Abstract
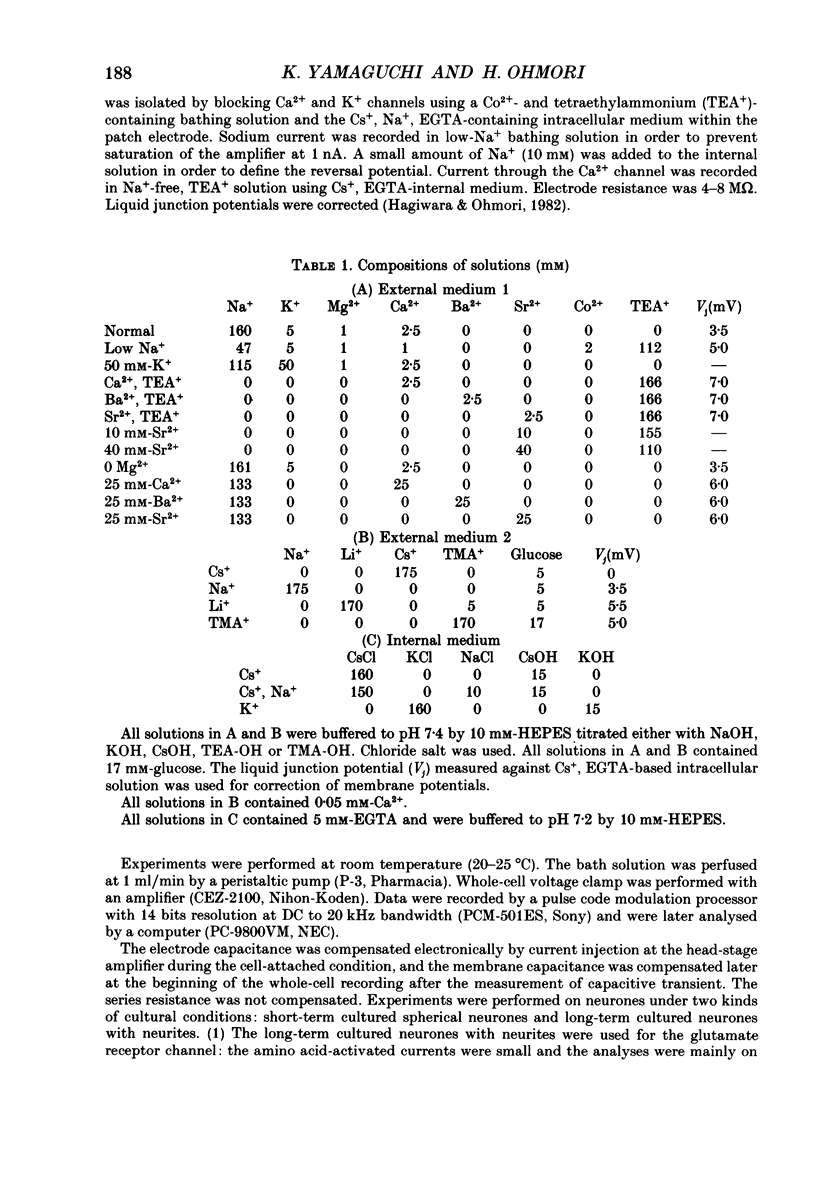
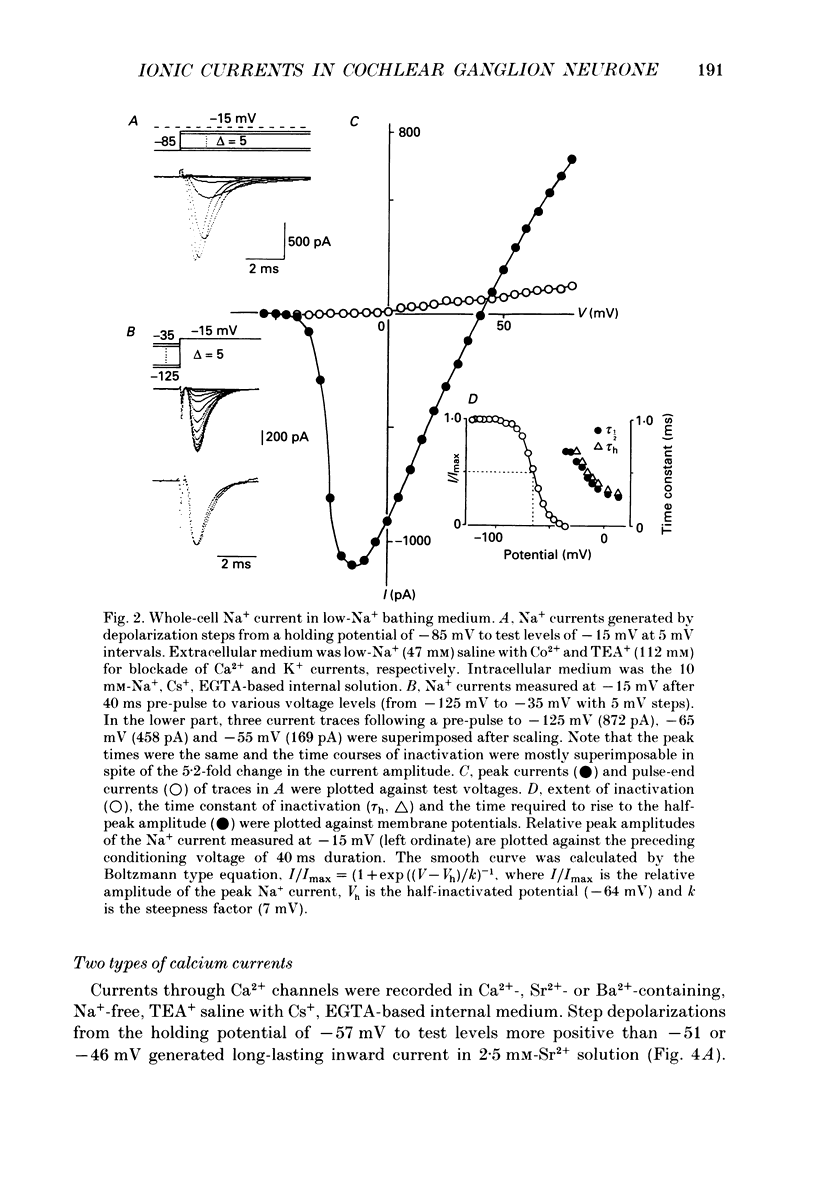
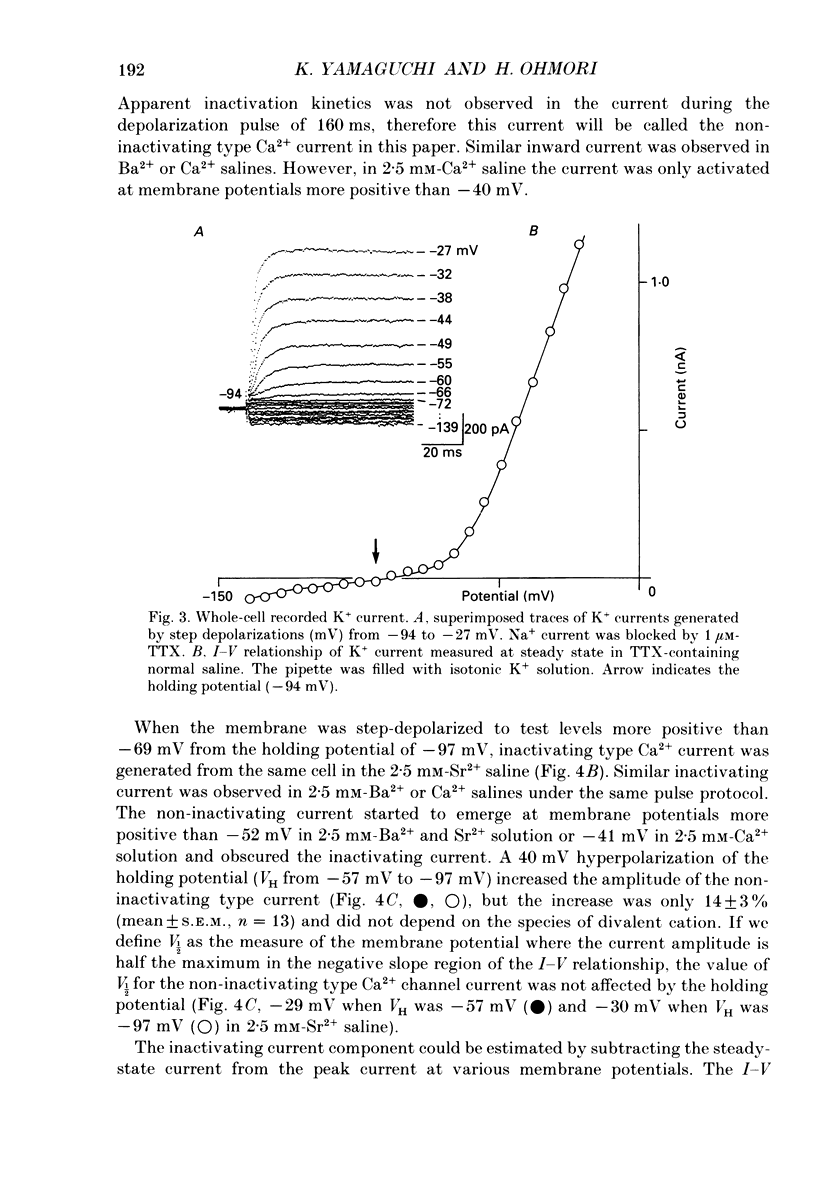
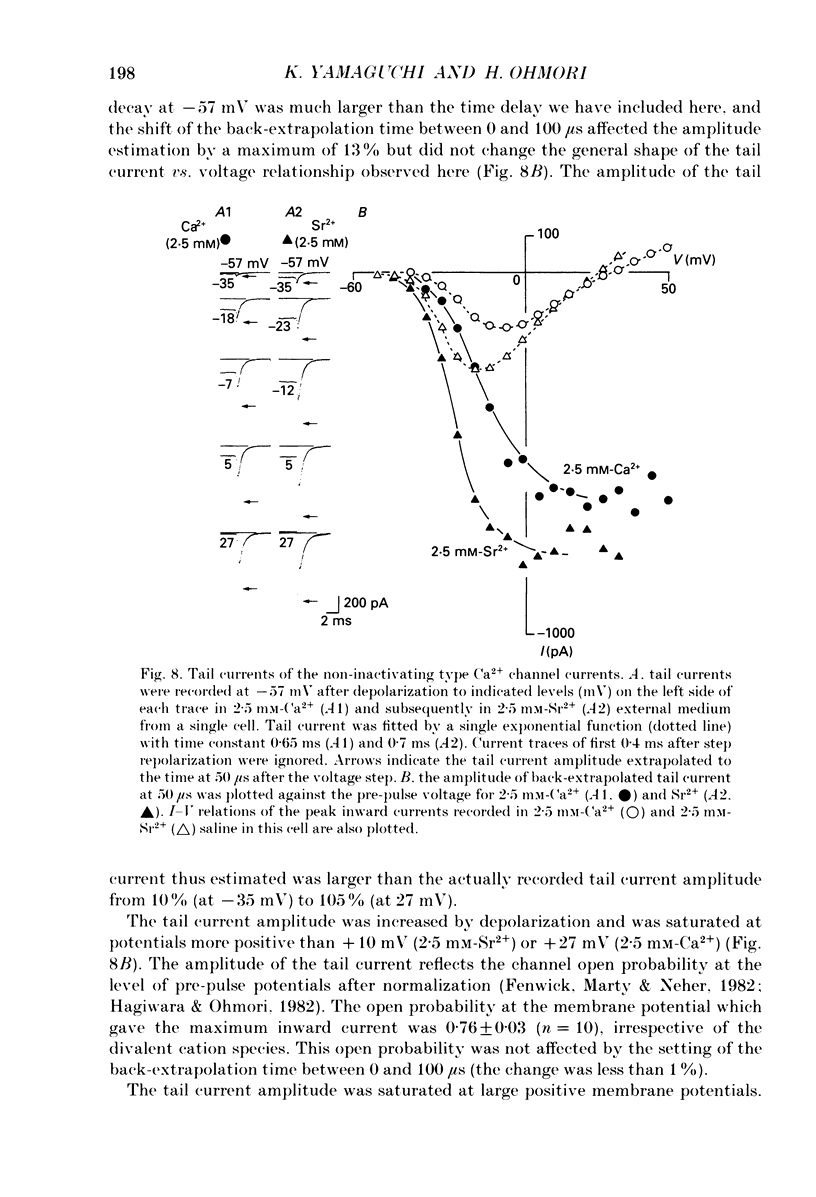
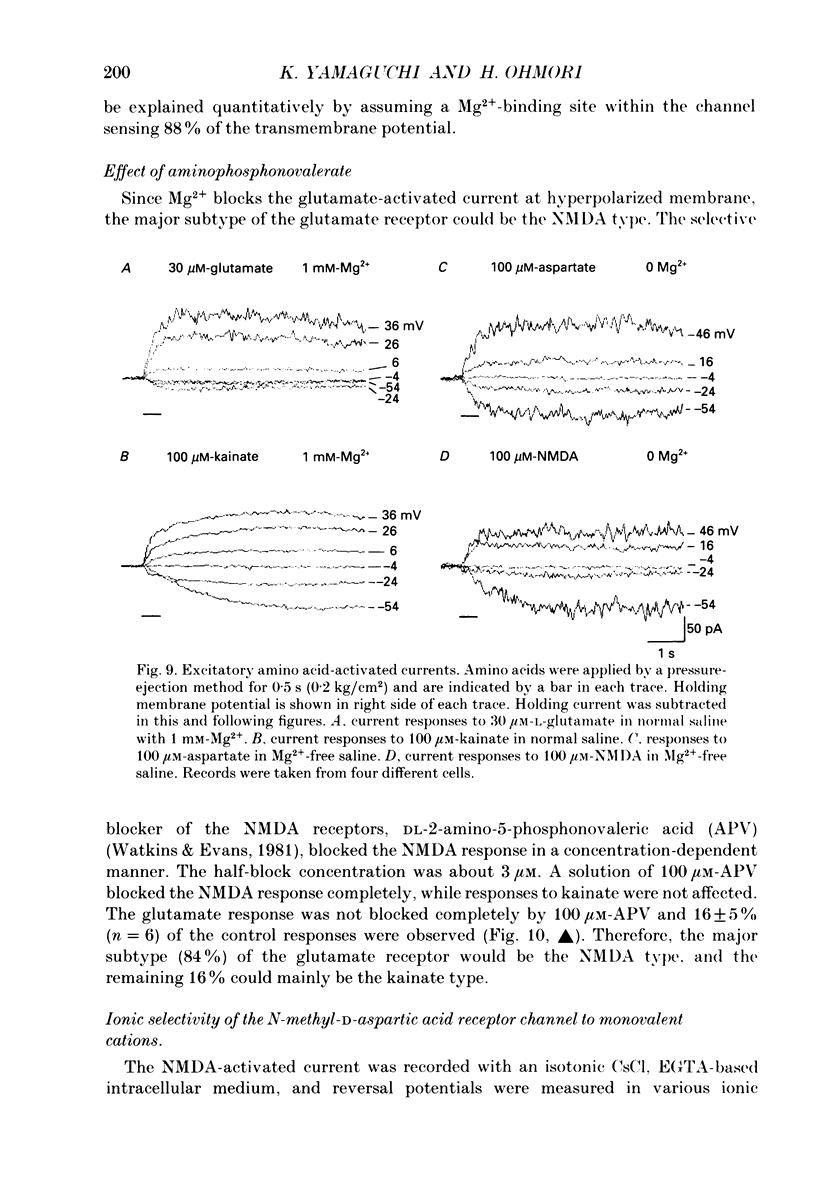
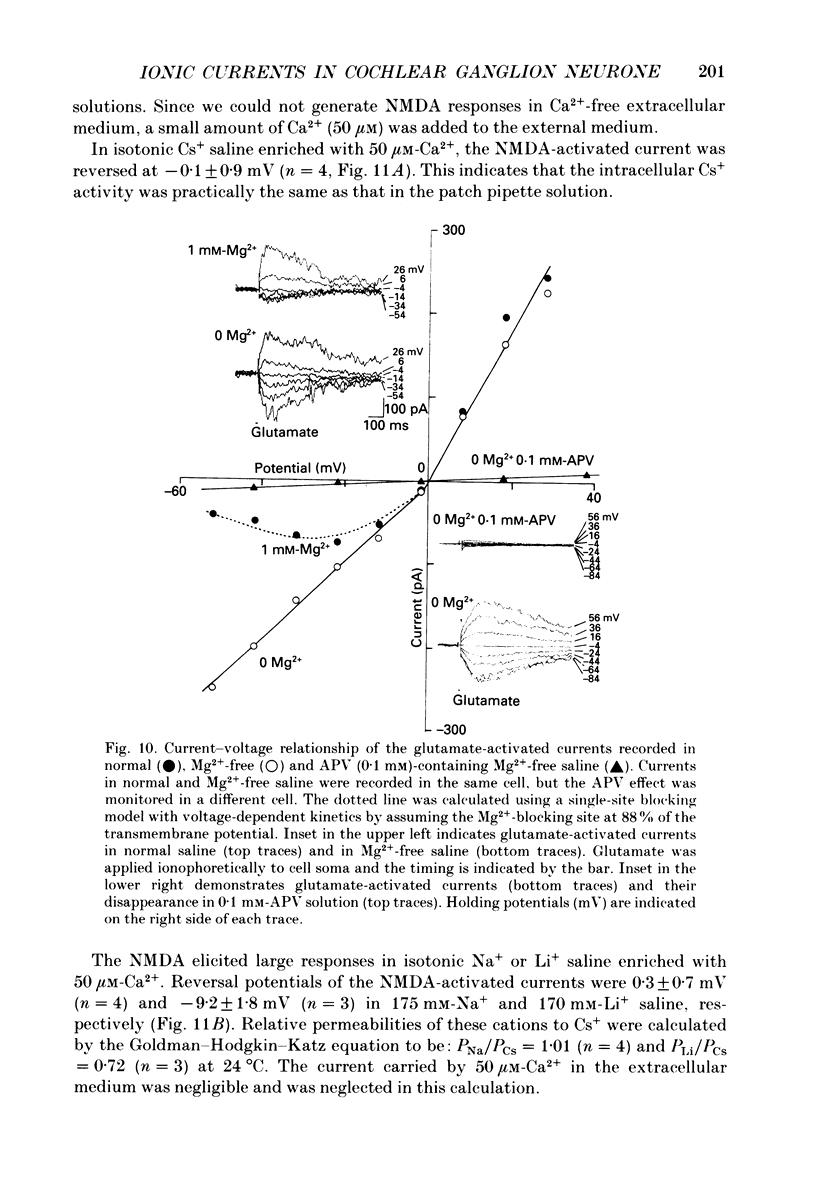
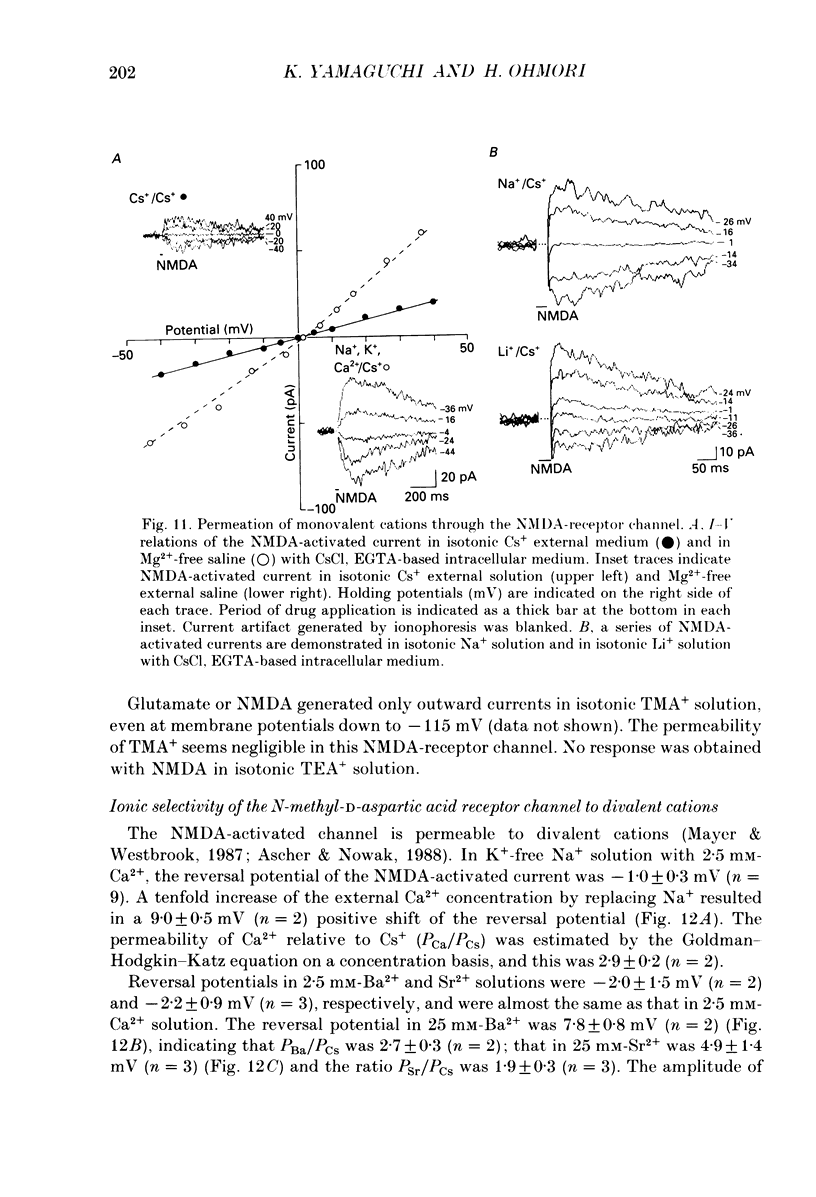
1. Electrophysiological properties of ionic channels of isolated or cultured cochlear ganglion (CG) neurones from chick embryo were studied under voltage-clamp conditions using a patch electrode. 2. Tetrodotoxin-sensitive Na+ current was activated by a step depolarization more positive than -40 mV, and was inactivated rapidly. 3. Outward-going K+ current was activated by step depolarization to membrane potentials more positive than -62 mV. 4. Two types of Ca2+ currents were demonstrated, an inactivating and a non-inactivating type. The inactivating type was activated by step depolarizations more positive than -69 mV and was inactivated rapidly. The non-inactivating type was activated by step depolarizations more positive than -52 or -41 mV depending on the external divalent cation species. 5. The I-V relationship and the activation kinetics of the non-inactivating type Ca2+ channel was shifted in a positive direction along the voltage axis by 12 mV when extracellular 2.5 mM-Sr2+ or Ba2+ were replaced by Ca2+. This shift was not observed in the inactivating type Ca2+ channel. 6. The amplitude of peak current through the inactivating type Ca2+ channel was in the order of Ca2+ greater than Sr2+ greater than Ba2+. The order of relative permeability through the non-inactivating type estimated from the tail current amplitude was Ba2+ greater than Sr2+ greater than Ca2+. 7. After 5 days in culture, glutamate (30 microM), aspartate (100 microM), kainate (100 microM) and N-methyl-D-aspartic acid (NMDA; 100 microM) elicited ionic currents. The glutamate response was depressed by 1 mM-Mg2+ in a voltage-dependent manner at negative membrane potentials and was almost extinguished by amino-phosphonovalerate (APV) (0.1 mM). The major subtype of glutamate receptor could be of the NMDA type. 8. The permeability of the NMDA receptor channel to Na+ and Li+ was estimated from the reversal potential and was 1.0 and 0.7 compared with that of Cs+, respectively. 9. Divalent cations were more permeable than the monovalent cations through the NMDA receptor channel: PCa greater than or equal to PBa greater than PSr greater than PCs.
Full text
PDF




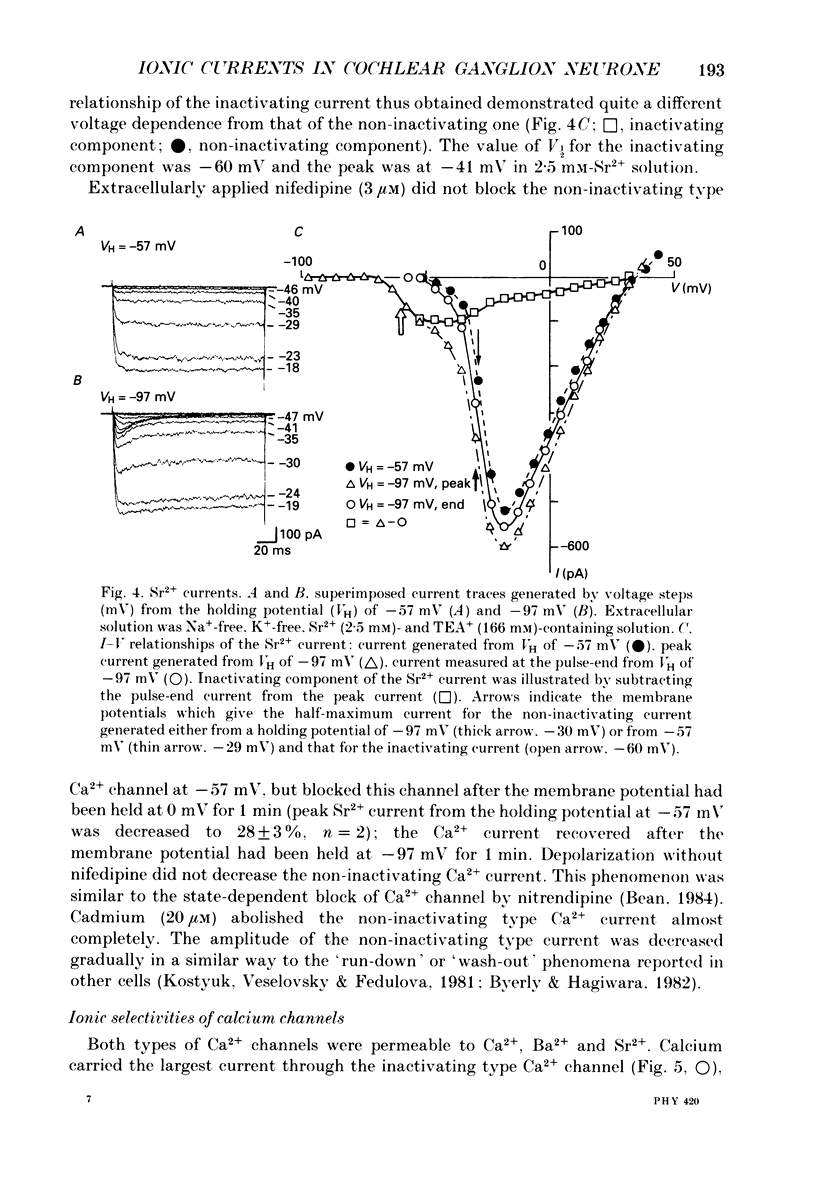
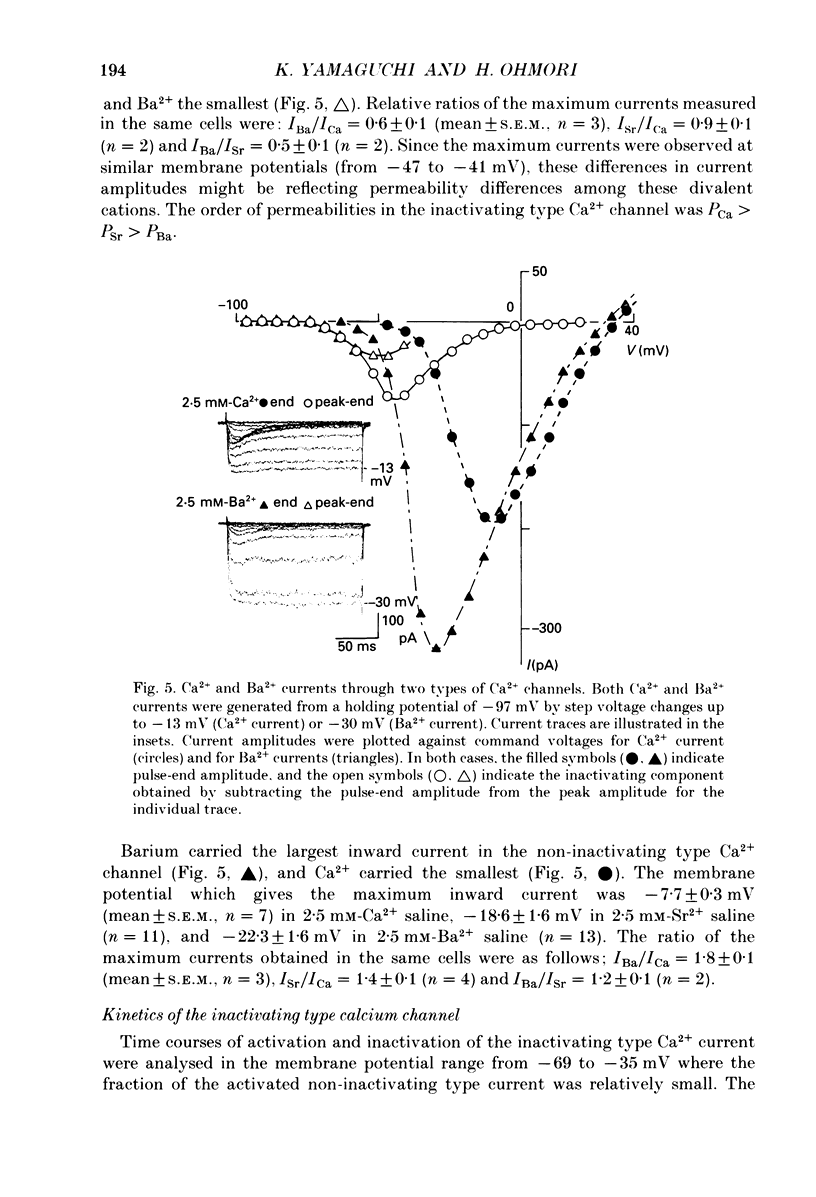

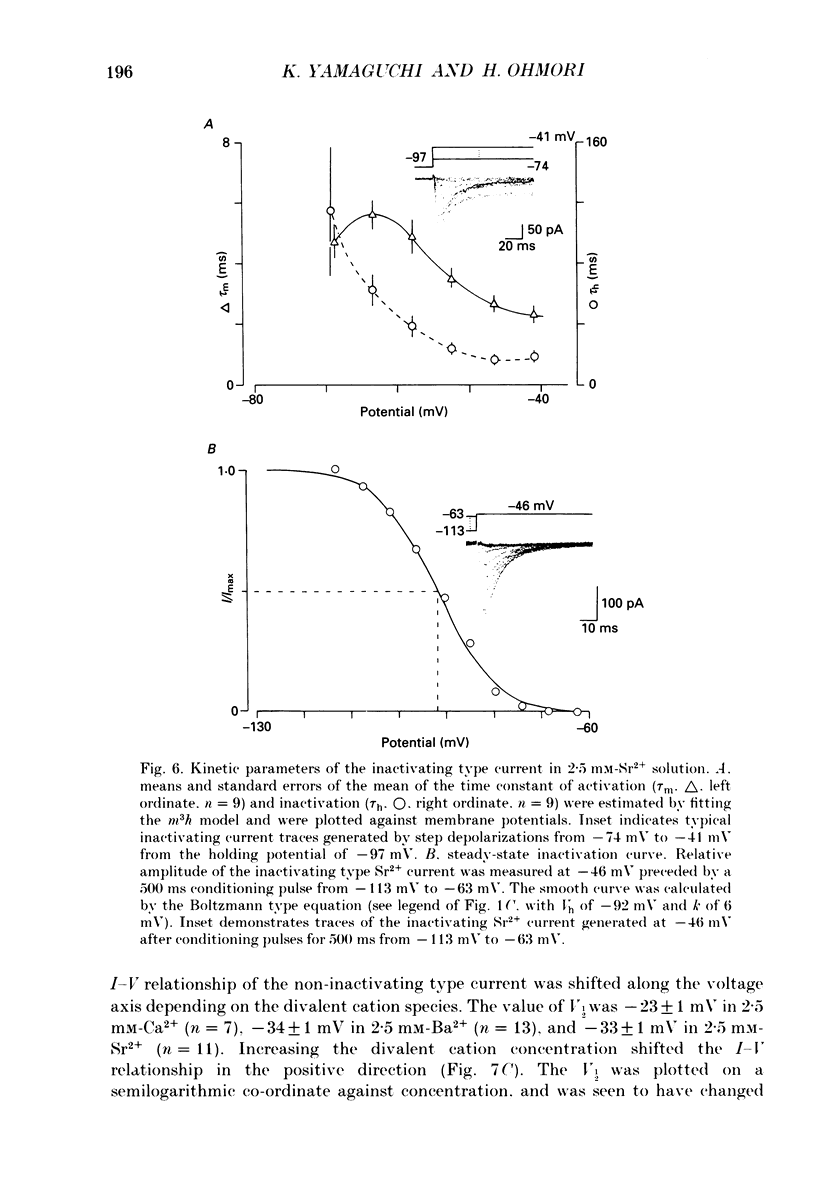
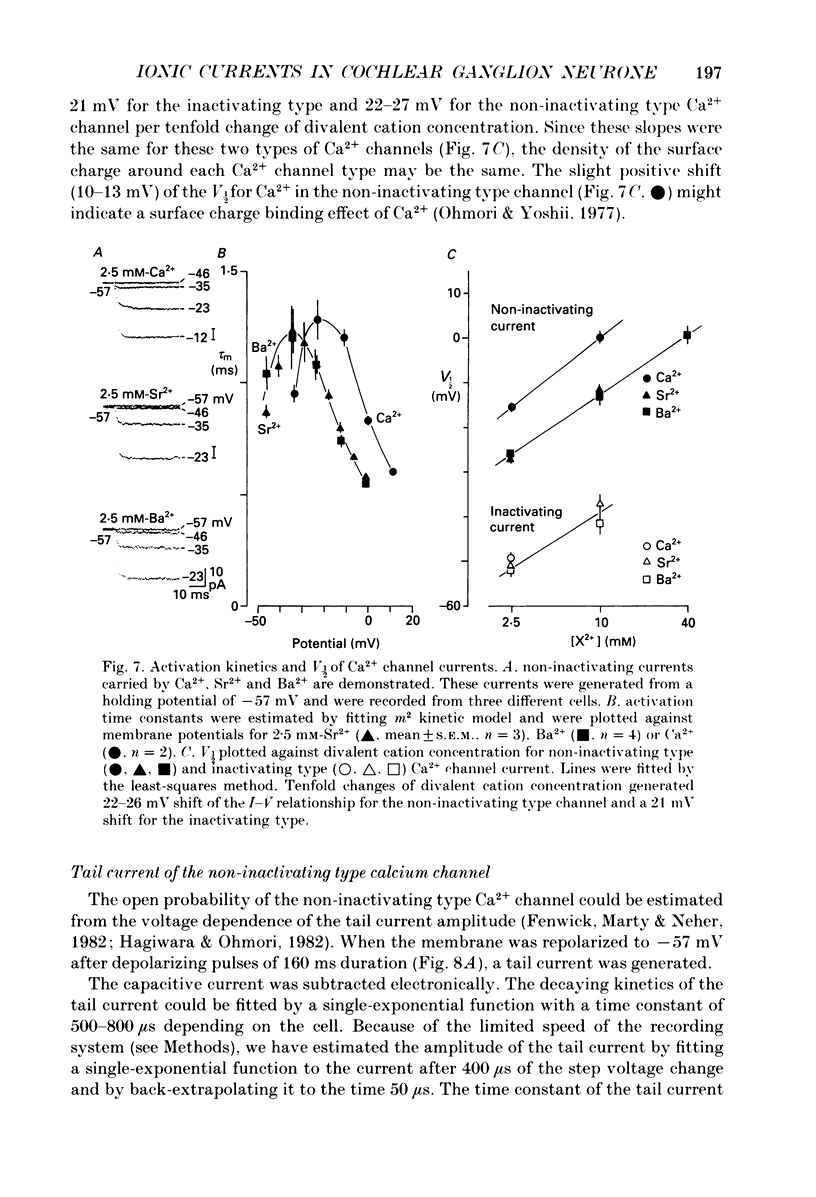

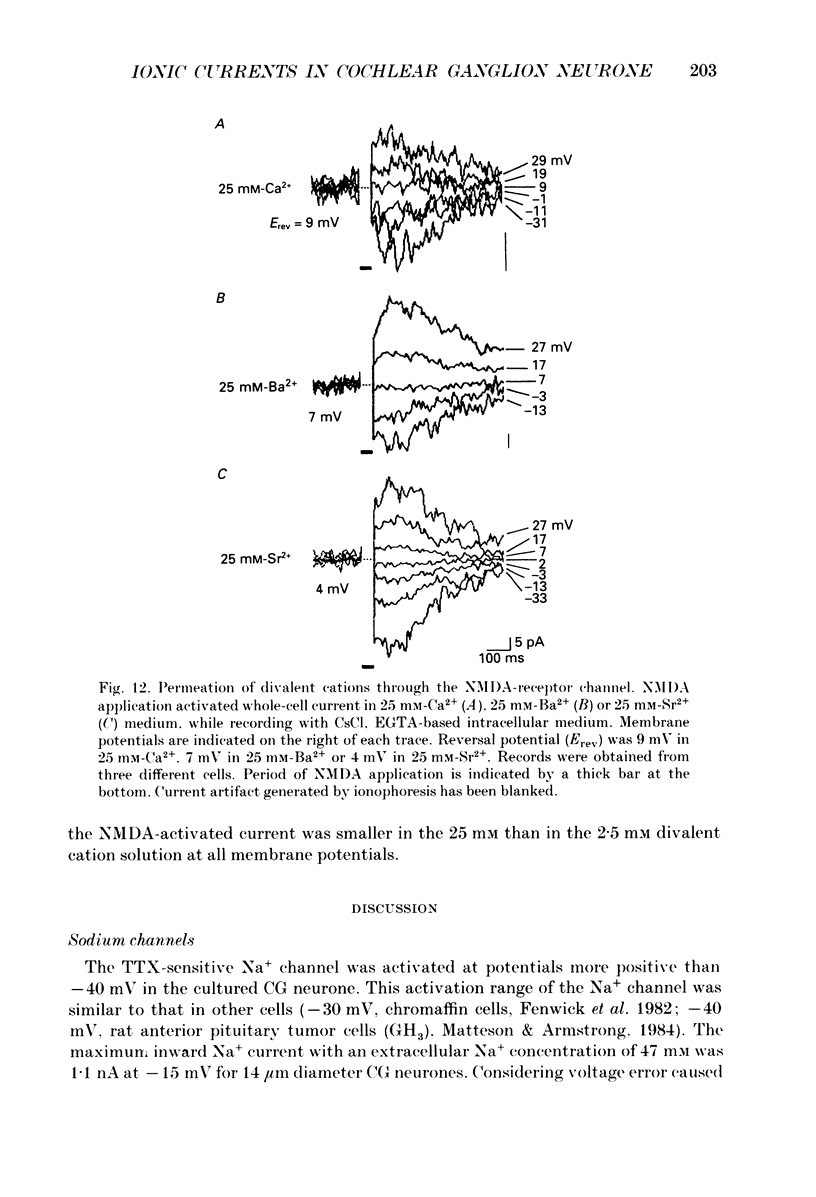



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annoni J. M., Cochran S. L., Precht W. Pharmacology of the vestibular hair cell-afferent fiber synapse in the frog. J Neurosci. 1984 Aug;4(8):2106–2116. doi: 10.1523/JNEUROSCI.04-08-02106.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Hagiwara S. Calcium currents in internally perfused nerve cell bodies of Limnea stagnalis. J Physiol. 1982 Jan;322:503–528. doi: 10.1113/jphysiol.1982.sp014052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol. 1987 Dec;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Fedulova S. A. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-II. Calcium currents. Neuroscience. 1981;6(12):2431–2437. doi: 10.1016/0306-4522(81)90089-0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981 Jun;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Na and Ca channels in a transformed line of anterior pituitary cells. J Gen Physiol. 1984 Mar;83(3):371–394. doi: 10.1085/jgp.83.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni F., Luzzi S., Franchi-Micheli S., Zilletti L. The presence of N-methyl-D-aspartate-type receptors for glutamic acid in the guinea pig myenteric plexus. Neurosci Lett. 1986 Jul 11;68(1):57–62. doi: 10.1016/0304-3940(86)90229-6. [DOI] [PubMed] [Google Scholar]
- Nachshen D. A. Selectivity of the Ca binding site in synaptosome Ca channels. Inhibition of Ca influx by multivalent metal cations. J Gen Physiol. 1984 Jun;83(6):941–967. doi: 10.1085/jgp.83.6.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- O'Brien R. J., Fischbach G. D. Characterization of excitatory amino acid receptors expressed by embryonic chick motoneurons in vitro. J Neurosci. 1986 Nov;6(11):3275–3283. doi: 10.1523/JNEUROSCI.06-11-03275.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yamashita N. Ionic currents through the membrane of the mammalian oocyte and their comparison with those in the tunicate and sea urchin. J Physiol. 1977 May;267(2):465–495. doi: 10.1113/jphysiol.1977.sp011822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt F. N., Narahashi T. Modification of single Na+ channels by batrachotoxin. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6732–6736. doi: 10.1073/pnas.79.21.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt T. E. Mediation of thalamic sensory input by both NMDA receptors and non-NMDA receptors. Nature. 1986 Jul 17;322(6076):263–265. doi: 10.1038/322263a0. [DOI] [PubMed] [Google Scholar]
- Sewell W. F., Mroz E. A. Neuroactive substances in inner ear extracts. J Neurosci. 1987 Aug;7(8):2465–2475. [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J., Neher E. Single Na+ channel currents observed in cultured rat muscle cells. Nature. 1980 Oct 2;287(5781):447–449. doi: 10.1038/287447a0. [DOI] [PubMed] [Google Scholar]
- Soto E., Vega R. Actions of excitatory amino acid acid agonists and antagonists on the primary afferents of the vestibular system of the axolotl (Ambystoma mexicanum). Brain Res. 1988 Oct 11;462(1):104–111. doi: 10.1016/0006-8993(88)90591-4. [DOI] [PubMed] [Google Scholar]
- Weiss R. E., Horn R. Functional differences between two classes of sodium channels in developing rat skeletal muscle. Science. 1986 Jul 18;233(4761):361–364. doi: 10.1126/science.2425432. [DOI] [PubMed] [Google Scholar]