Abstract
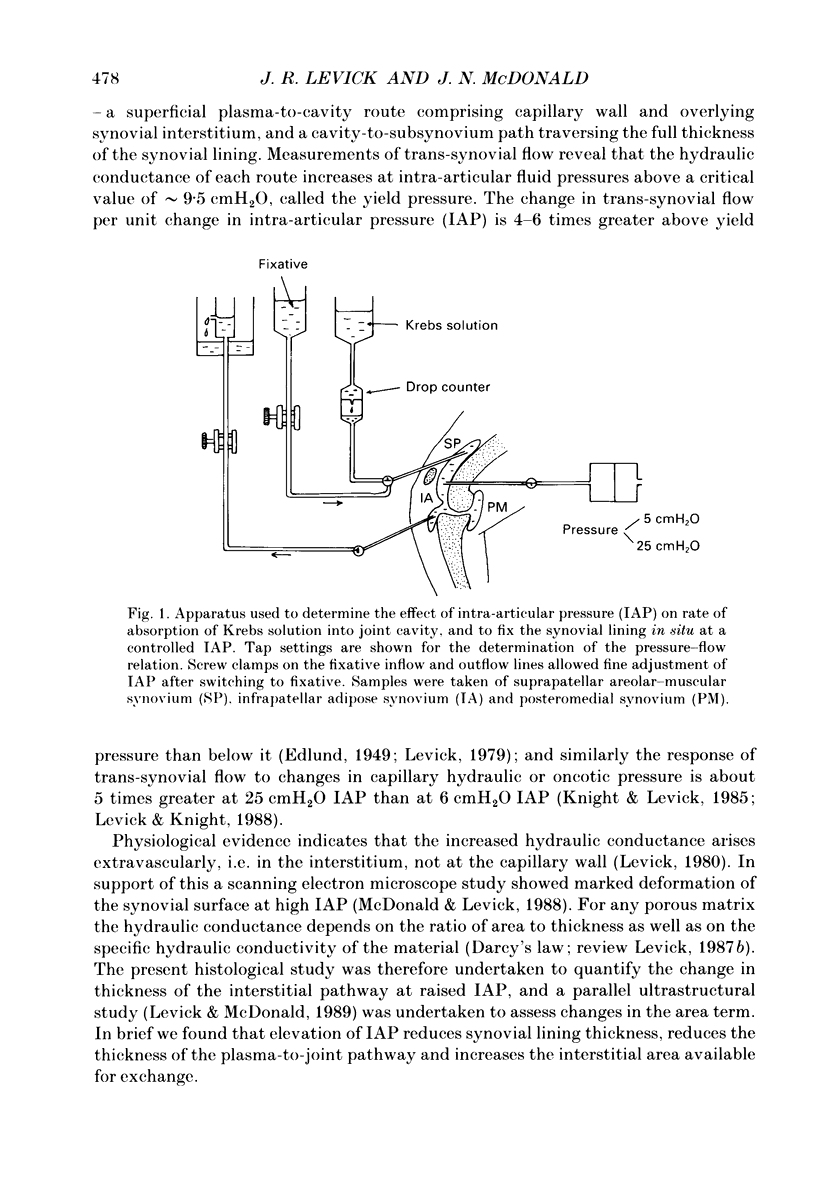
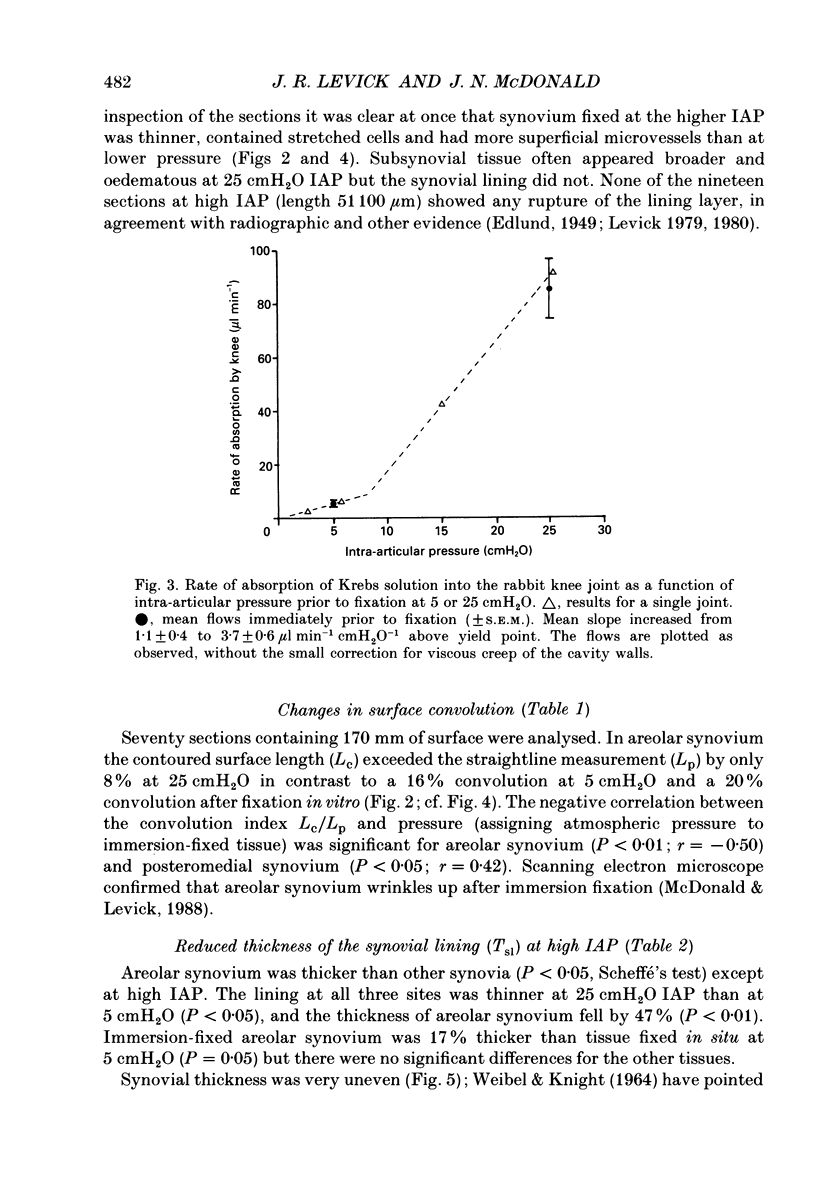
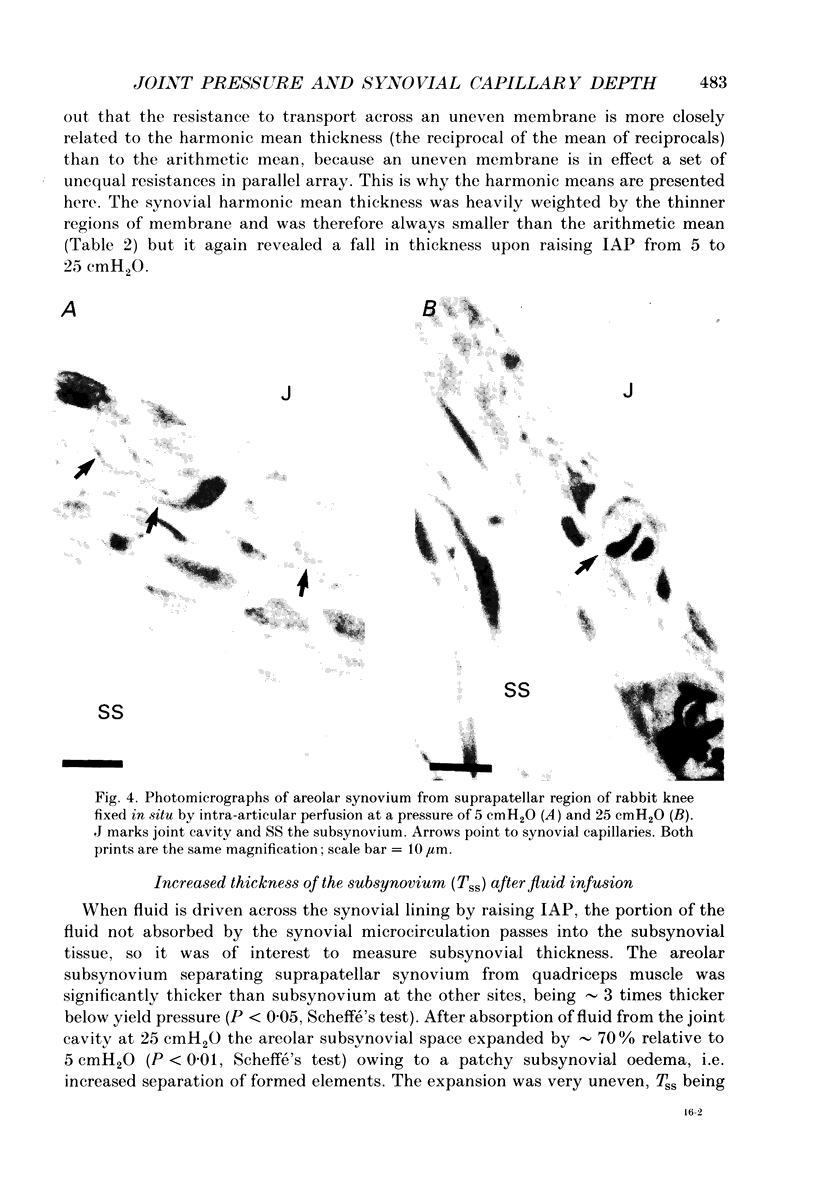
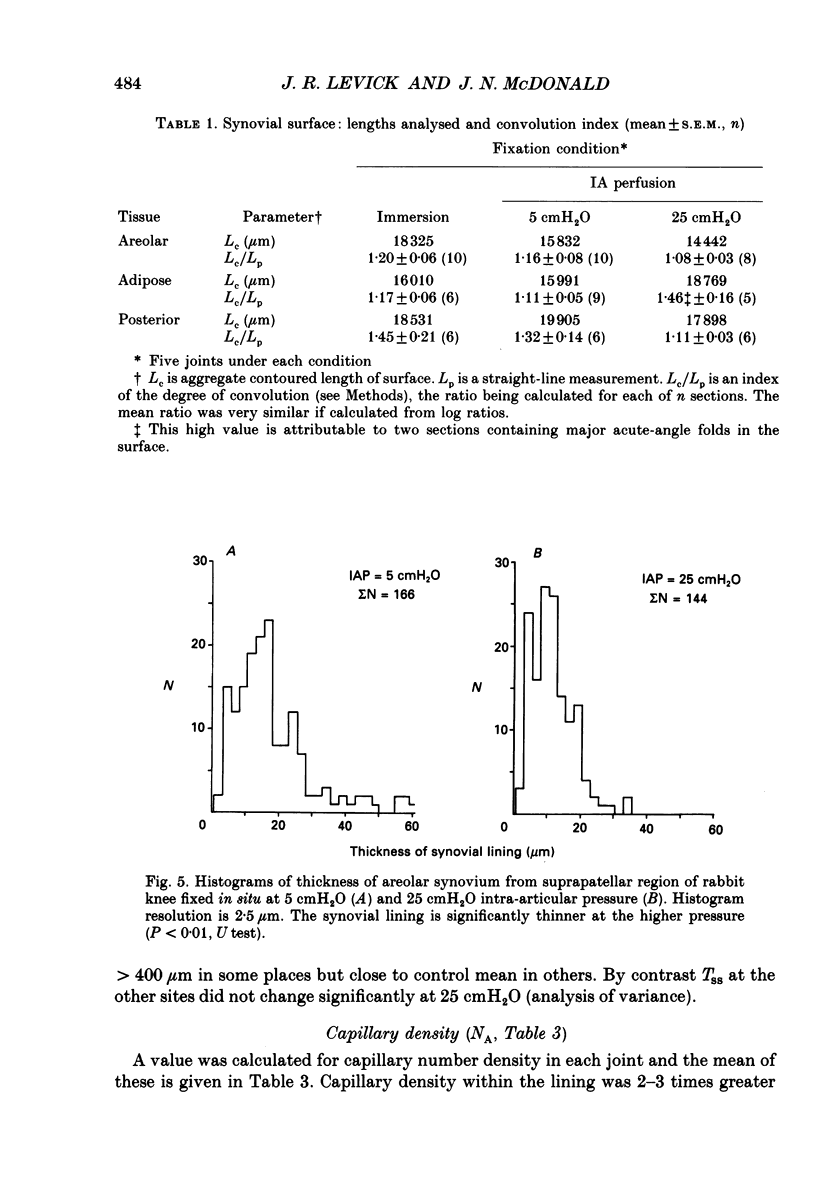
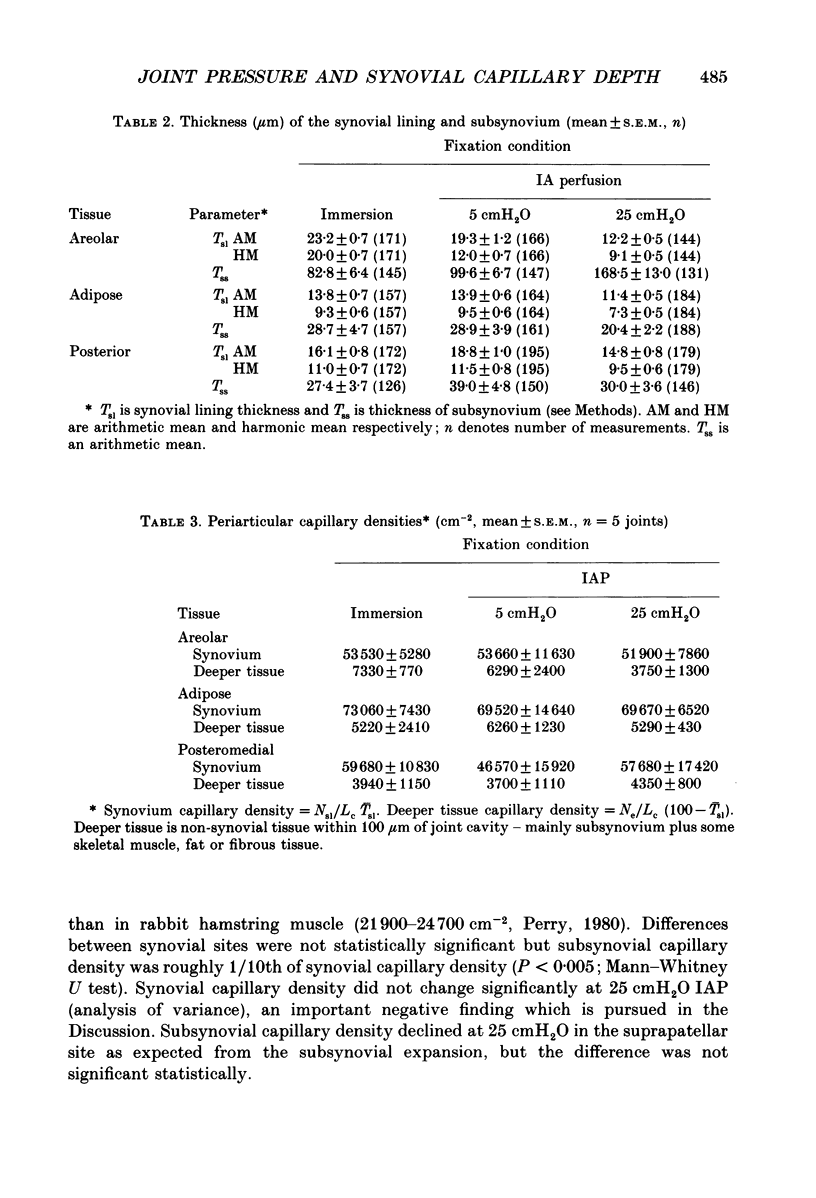
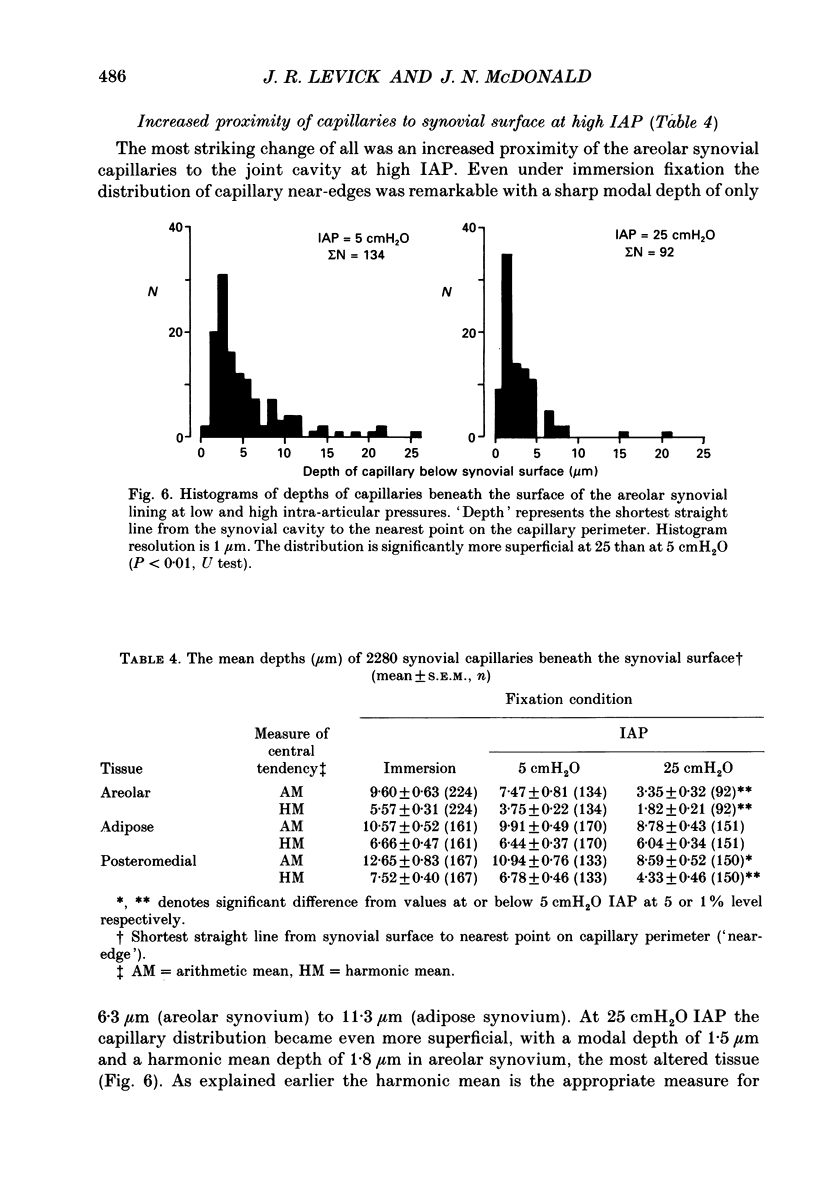
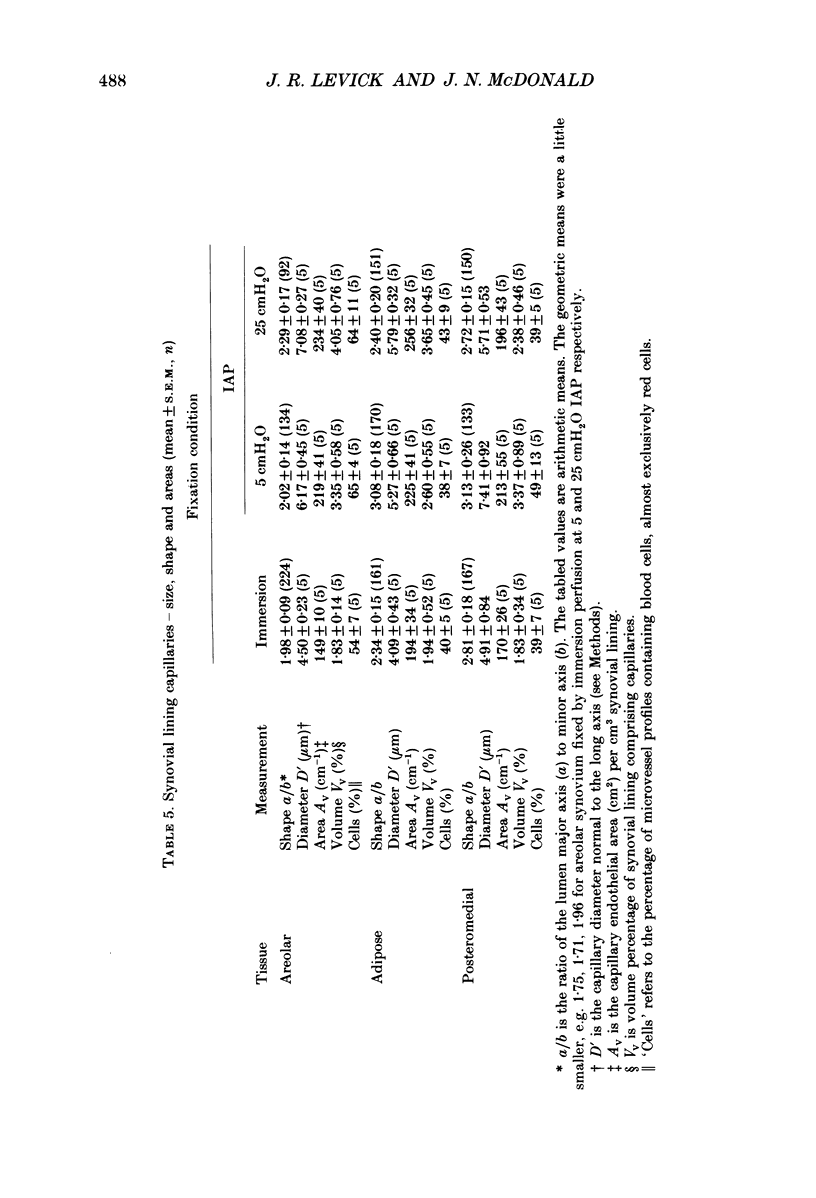
1. The hydraulic conductance of the synovial lining of the rabbit knee increases greatly at intra-articular pressures (IAP) above 9 cmH2O. A structural cause was sought by fixing synovium in situ at less than or equal to 5 cmH2O IAP (ten animals) or 25 cmH2O IAP (five animals) and examining histological sections morphometrically. 2. The synovial lining was found to be a highly deformable sheet of very vascular connective tissue, with 47 x 10(3)-73 X 10(3) capillaries per cm2 section. 150-260 cm2 endothelial surface per cm3 tissue and a vascular volume of 2.4-5.7%. 3. The thickness of the lining averaged 14-19 microns at low IAP and was reduced at high IAP; in suprapatellar synovium, where changes were most marked, thickness fell by 24-47%. The loose subsynovial space expanded. 4. The average distance separating capillary near-edges from the joint cavity approximately halved from 3.75 and 7.47 microns at low IAP (harmonic and arithmetical means respectively) to 1.82 and 3.35 microns at high IAP. Capillaries remained patent and their number density did not change significantly at high IAP. 5. It is concluded that a reduction in the extravascular path length for fluid exchange contributes to the increase synovial conductance at high IAP, but the path length changes were not sufficient to account fully for the conductance changes.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bone Q., Denton E. J. The osmotic effects of electron microscope fixatives. J Cell Biol. 1971 Jun;49(3):571–581. doi: 10.1083/jcb.49.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson E. C. Topographical specificity in isolated retinal capillary basement membranes: a high-resolution scanning electron microscope analysis. Microvasc Res. 1988 Mar;35(2):221–235. doi: 10.1016/0026-2862(88)90064-7. [DOI] [PubMed] [Google Scholar]
- Elias H., Hennig A., Schwartz D. E. Stereology: applications to biomedicalresearch. Physiol Rev. 1971 Jan;51(1):158–200. doi: 10.1152/physrev.1971.51.1.158. [DOI] [PubMed] [Google Scholar]
- Jilani M., Ghadially F. N. An ultrastructural study of age-associated changes in the rabbit synovial membrane. J Anat. 1986 Jun;146:201–215. [PMC free article] [PubMed] [Google Scholar]
- Knight A. D., Levick J. R. Effect of fluid pressure on the hydraulic conductance of interstitium and fenestrated endothelium in the rabbit knee. J Physiol. 1985 Mar;360:311–332. doi: 10.1113/jphysiol.1985.sp015619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A. D., Levick J. R. Physiological compartmentation of fluid within the synovial cavity of the rabbit knee. J Physiol. 1982 Oct;331:1–15. doi: 10.1113/jphysiol.1982.sp014361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A. D., Levick J. R. Pressure-volume relationships above and below atmospheric pressure in the synovial cavity of the rabbit knee. J Physiol. 1982 Jul;328:403–420. doi: 10.1113/jphysiol.1982.sp014273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A. D., Levick J. R. The density and distribution of capillaries around a synovial cavity. Q J Exp Physiol. 1983 Oct;68(4):629–644. doi: 10.1113/expphysiol.1983.sp002753. [DOI] [PubMed] [Google Scholar]
- Knox P., Levick J. R., McDonald J. N. Synovial fluid--its mass, macromolecular content and pressure in major limb joints of the rabbit. Q J Exp Physiol. 1988 Jan;73(1):33–45. doi: 10.1113/expphysiol.1988.sp003121. [DOI] [PubMed] [Google Scholar]
- Levick J. R. Contributions of the lymphatic and microvascular systems to fluid absorption from the synovial cavity of the rabbit knee. J Physiol. 1980 Sep;306:445–461. doi: 10.1113/jphysiol.1980.sp013406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J. R. Flow through interstitium and other fibrous matrices. Q J Exp Physiol. 1987 Oct;72(4):409–437. doi: 10.1113/expphysiol.1987.sp003085. [DOI] [PubMed] [Google Scholar]
- Levick J. R., Knight A. D. Interaction of plasma colloid osmotic pressure and joint fluid pressure across the endothelium-synovium layer: significance of extravascular resistance. Microvasc Res. 1988 Jan;35(1):109–121. doi: 10.1016/0026-2862(88)90054-4. [DOI] [PubMed] [Google Scholar]
- Levick J. R., McDonald J. N. Ultrastructure of transport pathways in stressed synovium of the knee in anaesthetized rabbits. J Physiol. 1989 Dec;419:493–508. doi: 10.1113/jphysiol.1989.sp017882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J. R., Michel C. C. A densitometric method for determining the filtration coefficients of single capillaries in the frog mesentery. Microvasc Res. 1977 Mar;13(2):141–151. doi: 10.1016/0026-2862(77)90081-4. [DOI] [PubMed] [Google Scholar]
- Levick J. R. The influence of hydrostatic pressure on trans-synovial fluid movement and on capsular expansion in the rabbit knee. J Physiol. 1979 Apr;289:69–82. doi: 10.1113/jphysiol.1979.sp012725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. N., Levick J. R. Morphology of surface synoviocytes in situ at normal and raised joint pressure, studied by scanning electron microscopy. Ann Rheum Dis. 1988 Mar;47(3):232–240. doi: 10.1136/ard.47.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry M. A. Capillary filtration and permeability coefficients calculated from measurements of interendothelial cell junctions in rabbit lung and skeletal muscle. Microvasc Res. 1980 Mar;19(2):142–157. doi: 10.1016/0026-2862(80)90036-9. [DOI] [PubMed] [Google Scholar]
- SHAW N. E., MARTIN B. F. Histological and histochemical studies on mammalian knee-joint tissues. J Anat. 1962 Jul;96:359–373. [PMC free article] [PubMed] [Google Scholar]
- Smaje L. H., Fraser P. A., Clough G. The distensibility of single capillaries and venules in the cat mesentery. Microvasc Res. 1980 Nov;20(3):358–370. doi: 10.1016/0026-2862(80)90064-3. [DOI] [PubMed] [Google Scholar]
- Tedgui A., Lever M. J. Filtration through damaged and undamaged rabbit thoracic aorta. Am J Physiol. 1984 Nov;247(5 Pt 2):H784–H791. doi: 10.1152/ajpheart.1984.247.5.H784. [DOI] [PubMed] [Google Scholar]
- WEIBEL E. R., KNIGHT B. W. A MORPHOMETRIC STUDY ON THE THICKNESS OF THE PULMONARY AIR-BLOOD BARRIER. J Cell Biol. 1964 Jun;21:367–396. doi: 10.1083/jcb.21.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]




