Abstract
1. The hydraulic conductance of the synovial lining of a rabbit knee increases greatly when intra-articular pressure (IAP) is raised above approximately 9 cmH2O (yield point). To investigate the cause, synovium was fixed in situ by perfusion at controlled IAP and prepared for transmission electron microscopy. Micrographs of synovium fixed below yield pressure (atmospheric pressure and 5 cmH2O IAP, ten joints) and above it (25 cmH2O IAP, five joints) were analysed by morphometry. 2. The discontinuous cellular lining consisted of fibroblast-like cells (67%) and macrophage-like cells (33%) separated by interstitium-filled gaps. Interstitium formed 26-36% of the surface below yield pressure. Depending on sample site the surface gaps averaged 1.9 +/- 0.2 to 2.4 +/- 0.2 microns wide below yield pressure (mean +/- S.E.M. throughout). Above yield pressure the mean gap width increased by 42-64% (P less than 0.05, analysis of variance). 3. The qualitative and quantitative composition of the lining varied with distance below the surface. In a plane 5 microns deep, the intercellular distances and interstitial area fraction were almost double those at the surface. Classic periodic collagen fibrils (diameter 50 +/- 3 nm) abounded at 5 microns depth whereas the surface interstitium was richer in Ruthenium Red-staining microfibrils (diameter 9.3 +/- 0.7 nm) associated with 93 nm period fibrous long-spacing bundles. 4. Averaging over all the tissue between the surface and the 5 microns deep plane, the mean interstitial volume fraction was 0.61 +/- 0.05 at 5 cmH2O and 0.67 +/- 0.02 at 25 cmH2O (n.s.). 5. Capillary fenestrae (8.5 +/- 1.1 per fenestrated profile) and intercellular junctions were unaltered at high IAP. The tortuosity of the capillary-to-joint cavity path was 1.50 +/- 0.01 below yield pressure and 1.86 +/- 0.24 at 25 cmH2O (n.s.). 6. Intra-articular tracers (ferrocyanide, ferritin and glycogen) permeated synovial interstitium without evidence of preferential pathways. Ferrocyanide delineated the capillary intercellular junction as a permeable channel. Ferritin and glycogen were phagocytosed by the macrophages. 7. In suprapatellar areolar synovium, the most extensive and most altered tissue, the ratio of interstitial area to path length increased maximally 4.1 times between 5 and 25 cmH2O IAP. This represents a substantial contribution to the physiologically estimated rise in interstitial conductance (14 x) but does not wholly explain it.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLAND P., NOVIKOFF A. B., HAMERMAN D. Electron microscopy of the human synovial membrane. J Cell Biol. 1962 Aug;14:207–220. doi: 10.1083/jcb.14.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning J. Demarcation of tissue channels by ferrocyanide deposits: use of an alternative precipitant. Microvasc Res. 1980 May;19(3):380–384. doi: 10.1016/0026-2862(80)90057-6. [DOI] [PubMed] [Google Scholar]
- CASTOR C. W. The microscopic structure of normal human synovial tissue. Arthritis Rheum. 1960 Apr;3:140–151. doi: 10.1002/art.1780030205. [DOI] [PubMed] [Google Scholar]
- Casley-Smith J. R. An electron microscopical study of the passage of ions through the endothelium of lymphatic and blood capillaries, and through the mesothelium. Q J Exp Physiol Cogn Med Sci. 1967 Apr;52(2):105–113. doi: 10.1113/expphysiol.1967.sp001892. [DOI] [PubMed] [Google Scholar]
- Casley-Smith J. R., Vincent A. H. The quantitative morphology of interstitial tissue channels in some tissues of the rat and rabbit. Tissue Cell. 1978;10(3):571–584. doi: 10.1016/s0040-8166(16)30350-0. [DOI] [PubMed] [Google Scholar]
- Fisher R. F. The water permeability of basement membrane under increasing pressure: evidence for a new theory of permeability. Proc R Soc Lond B Biol Sci. 1982 Nov 22;216(1205):475–496. doi: 10.1098/rspb.1982.0087. [DOI] [PubMed] [Google Scholar]
- Ghadially F. N., Roy S. Ultrastructure of rabbit synovial membrane. Ann Rheum Dis. 1966 Jul;25(4):318–326. doi: 10.1136/ard.25.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graabaek P. M. Ultrastructural evidence for two distinct types of synoviocytes in rat synovial membrane. J Ultrastruct Res. 1982 Mar;78(3):321–339. doi: 10.1016/s0022-5320(82)80006-3. [DOI] [PubMed] [Google Scholar]
- Henderson B., Pettipher E. R. The synovial lining cell: biology and pathobiology. Semin Arthritis Rheum. 1985 Aug;15(1):1–32. doi: 10.1016/0049-0172(85)90007-1. [DOI] [PubMed] [Google Scholar]
- Jilani M., Ghadially F. N. An ultrastructural study of age-associated changes in the rabbit synovial membrane. J Anat. 1986 Jun;146:201–215. [PMC free article] [PubMed] [Google Scholar]
- Knight A. D., Levick J. R. Effect of fluid pressure on the hydraulic conductance of interstitium and fenestrated endothelium in the rabbit knee. J Physiol. 1985 Mar;360:311–332. doi: 10.1113/jphysiol.1985.sp015619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A. D., Levick J. R. Morphometry of the ultrastructure of the blood-joint barrier in the rabbit knee. Q J Exp Physiol. 1984 Apr;69(2):271–288. doi: 10.1113/expphysiol.1984.sp002805. [DOI] [PubMed] [Google Scholar]
- Krey P. R., Cohen A. S. Fine structural analysis of rabbit synovial cells. I. The normal synovium and changes in organ culture. Arthritis Rheum. 1973 May-Jun;16(3):324–340. doi: 10.1002/art.1780160306. [DOI] [PubMed] [Google Scholar]
- Levick J. R. Contributions of the lymphatic and microvascular systems to fluid absorption from the synovial cavity of the rabbit knee. J Physiol. 1980 Sep;306:445–461. doi: 10.1113/jphysiol.1980.sp013406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J. R. Flow through interstitium and other fibrous matrices. Q J Exp Physiol. 1987 Oct;72(4):409–437. doi: 10.1113/expphysiol.1987.sp003085. [DOI] [PubMed] [Google Scholar]
- Levick J. R., Knight A. D. Interaction of plasma colloid osmotic pressure and joint fluid pressure across the endothelium-synovium layer: significance of extravascular resistance. Microvasc Res. 1988 Jan;35(1):109–121. doi: 10.1016/0026-2862(88)90054-4. [DOI] [PubMed] [Google Scholar]
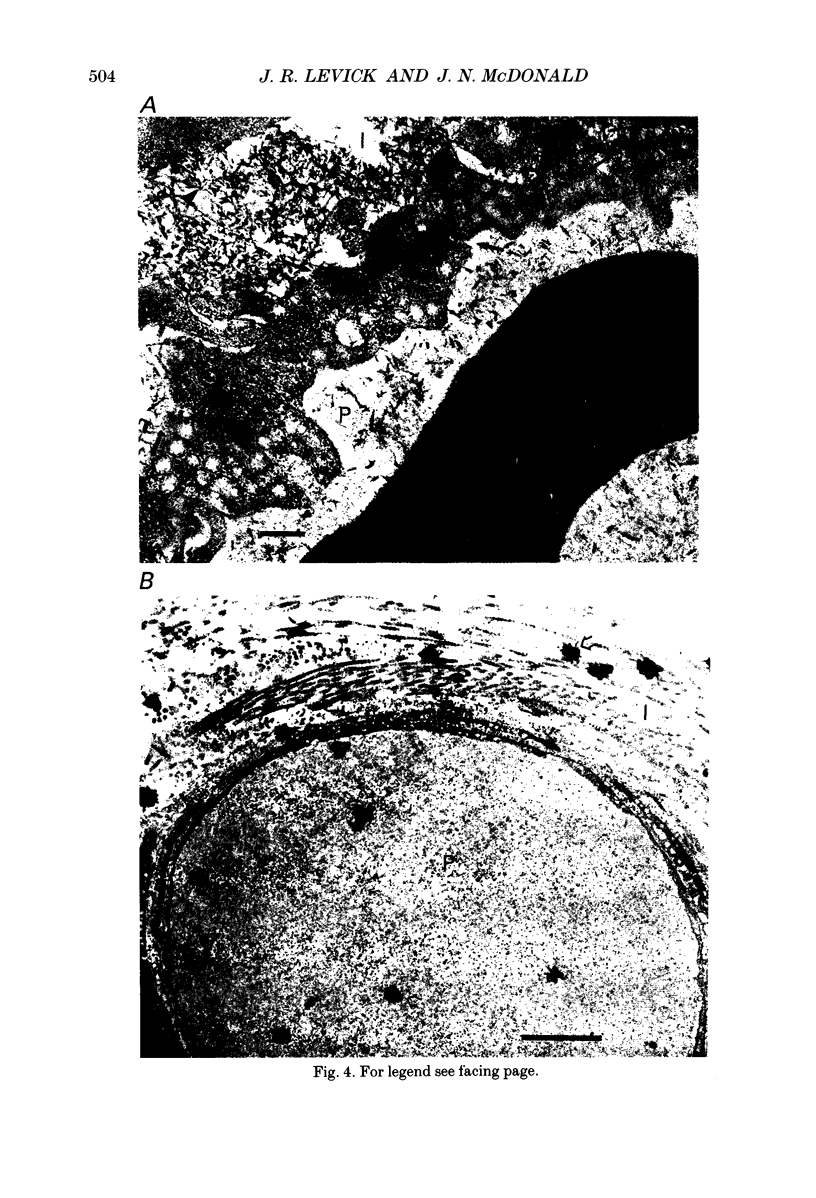
- Levick J. R., McDonald J. N. Synovial capillary distribution in relation to altered pressure and permeability in knees of anaesthetized rabbits. J Physiol. 1989 Dec;419:477–492. doi: 10.1113/jphysiol.1989.sp017881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J. R., Smaje L. H. An analysis of the permeability of a fenestra. Microvasc Res. 1987 Mar;33(2):233–256. doi: 10.1016/0026-2862(87)90020-3. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- Mapp P. I., Revell P. A. Ultrastructural localisation of muramidase in the human synovial membrane. Ann Rheum Dis. 1987 Jan;46(1):30–37. doi: 10.1136/ard.46.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A., Bannon C. Measurement of swelling pressure in cartilage and comparison with the osmotic pressure of constituent proteoglycans. Biorheology. 1981;18(3-6):619–632. doi: 10.3233/bir-1981-183-624. [DOI] [PubMed] [Google Scholar]
- McDonald J. N., Levick J. R. Morphology of surface synoviocytes in situ at normal and raised joint pressure, studied by scanning electron microscopy. Ann Rheum Dis. 1988 Mar;47(3):232–240. doi: 10.1136/ard.47.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers D. B. Electron microscopic autoradiography of 35SO4-labelled material closely associated with collagen fibrils in mammalian synovium and ear cartilage. Histochem J. 1976 Mar;8(2):191–199. doi: 10.1007/BF01007168. [DOI] [PubMed] [Google Scholar]
- Okada Y., Nakanishi I., Kajikawa K. Ultrastructure of the mouse synovial membrane. Development and organization of the extracellular matrix. Arthritis Rheum. 1981 Jun;24(6):835–843. doi: 10.1002/art.1780240611. [DOI] [PubMed] [Google Scholar]
- SUTER E. R., MAJNO G. ULTRASTRUCTURE OF THE JOINT CAPSULE IN THE RAT: PRESENCE OF TWO KINDS OF CAPILLARIES. Nature. 1964 May 30;202:920–921. doi: 10.1038/202920b0. [DOI] [PubMed] [Google Scholar]





