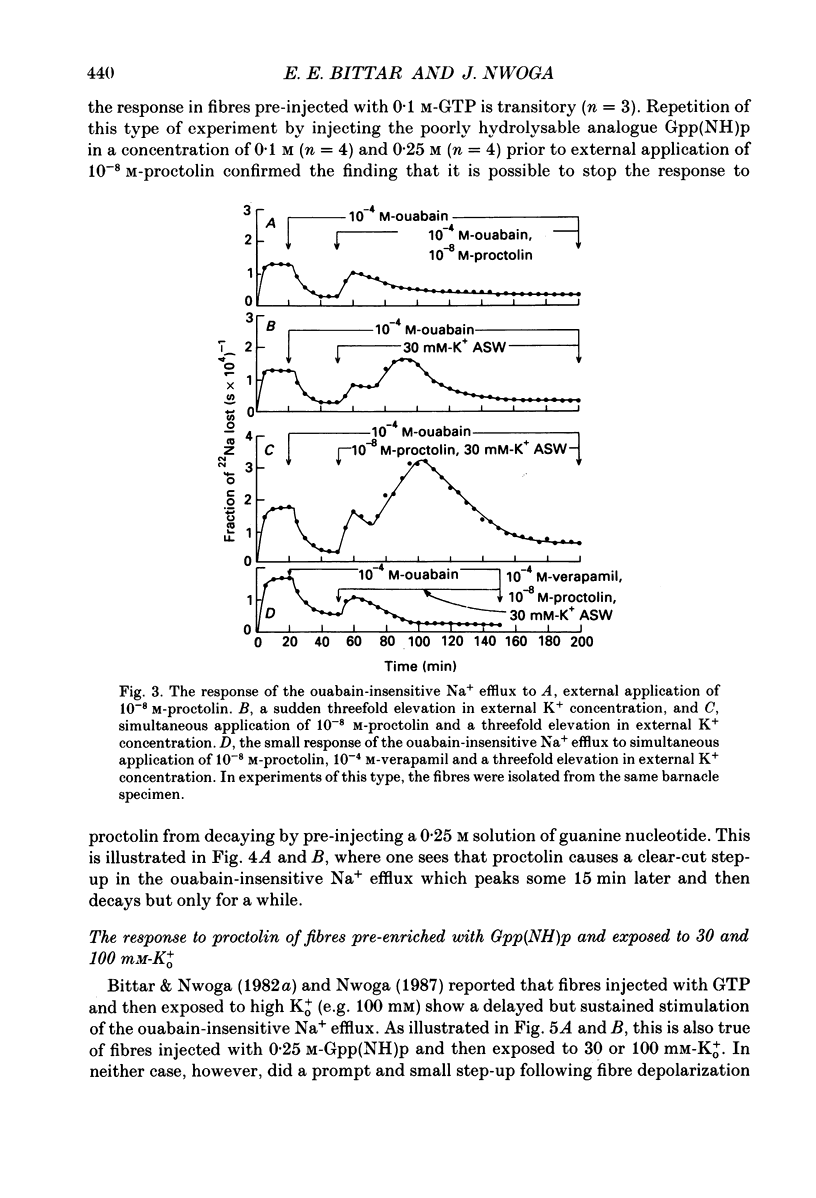
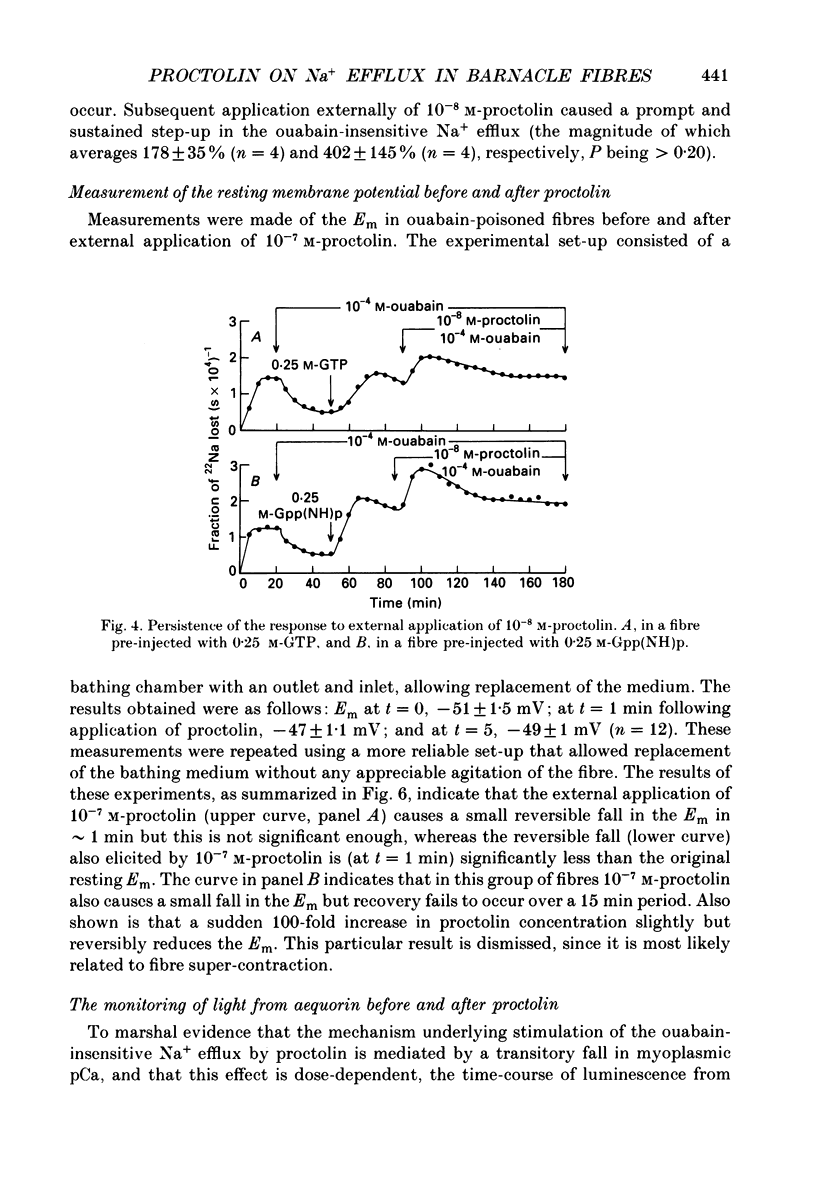
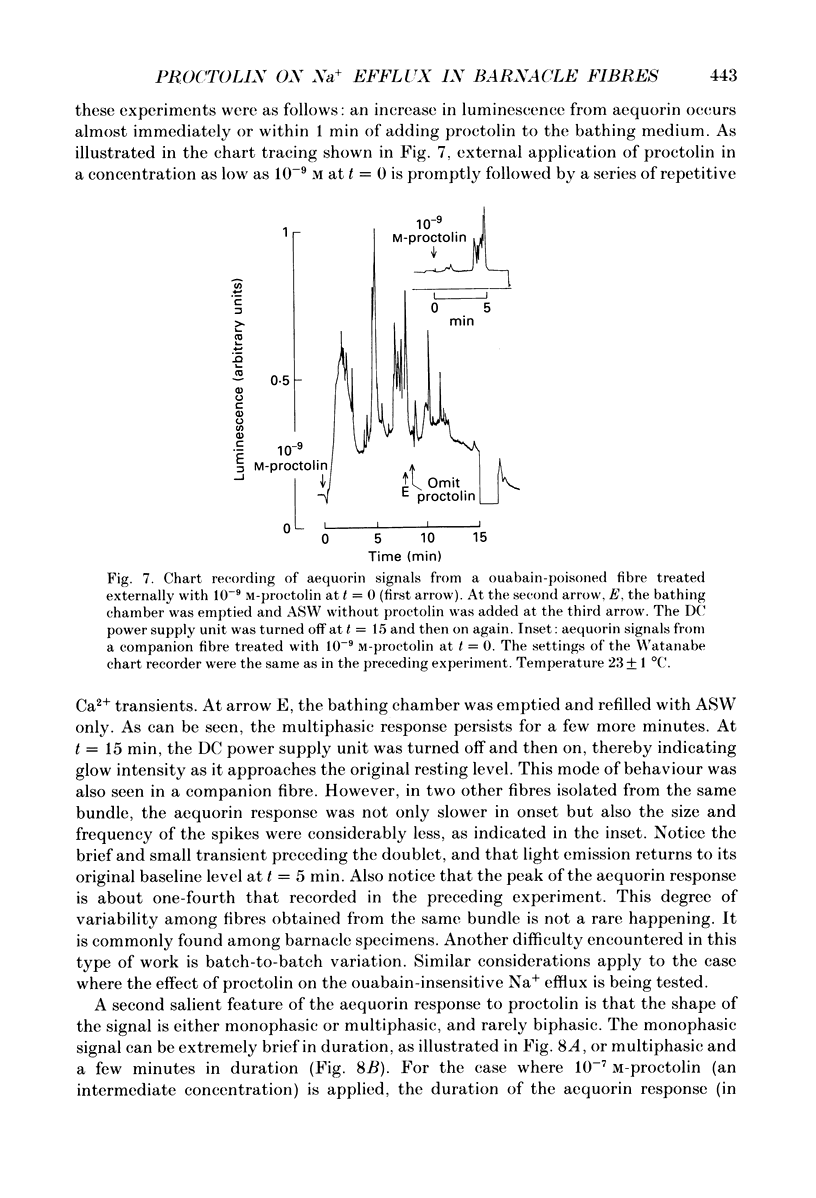
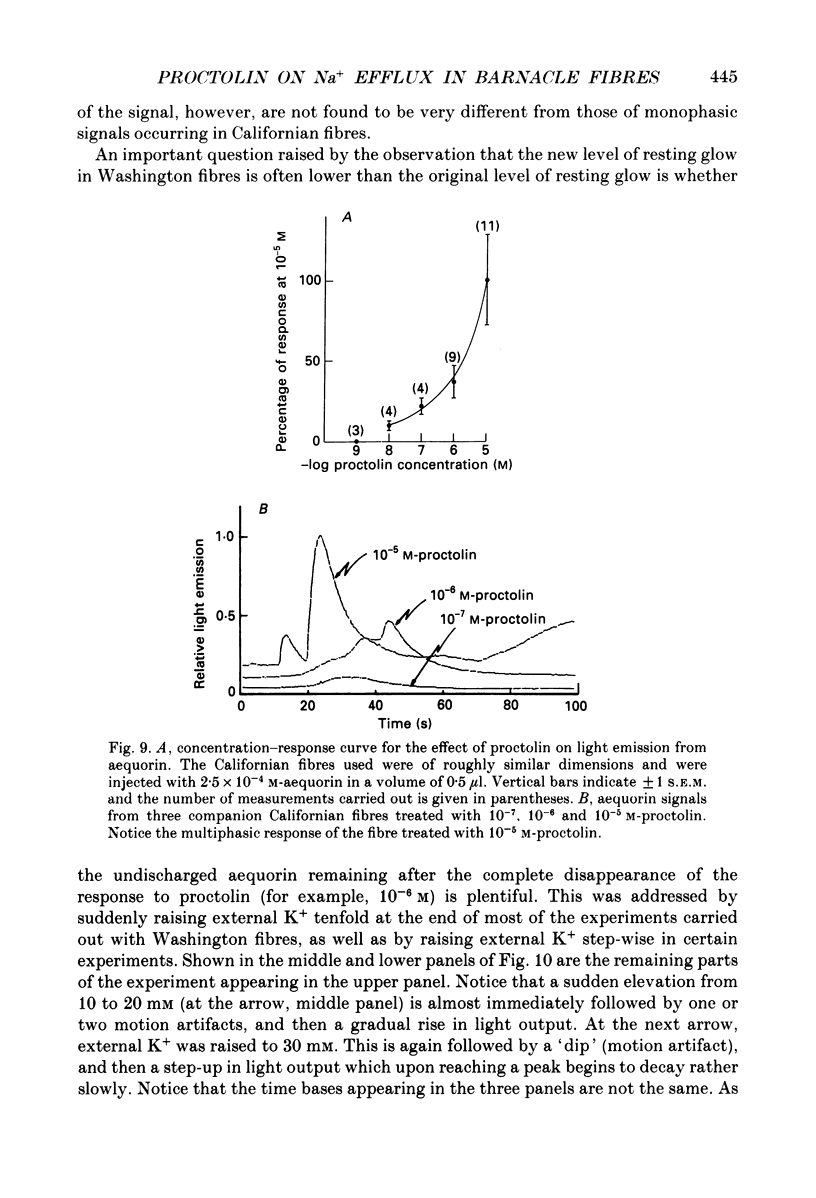
1. A further study has been made of the stimulatory action of proctolin on the ouabain-insensitive Na+ efflux in single muscle fibres from the barnacle, Balanus nubilus. 2. (i) Strontium (Sr2+) behaves as a substitute for external Ca2+. In this case, however, the response to proctolin fails to decay. (ii) Injection of Sr2+ stimulates the ouabain-insensitive Na+ efflux. This effect is mimicked by injecting Ca2+. 3. Depolarization of the fibre membrane with 30 and 100 mM-external K+ augments the response to proctolin. 4. Pre-injection of GTP or Gpp(NH)p (sodium 5-guanylylimidodiphosphate) prevents the response to proctolin from completely decaying. 5. Pre-injection of guanine nucleotides in conjunction with membrane depolarization stops the response to proctolin from decaying. 6. Measurements of Em before and during treatment with proctolin indicate a prompt but small and reversible fall in the membrane potential. 7. (i) The aequorin response of fibres pre-treated with ouabain to proctolin is monophasic or multiphasic, and concentration dependent, the minimal effective concentration being in the nanomolar range. (ii) The duration of these signals is usually less than 5 min; this is about half the time it takes for the stimulated Na+ efflux to reach a maximum. (iii) The aequorin response to proctolin occurs quite often in fibres suspended in nominally Ca2(+)-free artificial sea water. (iv) Sudden graded elevations in external K+ following complete decay of the aequorin response to proctolin are rapidly followed by stepwise transitory increments in light emission. (v) The aequorin response to 100 mM-external K+ is frequently a triplet. 8. (i) Together, these results are in line with the view that the action of proctolin on the ouabain-insensitive Na+ efflux is the result of a temporary fall in internal pCa and that its point of action is the Ca2+ channel, where a putative G protein in the presence of GTP or Gpp(NH)p is able to maintain constancy of the hormonal effect. (ii). They strengthen the argument that the barnacle muscle fibre as a preparation is especially suitable for studies of this kind.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Blinks J. R., Prendergast F. G. Aequorin luminescence: relation of light emission to calcium concentration--a calcium-independent component. Science. 1977 Mar 11;195(4282):996–998. doi: 10.1126/science.841325. [DOI] [PubMed] [Google Scholar]
- Ashley C. C., Ellory J. C. The efflux of magnesium from single crustacean muscle fibres. J Physiol. 1972 Nov;226(3):653–674. doi: 10.1113/jphysiol.1972.sp010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Chambers G. The modulatory action of 5-hydroxytryptamine on sodium efflux: the barnacle muscle fibre as a model system. Comp Biochem Physiol C. 1985;80(2):421–429. doi: 10.1016/0742-8413(85)90079-9. [DOI] [PubMed] [Google Scholar]
- Bittar E. E., Keh T. An investigation of myoplasmic magnesium adenosine triphosphate in barnacle muscle fibres with the firefly method. J Physiol. 1980 May;302:73–88. doi: 10.1113/jphysiol.1980.sp013230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Nwoga J. Some further observations on the stimulation by high external potassium of the sodium efflux in barnacle muscle fibers. Pflugers Arch. 1982 Dec;395(4):318–325. doi: 10.1007/BF00580796. [DOI] [PubMed] [Google Scholar]
- Bittar E. E., Nwoga J. Stimulation by injected guanosine triphosphate of the sodium efflux in barnacle muscle fibres. J Physiol. 1982 Jan;322:389–397. doi: 10.1113/jphysiol.1982.sp014043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Tallitsch R. B. Stimulation by aldosterone of the sodium efflux in barnacle muscle fibres: effects of RNA inhibitors and spironolactone. J Physiol. 1975 Sep;250(2):331–346. doi: 10.1113/jphysiol.1975.sp011057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Birnbaumer L. Direct G protein gating of ion channels. Am J Physiol. 1988 Mar;254(3 Pt 2):H401–H410. doi: 10.1152/ajpheart.1988.254.3.H401. [DOI] [PubMed] [Google Scholar]
- Brown B. E. Proctolin: a peptide transmitter candidate in insects. Life Sci. 1975 Oct 15;17(8):1241–1252. doi: 10.1016/0024-3205(75)90133-2. [DOI] [PubMed] [Google Scholar]
- CALDWELL P. C., WALSTER G. STUDIES ON THE MICRO-INJECTION OF VARIOUS SUBSTANCES INTO CRAB MUSCLE FIBRES. J Physiol. 1963 Nov;169:353–372. doi: 10.1113/jphysiol.1963.sp007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. P., Leung A. T., Sharp A. H. The biochemistry and molecular biology of the dihydropyridine-sensitive calcium channel. Trends Neurosci. 1988 Oct;11(10):425–430. doi: 10.1016/0166-2236(88)90193-2. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Allen D. G. Model of calcium movements during activation in the sarcomere of frog skeletal muscle. Biophys J. 1984 May;45(5):913–925. doi: 10.1016/S0006-3495(84)84238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P., Soldati L., Carafoli E. The Na+/Ca2+ exchanger of heart sarcolemma is regulated by a phosphorylation-dephosphorylation process. Soc Gen Physiol Ser. 1984;38:149–160. [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Characterization of the reverse Na/Ca exchange in squid axons and its modulation by Cai and ATP. Cai-dependent Nai/Cao and Nai/Nao exchange modes. J Gen Physiol. 1987 Oct;90(4):505–525. doi: 10.1085/jgp.90.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson S. K., Goldberg N. D., Walseth T. F., Huetteman D. A. Voltage dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in peeled skeletal muscle fibers. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5749–5753. doi: 10.1073/pnas.85.15.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill M., Coronado R. Ryanodine receptor channel of sarcoplasmic reticulum. Trends Neurosci. 1988 Oct;11(10):453–457. doi: 10.1016/0166-2236(88)90198-1. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol. 1956 Mar 28;131(3):592–616. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Fukuda J., Eaton D. C. Membrane currents carried by Ca, Sr, and Ba in barnacle muscle fiber during voltage clamp. J Gen Physiol. 1974 May;63(5):564–578. doi: 10.1085/jgp.63.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto N., Antoniu B., Kim D. H. Rapid calcium release from the isolated sarcoplasmic reticulum is triggered via the attached transverse tubular system. J Biol Chem. 1984 Nov 10;259(21):13151–13158. [PubMed] [Google Scholar]
- Lea T. J., Griffiths P. J., Tregear R. T., Ashley C. C. An examination of the ability of inositol 1,4,5-trisphosphate to induce calcium release and tension development in skinned skeletal muscle fibres of frog and crustacea. FEBS Lett. 1986 Oct 20;207(1):153–161. doi: 10.1016/0014-5793(86)80031-x. [DOI] [PubMed] [Google Scholar]
- Mason-Sharp D., Bittar E. E. Stimulation by high external potassium of the sodium efflux in barnacle muscle fibers. J Membr Biol. 1981 Feb 28;58(3):213–226. doi: 10.1007/BF01870907. [DOI] [PubMed] [Google Scholar]
- Mattera R., Graziano M. P., Yatani A., Zhou Z., Graf R., Codina J., Birnbaumer L., Gilman A. G., Brown A. M. Splice variants of the alpha subunit of the G protein Gs activate both adenylyl cyclase and calcium channels. Science. 1989 Feb 10;243(4892):804–807. doi: 10.1126/science.2536957. [DOI] [PubMed] [Google Scholar]
- Nwoga J., Bittar E. E. Stimulation by proctolin of the ouabain-insensitive sodium efflux in single barnacle muscle fibers. Comp Biochem Physiol C. 1985;81(2):345–350. doi: 10.1016/0742-8413(85)90018-0. [DOI] [PubMed] [Google Scholar]
- Nwoga J. Concerning stimulation by high external potassium and calcium injection of the ouabain-insensitive sodium efflux in barnacle muscle fibers. Comp Biochem Physiol A Comp Physiol. 1987;86(4):733–737. doi: 10.1016/0300-9629(87)90633-5. [DOI] [PubMed] [Google Scholar]
- O'Callahan C. M., Hosey M. M. Multiple phosphorylation sites in the 165-kilodalton peptide associated with dihydropyridine-sensitive calcium channels. Biochemistry. 1988 Aug 9;27(16):6071–6077. doi: 10.1021/bi00416a036. [DOI] [PubMed] [Google Scholar]
- Rasgado-Flores H., Blaustein M. P. Na/Ca exchange in barnacle muscle cells has a stoichiometry of 3 Na+/1 Ca2+. Am J Physiol. 1987 May;252(5 Pt 1):C499–C504. doi: 10.1152/ajpcell.1987.252.5.C499. [DOI] [PubMed] [Google Scholar]
- Rojas E., Nassar-Gentina V., Luxoro M., Pollard M. E., Carrasco M. A. Inositol 1,4,5-trisphosphate-induced Ca2+ release from the sarcoplasmic reticulum and contraction in crustacean muscle. Can J Physiol Pharmacol. 1987 Apr;65(4):672–680. doi: 10.1139/y87-111. [DOI] [PubMed] [Google Scholar]
- Schulman H., Lou L. L. Multifunctional Ca2+/calmodulin-dependent protein kinase: domain structure and regulation. Trends Biochem Sci. 1989 Feb;14(2):62–66. doi: 10.1016/0968-0004(89)90045-5. [DOI] [PubMed] [Google Scholar]
- Schwarz T. L., Harris-Warrick R. M., Glusman S., Kravitz E. A. A peptide action in a lobster neuromuscular preparation. J Neurobiol. 1980 Nov;11(6):623–628. doi: 10.1002/neu.480110611. [DOI] [PubMed] [Google Scholar]
- Stewart A. A., Ingebritsen T. S., Manalan A., Klee C. B., Cohen P. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80). FEBS Lett. 1982 Jan 11;137(1):80–84. doi: 10.1016/0014-5793(82)80319-0. [DOI] [PubMed] [Google Scholar]
- Vergara J., Tsien R. Y., Delay M. Inositol 1,4,5-trisphosphate: a possible chemical link in excitation-contraction coupling in muscle. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6352–6356. doi: 10.1073/pnas.82.18.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. H., 3rd, Hoshi T. Proctolin induces rhythmic contractions and spikes in Limulus heart muscle. Am J Physiol. 1985 Oct;249(4 Pt 2):R490–R495. doi: 10.1152/ajpregu.1985.249.4.R490. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987 Feb;8(1):79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Yoshioka T., Somlyo A. P. Calcium and magnesium contents and volume of the terminal cisternae in caffeine-treated skeletal muscle. J Cell Biol. 1984 Aug;99(2):558–568. doi: 10.1083/jcb.99.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]


