Abstract
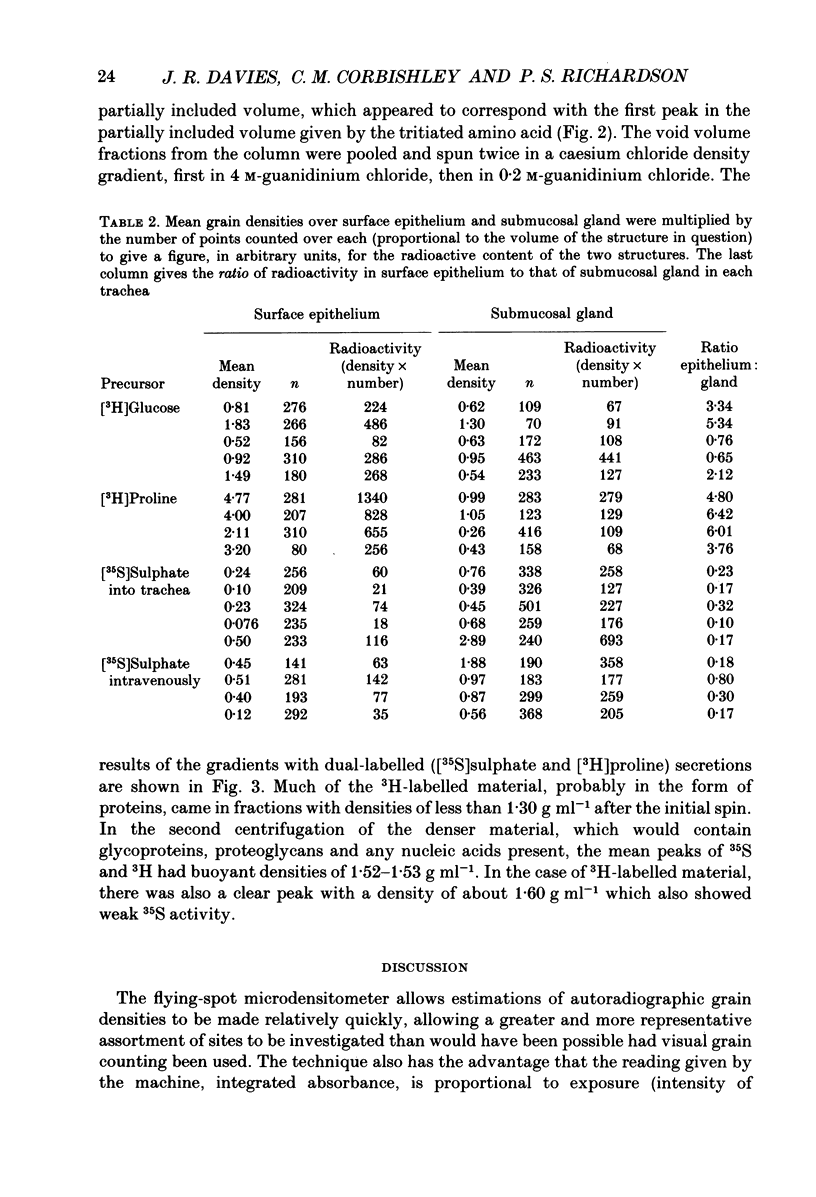
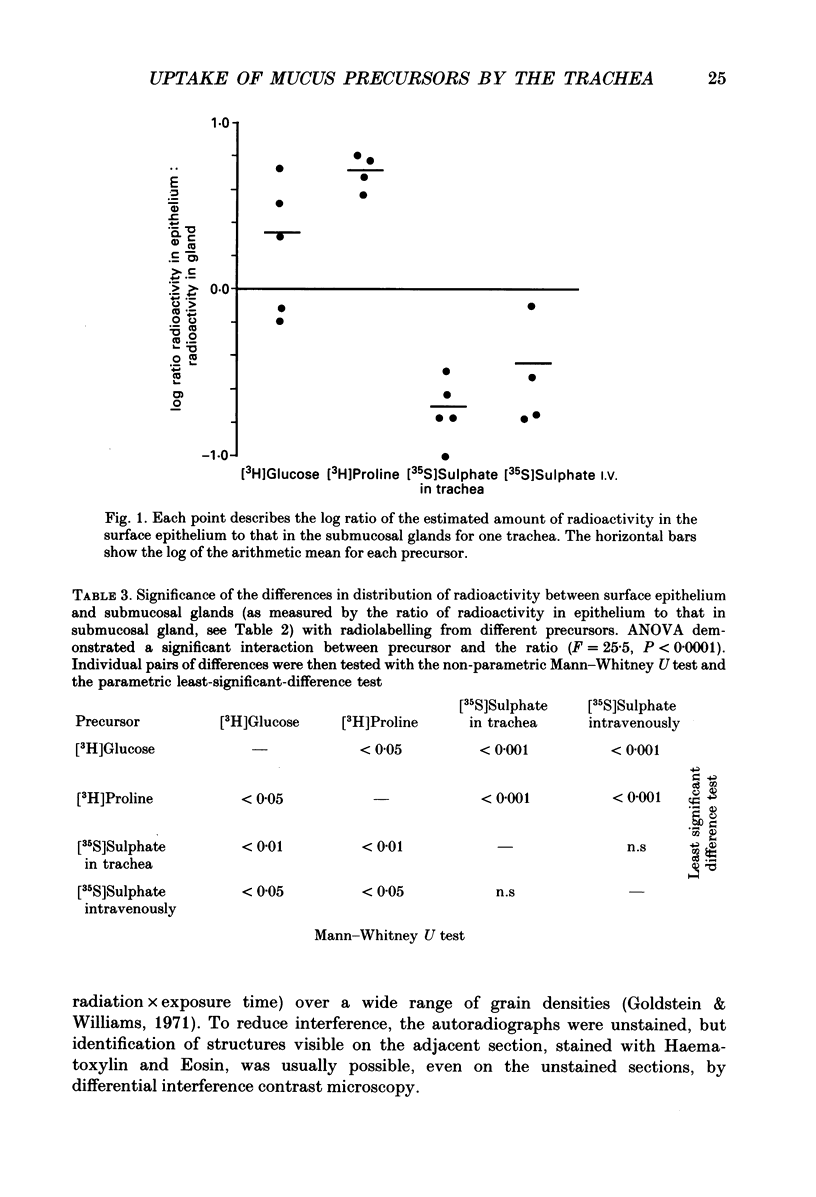
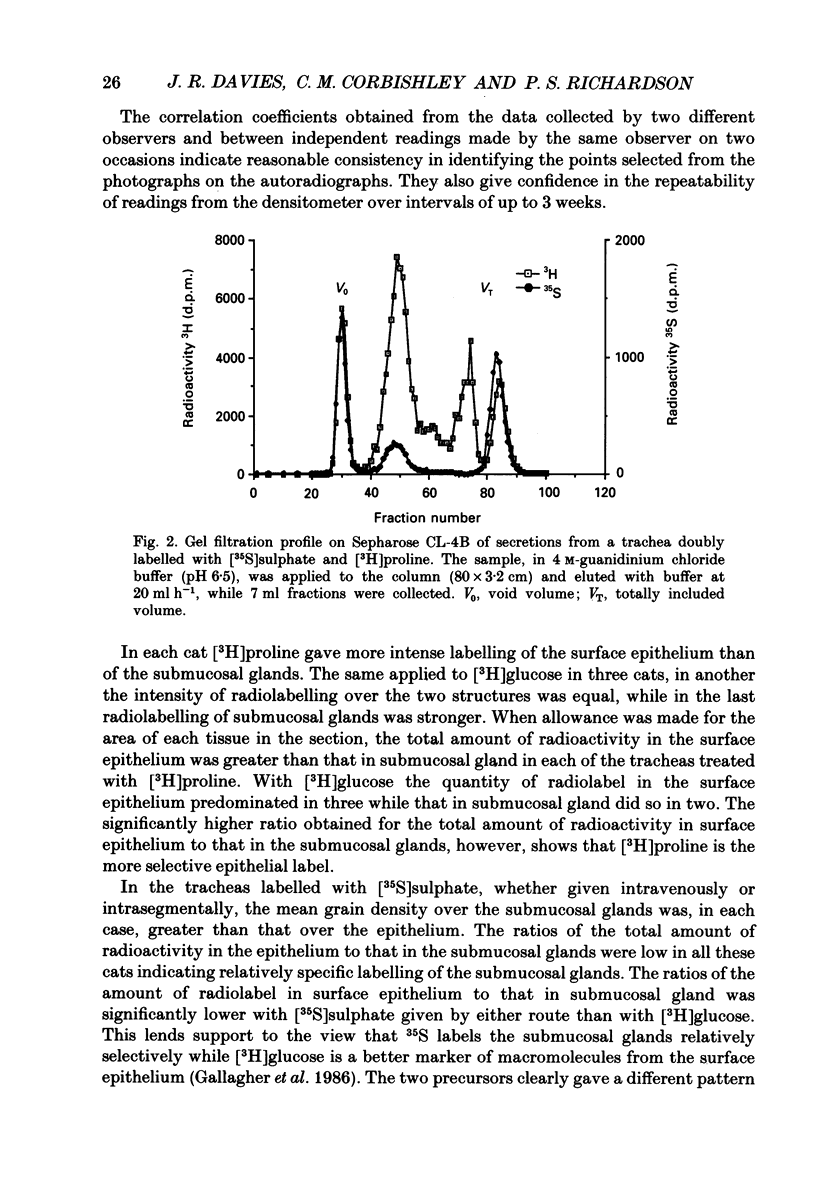
1. We gave one of three radiolabelled precursors of mucus glycoconjugates ([3H]proline, [3H]glucose and [35S]sulphate) into the tracheas of anaesthetized cats for 3 h. In other cats [35S]sulphate was given by intravenous injection. 2. After a further 2 h, tracheas were removed and fixed. Serial actions were cut and alternate sections were stained with Haematoxylin and Eosin or prepared as unstained autoradiographs. Points on submucosal gland and surface epithelium were chosen with a grid on photomicrographs of the stained sections. Absorbance, which is proportional to autoradiographic grain density, was estimated on corresponding points on unstained autoradiographs by flying-spot microdensitometry. 3. With [3H]proline as precursor, the grain densities were greater over surface epithelium than over submucosal gland. With [3H]glucose, grain densities were greater over the surface epithelium in three cases, equal in one and greater over submucosal gland in the last. [35S]Sulphate, given either into the tracheal segment or intravenously, yielded grain densities that were greater over the submucosal glands than over surface epithelium. 4. The areas of submucosal gland the surface epithelium were estimated by point counting and the total content of radioactivity in the two structures estimated by multiplying mean absorbance by area. Ratios of the total radiolabel in surface epithelium to that in submucosal gland were consistently high when [3H]proline was the precursor and low with [35S]sulphate, given by either route. [3H]Glucose gave intermediate ratios. 5. Secretions washed from the trachea were subjected to gel-exclusion chromatography. Washings from tracheas labelled with [3H]proline contained some molecules eluting in the void volume of a Sepharose CL-4B column (suggesting a relative molecular mass of greater than 10(6) Da), but more of the radiolabel eluted in three peaks in the partially included volume. Density gradient ultracentrifugation of the void volume material gave radiolabelled peaks at densities of approximately 1.60 and 1.50 g ml-1, consistent with glycosylated proteins, as well as less dense material (less than 1.30 g ml-1), probably proteins with little or no glycosylation. 6. We discuss the justification of using these radiolabelled precursors to give relatively selective labelling of secretory products from submucosal gland and surface epithelium.
Full text
PDF








Images in this article
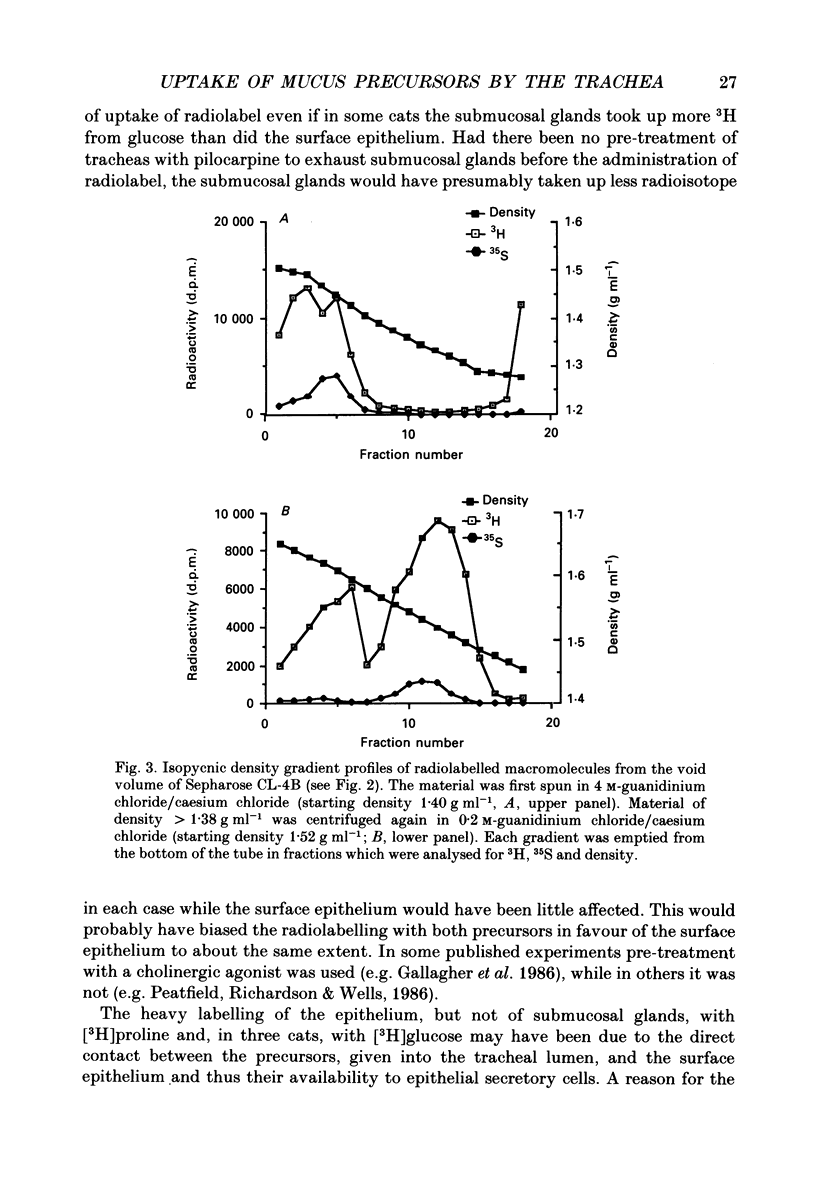
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basbaum C. B., Ueki I., Brezina L., Nadel J. A. Tracheal submucosal gland serous cells stimulated in vitro with adrenergic and cholinergic agonists. A morphometric study. Cell Tissue Res. 1981;220(3):481–498. doi: 10.1007/BF00216752. [DOI] [PubMed] [Google Scholar]
- Bhaskar K. R., O'Sullivan D. D., Opaskar-Hincman H., Reid L. M., Coles S. J. Density gradient analysis of secretions produced in vitro by human and canine airway mucosa: identification of lipids and proteoglycans in such secretions. Exp Lung Res. 1986;10(4):401–422. doi: 10.3109/01902148609058290. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Lindgren H., Sheehan J. K., Ulmsten U., Wingerup L. Isolation and characterization of human cervical-mucus glycoproteins. Biochem J. 1983 Apr 1;211(1):13–22. doi: 10.1042/bj2110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt I., Sheehan J. K. Is the macromolecular architecture of cervical, respiratory and gastric mucins the same? Biochem Soc Trans. 1984 Aug;12(4):615–617. doi: 10.1042/bst0120615. [DOI] [PubMed] [Google Scholar]
- Chakrin L. W., Baker A. P., Spicer S. S., Wardell J. R., Jr, De Sanctis N., Dries C. Synthesis and secretion of macromolecules by canine trachea. Am Rev Respir Dis. 1972 Mar;105(3):368–381. doi: 10.1164/arrd.1972.105.3.368. [DOI] [PubMed] [Google Scholar]
- Feldhoff P. A., Bhavanandan V. P., Davidson E. A. Purification, properties, and analysis of human asthmatic bronchial mucin. Biochemistry. 1979 May 29;18(11):2430–2436. doi: 10.1021/bi00578a044. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Hall R. L., Phipps R. J., Jeffery P. K., Kent P. W., Richardson P. S. Mucus-glycoproteins (mucins) of the cat trachea: characterisation and control of secretion. Biochim Biophys Acta. 1986 Apr 29;886(2):243–254. doi: 10.1016/0167-4889(86)90142-4. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Kent P. W., Passatore M., Phipps R. J., Richardson P. S. The composition of tracheal mucus and the nervous control of its secretion in the cat. Proc R Soc Lond B Biol Sci. 1975 Dec 31;192(1106):49–76. doi: 10.1098/rspb.1975.0151. [DOI] [PubMed] [Google Scholar]
- Goldstein D. J., Williams M. A. Quantitative autoradiography: an evaluation of visual grain counting, reflectance microscopy, gross absorbance measurements and flying-spot microdensitometry. J Microsc. 1971 Dec;94(3):215–239. doi: 10.1111/j.1365-2818.1971.tb02371.x. [DOI] [PubMed] [Google Scholar]
- KENT P. W., WHITEHOUSE M. W. Observations on the incorporation of 35S in duodenal mucosubstances. Q J Exp Physiol Cogn Med Sci. 1956 Jul;41(3):230–246. doi: 10.1113/expphysiol.1956.sp001185. [DOI] [PubMed] [Google Scholar]
- PELC S. R. The stripping-film technique of autoradiography. Int J Appl Radiat Isot. 1956 Nov;1(3):172–177. doi: 10.1016/0020-708x(56)90003-5. [DOI] [PubMed] [Google Scholar]
- Peatfield A. C., Richardson P. S., Wells U. M. The effect of airflow on mucus secretion into the trachea of the cat. J Physiol. 1986 Nov;380:429–439. doi: 10.1113/jphysiol.1986.sp016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps R. J., Nadel J. A., Davis B. Effect of alpha-adrenergic stimulation on mucus secretion and on ion transport in cat trachea in vitro. Am Rev Respir Dis. 1980 Feb;121(2):359–365. doi: 10.1164/arrd.1980.121.2.359. [DOI] [PubMed] [Google Scholar]
- Richardson P. S., Phipps R. J., Balfre K., Hall R. L. The roles of mediators, irritants and allergens in causing mucin secretion from the trachea. Ciba Found Symp. 1978;(54):111–131. doi: 10.1002/9780470720356.ch6. [DOI] [PubMed] [Google Scholar]
- Sturgess J., Reid L. An organ culture study of the effect of drugs on the secretory activity of the human bronchial submucosal gland. Clin Sci. 1972 Oct;43(4):533–543. doi: 10.1042/cs0430533. [DOI] [PubMed] [Google Scholar]
- Varsano S., Basbaum C. B., Forsberg L. S., Borson D. B., Caughey G., Nadel J. A. Dog tracheal epithelial cells in culture synthesize sulfated macromolecular glycoconjugates and release them from the cell surface upon exposure to extracellular proteinases. Exp Lung Res. 1987;13(2):157–184. doi: 10.3109/01902148709064316. [DOI] [PubMed] [Google Scholar]