Abstract
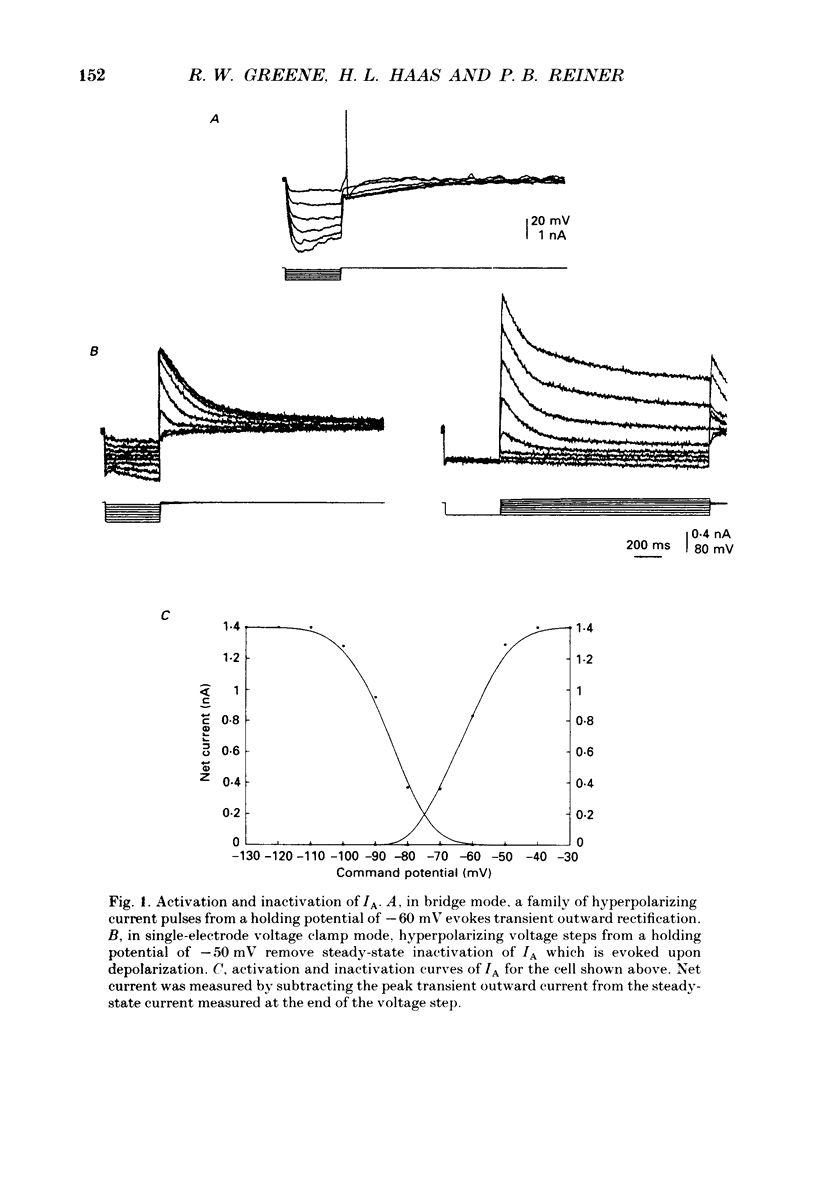
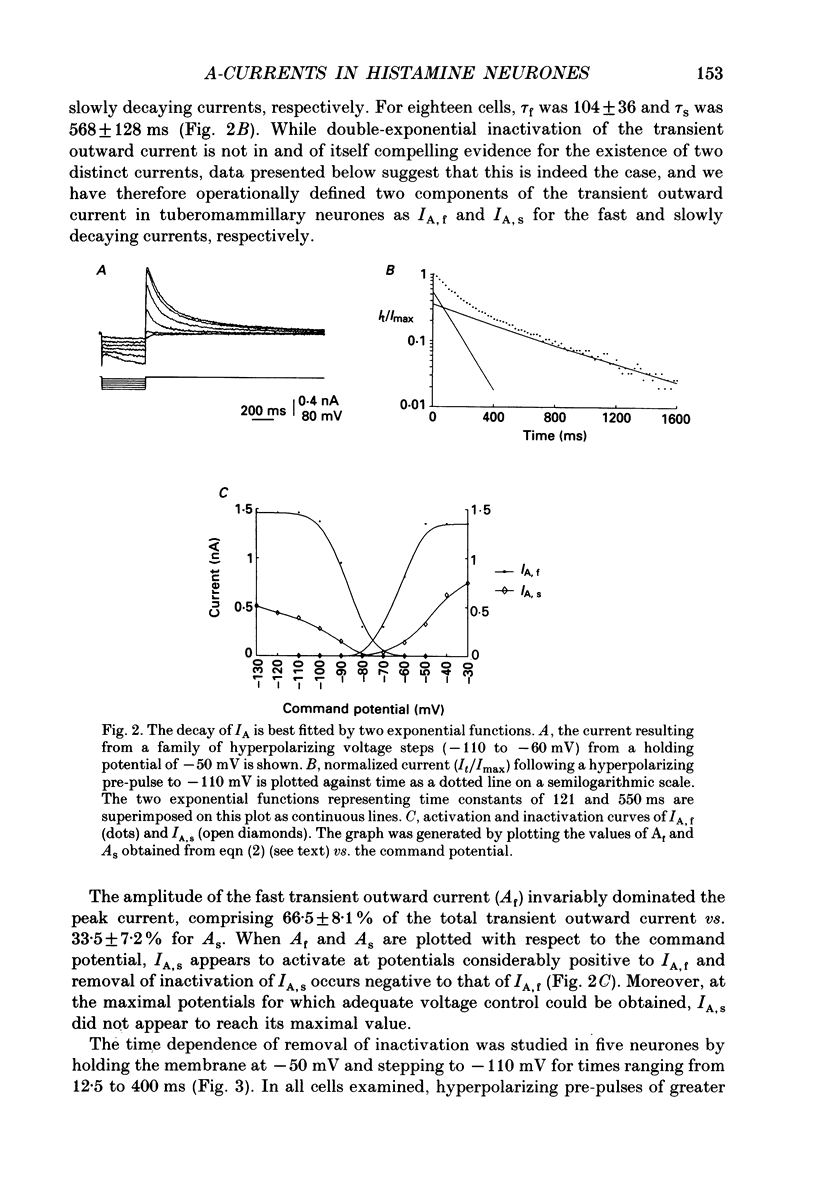
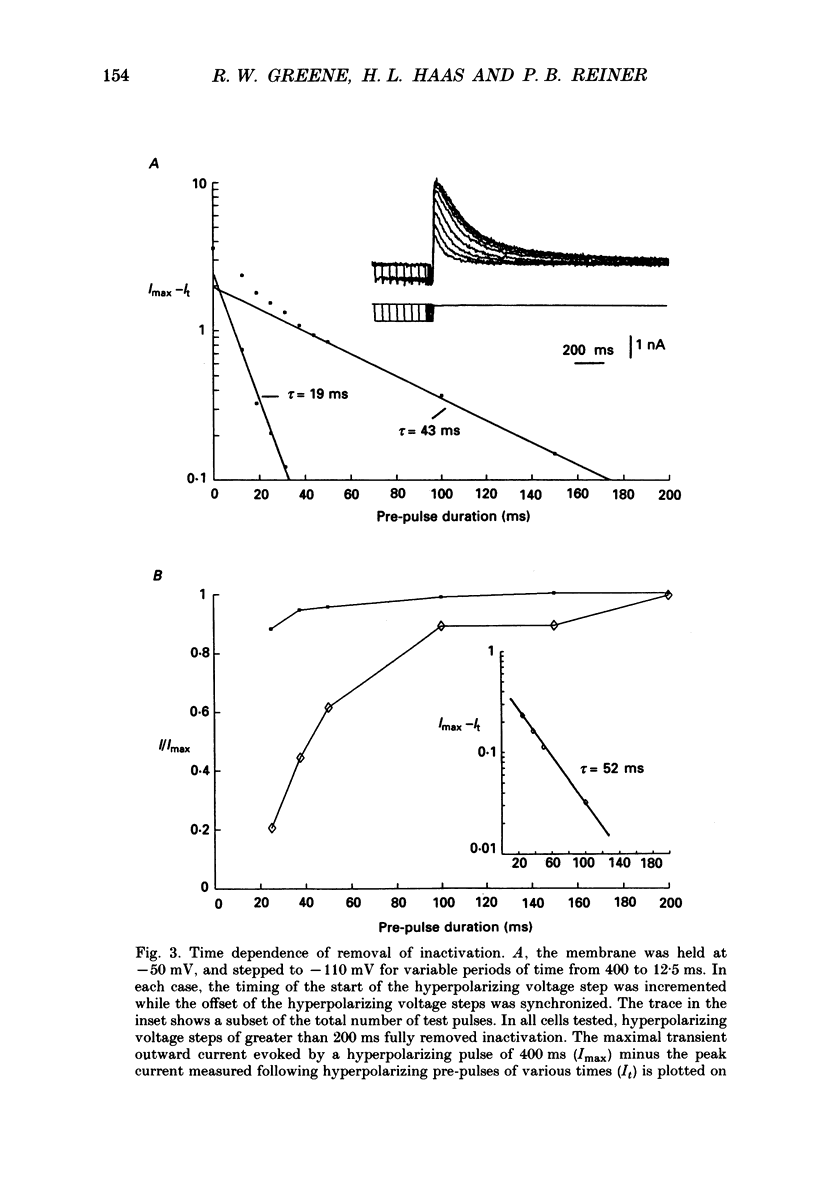
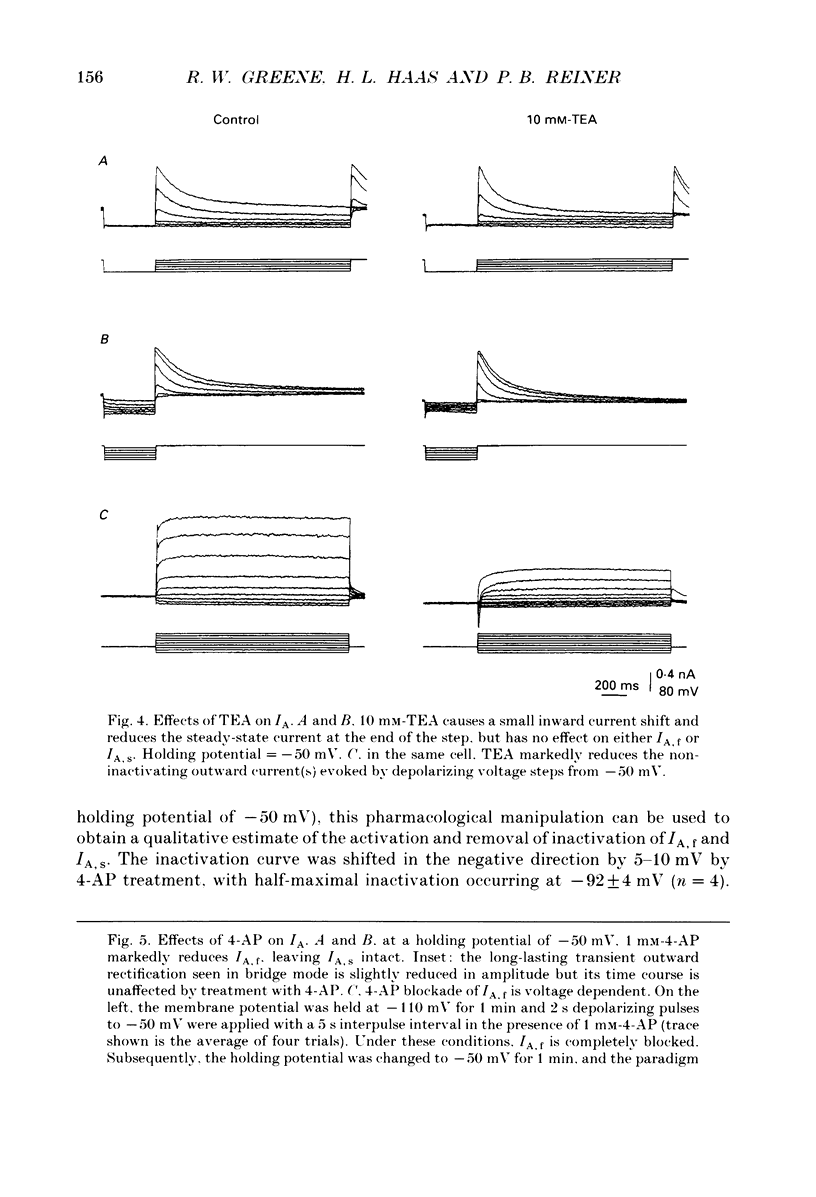
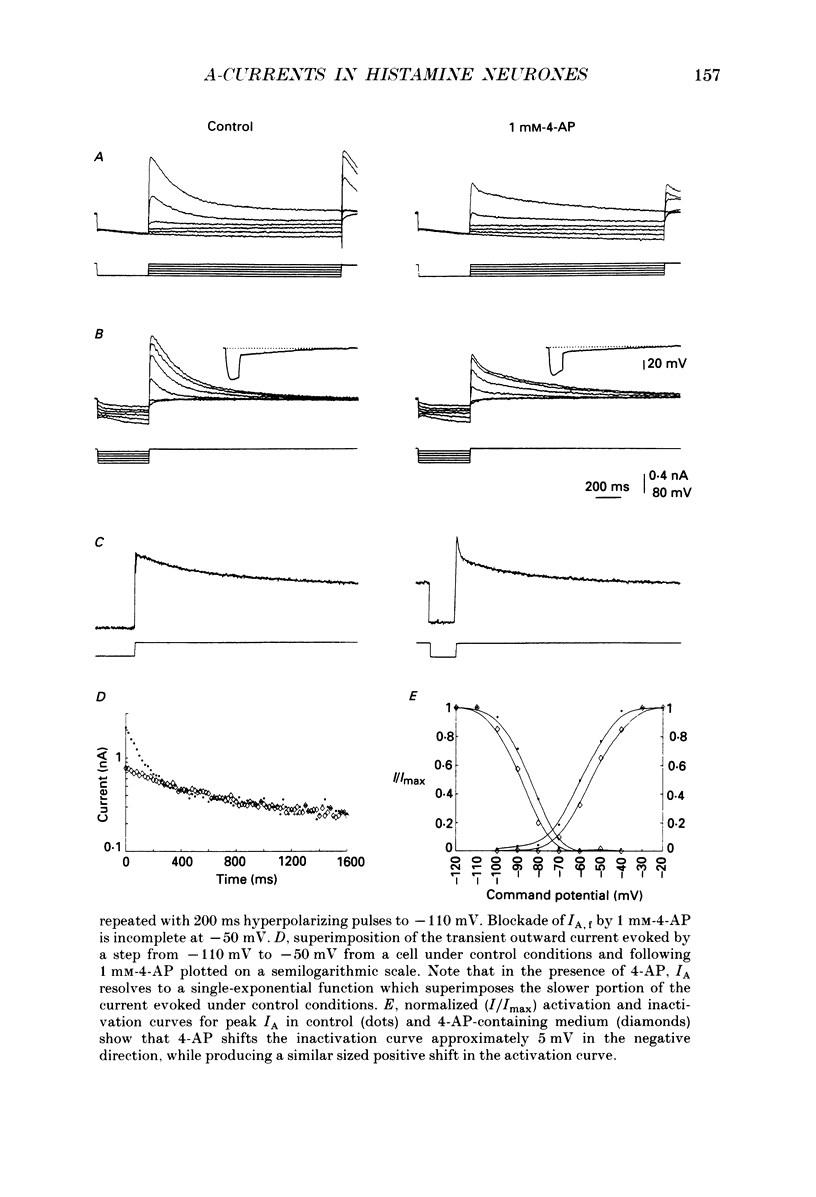
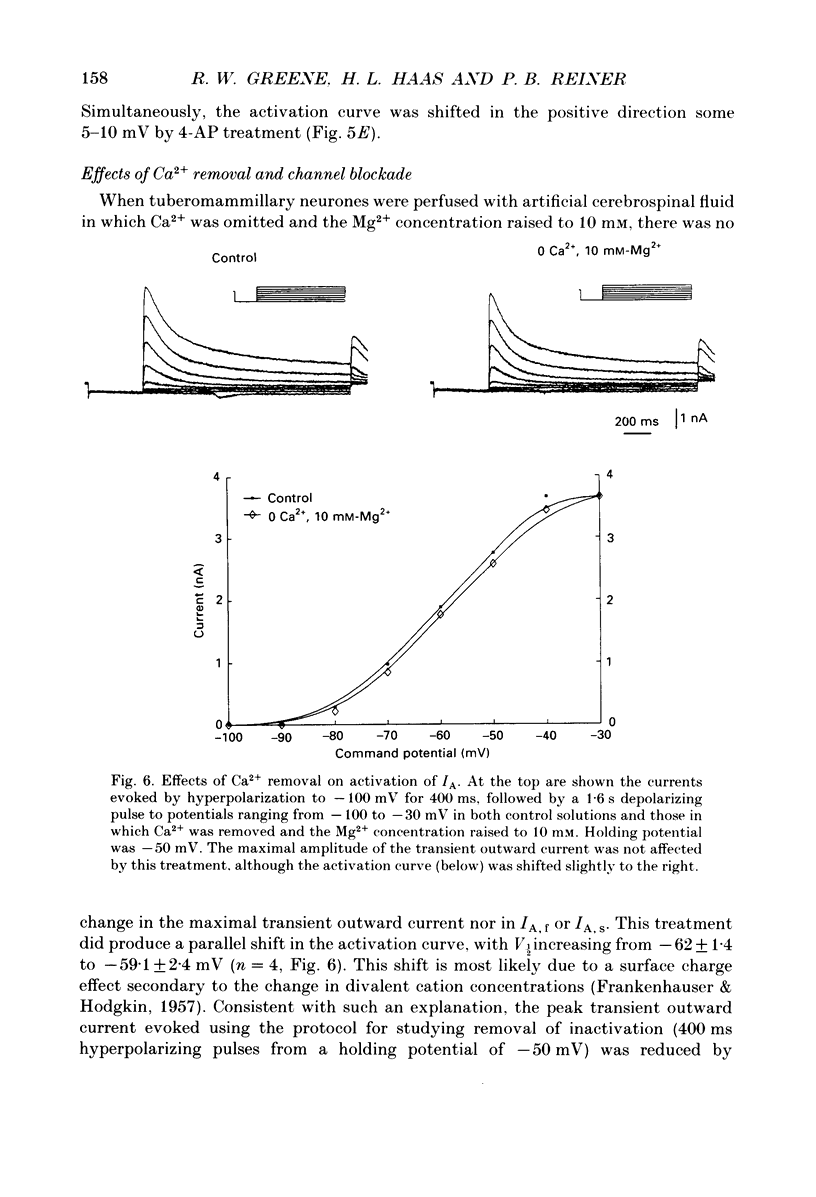
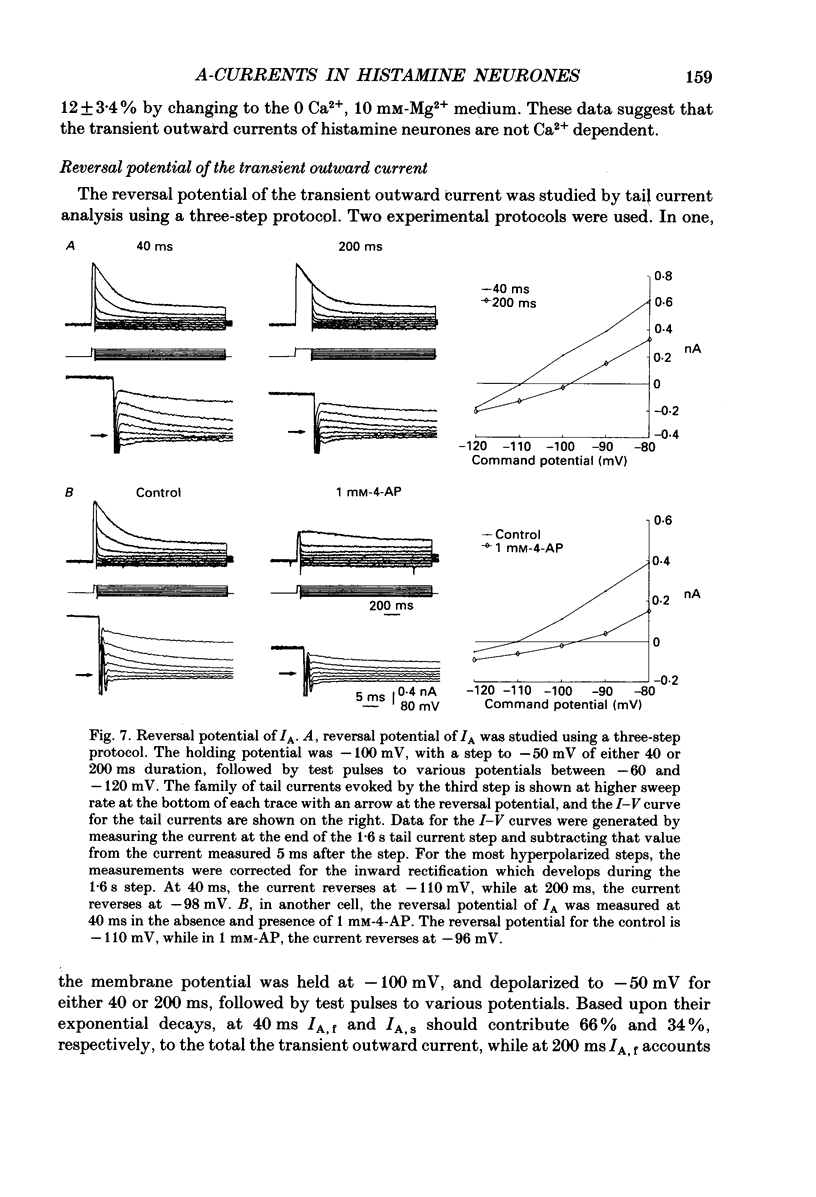
1. The transient outward current exhibited by the histamine neurones of the tuberomammillary nucleus was studied using the single-electrode voltage clamp technique in an in vitro rat hypothalamic slice preparation. 2. The transient outward current exhibited steady-state inactivation at the resting potential. Inactivation was removed by priming hyperpolarization with a V1/2 of -85 +/- 1.2 mV, while the V1/2 for activation was -60.3 +/- 2.1 mV. 3. The decay of the transient outward current was best fitted by two exponentials with time constants of 104 +/- 36 and 568 +/- 128 ms. These two components were provisionally termed IA,f and IA,s for the fast and slowly decaying currents, respectively. 4. Removal of inactivation was time dependent; inactivation was fully removed by hyperpolarizing pulses to -110 mV of 200 ms or greater duration. Removal of inactivation of IA,f was rapid, becoming complete with hyperpolarizing pre-pulses of 50 ms or greater, while removal of inactivation of IA,s was not complete until hyperpolarizing pre-pulses were 200 ms in duration. 5. The fast decaying current IA,f was selectively blocked by 1 mM-4-aminopyridine. Tetraethylammonium chloride (10 mM) had no effect on either IA,f or IA,s. 6. The inactivation curves for IA,s, determined both by using the values obtained from the amplitude of the computed slower exponential function as well as that of the current remaining in 1 mM 4-aminopyridine, were negative to those of IA,f. Similarly derived activation curves for IA,s were positive to those of IA,f. 7. Superfusion with a nominal 0 Ca2+ medium containing 10 mM-Mg2+ did not reduce the maximal transient outward current. 8. The reversal potential of IA,s with 2.5 mM-K+ in the medium was -95 +/- 3 mV; the reversal potential of IA,f was at least 15 mV negative to that of IA,s. 9. It is concluded that histaminergic tuberomammillary neurones possess at least two transient outward currents which can be distinguished on the basis of their rates of decay, 4-aminopyridine sensitivity, voltage dependence and reversal potentials.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian G. K. Modulation of a transient outward current in serotonergic neurones by alpha 1-adrenoceptors. Nature. 1985 Jun 6;315(6019):501–503. doi: 10.1038/315501a0. [DOI] [PubMed] [Google Scholar]
- Butler A., Wei A. G., Baker K., Salkoff L. A family of putative potassium channel genes in Drosophila. Science. 1989 Feb 17;243(4893):943–947. doi: 10.1126/science.2493160. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol. 1971 Feb;213(1):31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E., Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflugers Arch. 1982 Feb;392(4):352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H. L., Reiner P. B. Membrane properties of histaminergic tuberomammillary neurones of the rat hypothalamus in vitro. J Physiol. 1988 May;399:633–646. doi: 10.1113/jphysiol.1988.sp017100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H. L., Schaerer B., Vosmansky M. A simple perfusion chamber for the study of nervous tissue slices in vitro. J Neurosci Methods. 1979 Dec;1(4):323–325. doi: 10.1016/0165-0270(79)90021-9. [DOI] [PubMed] [Google Scholar]
- Hiraoka M., Kawano S. Calcium-sensitive and insensitive transient outward current in rabbit ventricular myocytes. J Physiol. 1989 Mar;410:187–212. doi: 10.1113/jphysiol.1989.sp017528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Dennis M. J. Two mutations of synaptic transmission in Drosophila. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):87–108. doi: 10.1098/rspb.1977.0087. [DOI] [PubMed] [Google Scholar]
- Johnston D., Brown T. H. Interpretation of voltage-clamp measurements in hippocampal neurons. J Neurophysiol. 1983 Aug;50(2):464–486. doi: 10.1152/jn.1983.50.2.464. [DOI] [PubMed] [Google Scholar]
- Krafte D. S., Snutch T. P., Leonard J. P., Davidson N., Lester H. A. Evidence for the involvement of more than one mRNA species in controlling the inactivation process of rat and rabbit brain Na channels expressed in Xenopus oocytes. J Neurosci. 1988 Aug;8(8):2859–2868. doi: 10.1523/JNEUROSCI.08-08-02859.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz P., Werblin F. A slowly inactivating potassium current truncates spike activity in ganglion cells of the tiger salamander retina. J Neurosci. 1988 Dec;8(12):4470–4481. doi: 10.1523/JNEUROSCI.08-12-04470.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner P. B., McGeer E. G. THA increases action potential duration of central histamine neurons in vitro. Eur J Pharmacol. 1988 Oct 18;155(3):265–270. doi: 10.1016/0014-2999(88)90512-2. [DOI] [PubMed] [Google Scholar]
- Reiner P. B., Semba K., Fibiger H. C., McGeer E. G. Ontogeny of histidine-decarboxylase-immunoreactive neurons in the tuberomammillary nucleus of the rat hypothalamus: time of origin and development of transmitter phenotype. J Comp Neurol. 1988 Oct 8;276(2):304–311. doi: 10.1002/cne.902760212. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Rudy B., Hoger J. H., Lester H. A., Davidson N. At least two mRNA species contribute to the properties of rat brain A-type potassium channels expressed in Xenopus oocytes. Neuron. 1988 Oct;1(8):649–658. doi: 10.1016/0896-6273(88)90164-x. [DOI] [PubMed] [Google Scholar]
- Salkoff L. Drosophila mutants reveal two components of fast outward current. Nature. 1983 Mar 17;302(5905):249–251. doi: 10.1038/302249a0. [DOI] [PubMed] [Google Scholar]
- Schwarz T. L., Tempel B. L., Papazian D. M., Jan Y. N., Jan L. Y. Multiple potassium-channel components are produced by alternative splicing at the Shaker locus in Drosophila. Nature. 1988 Jan 14;331(6152):137–142. doi: 10.1038/331137a0. [DOI] [PubMed] [Google Scholar]
- Solc C. K., Zagotta W. N., Aldrich R. W. Single-channel and genetic analyses reveal two distinct A-type potassium channels in Drosophila. Science. 1987 May 29;236(4805):1094–1098. doi: 10.1126/science.2437657. [DOI] [PubMed] [Google Scholar]
- Storm J. F. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988 Nov 24;336(6197):379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Tanouye M. A., Ferrus A., Fujita S. C. Abnormal action potentials associated with the Shaker complex locus of Drosophila. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6548–6552. doi: 10.1073/pnas.78.10.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. Aminopyridine block of transient potassium current. J Gen Physiol. 1982 Jul;80(1):1–18. doi: 10.1085/jgp.80.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpe L. C., Schwarz T. L., Tempel B. L., Papazian D. M., Jan Y. N., Jan L. Y. Expression of functional potassium channels from Shaker cDNA in Xenopus oocytes. Nature. 1988 Jan 14;331(6152):143–145. doi: 10.1038/331143a0. [DOI] [PubMed] [Google Scholar]
- Vanni-Mercier G., Sakai K., Jouvet M. Neurones spécifiques de l'éveil dans l'hypothalamus postérieur du chat. C R Acad Sci III. 1984;298(7):195–200. [PubMed] [Google Scholar]
- Williams J. T., North R. A., Shefner S. A., Nishi S., Egan T. M. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984 Sep;13(1):137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]
- Zbicz K. L., Weight F. F. Transient voltage and calcium-dependent outward currents in hippocampal CA3 pyramidal neurons. J Neurophysiol. 1985 Apr;53(4):1038–1058. doi: 10.1152/jn.1985.53.4.1038. [DOI] [PubMed] [Google Scholar]


