Abstract
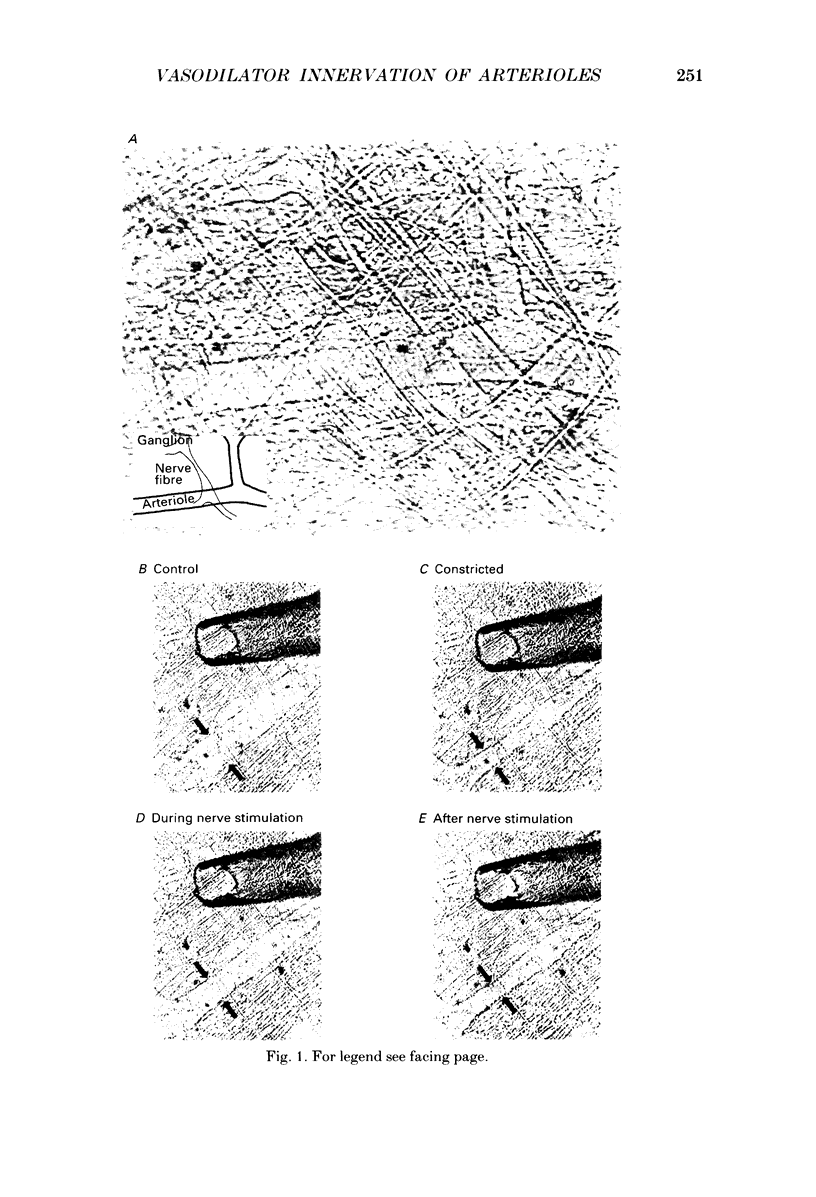
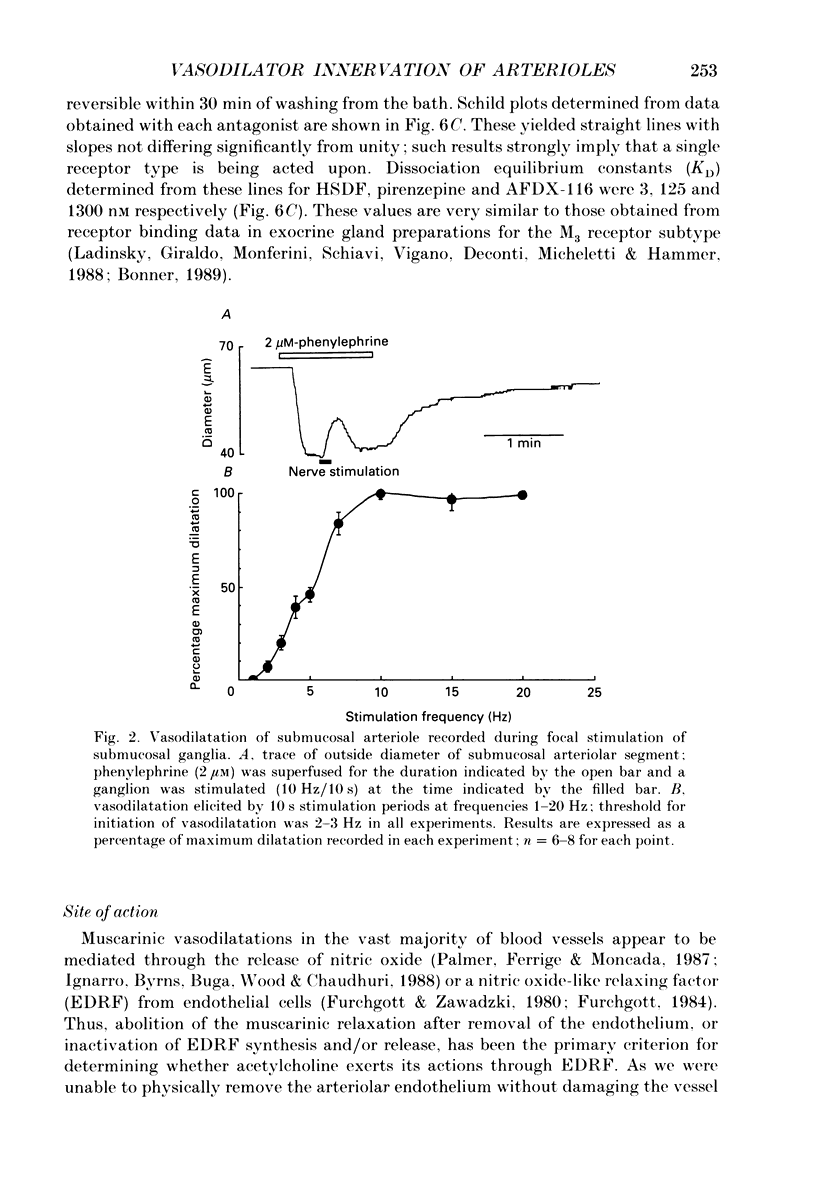
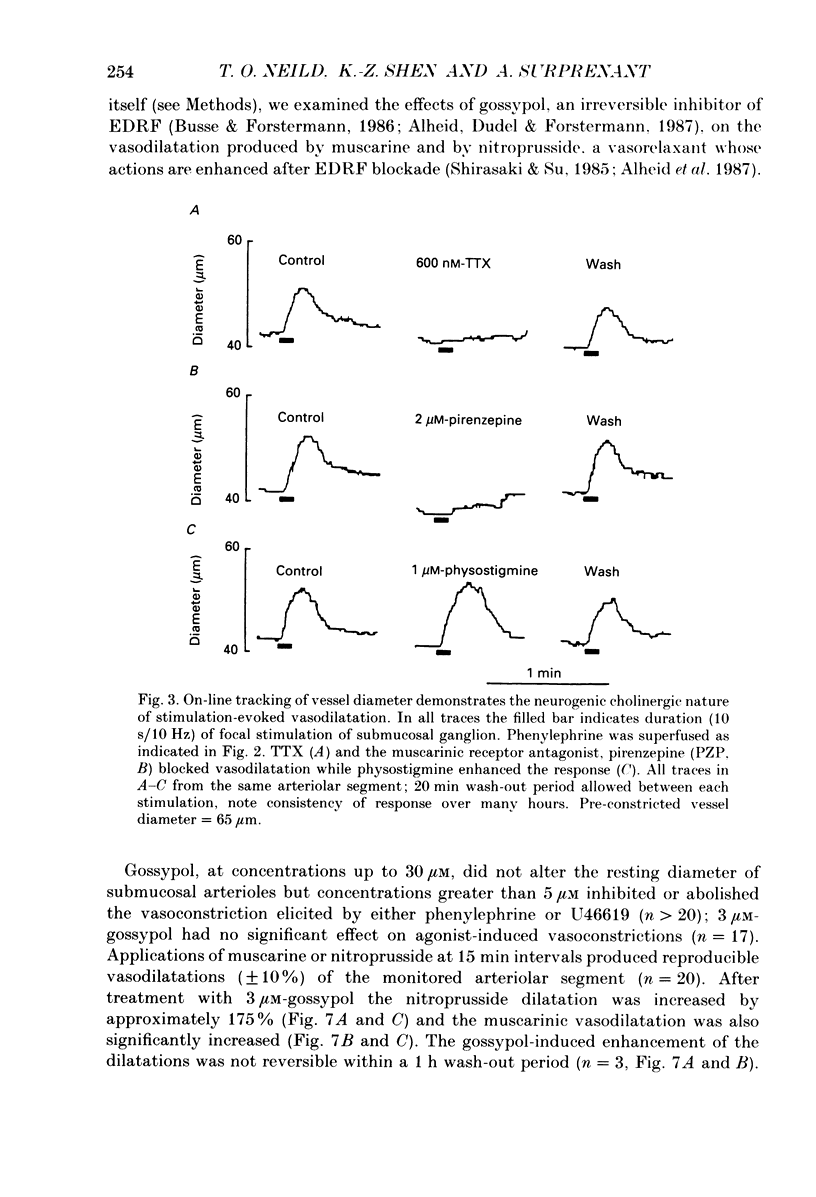
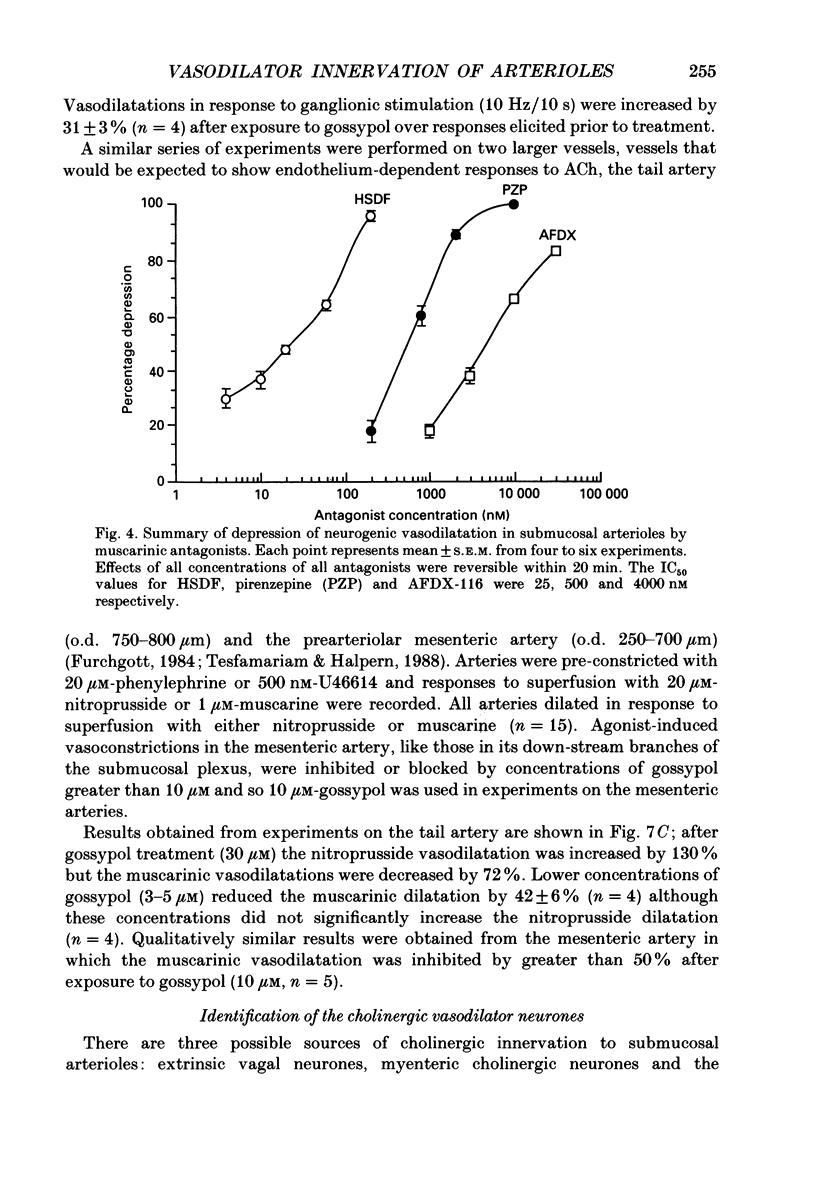
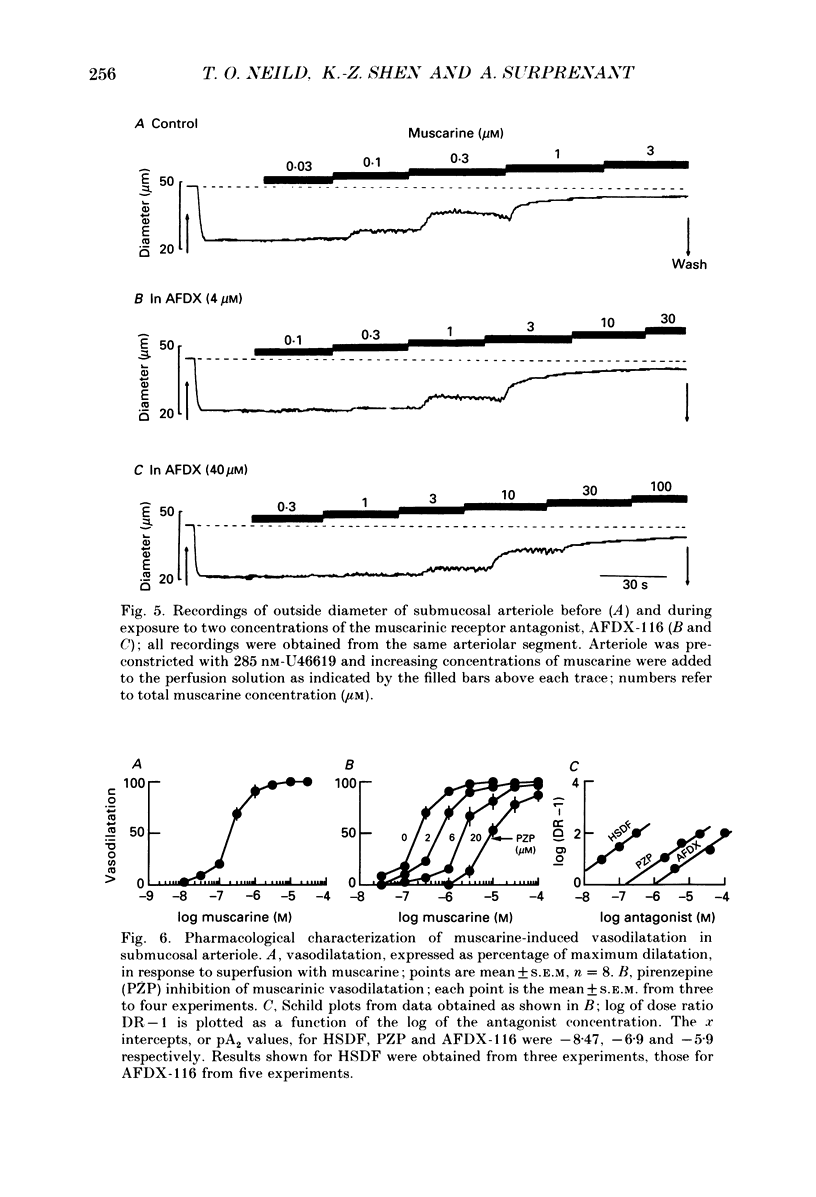
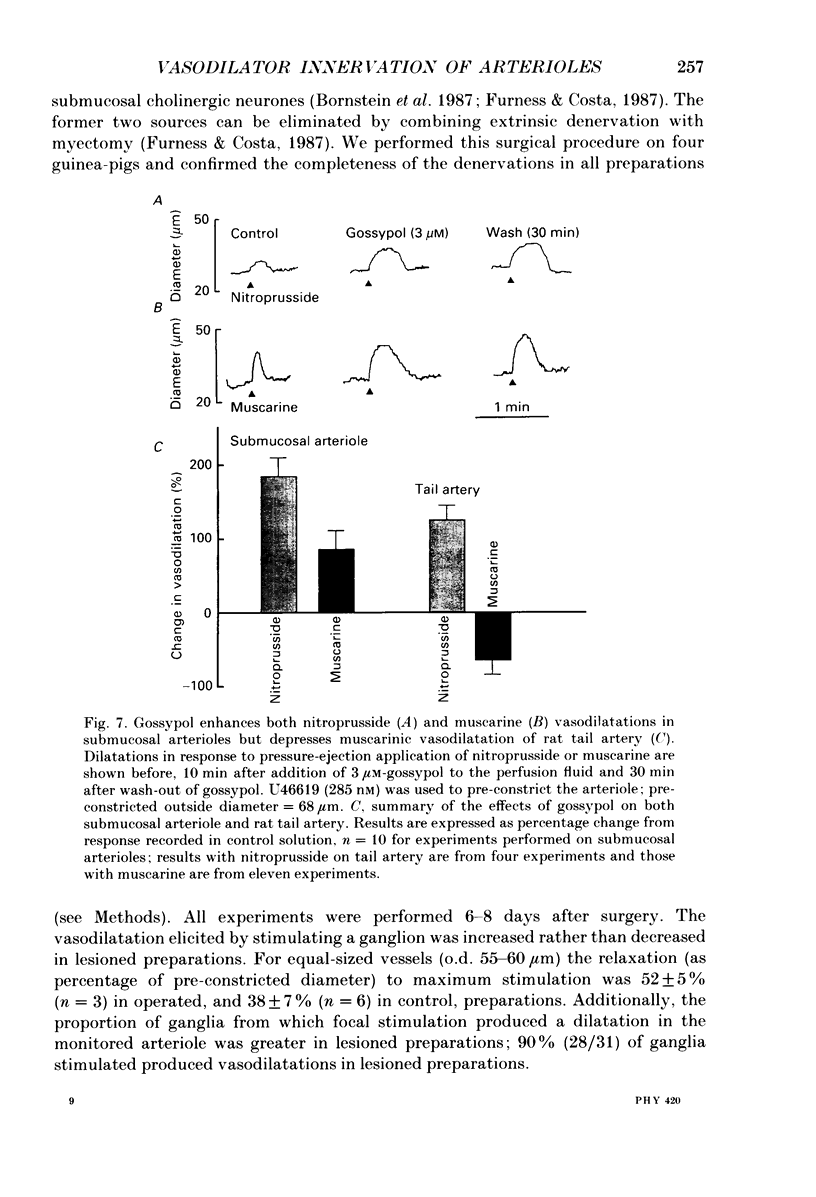
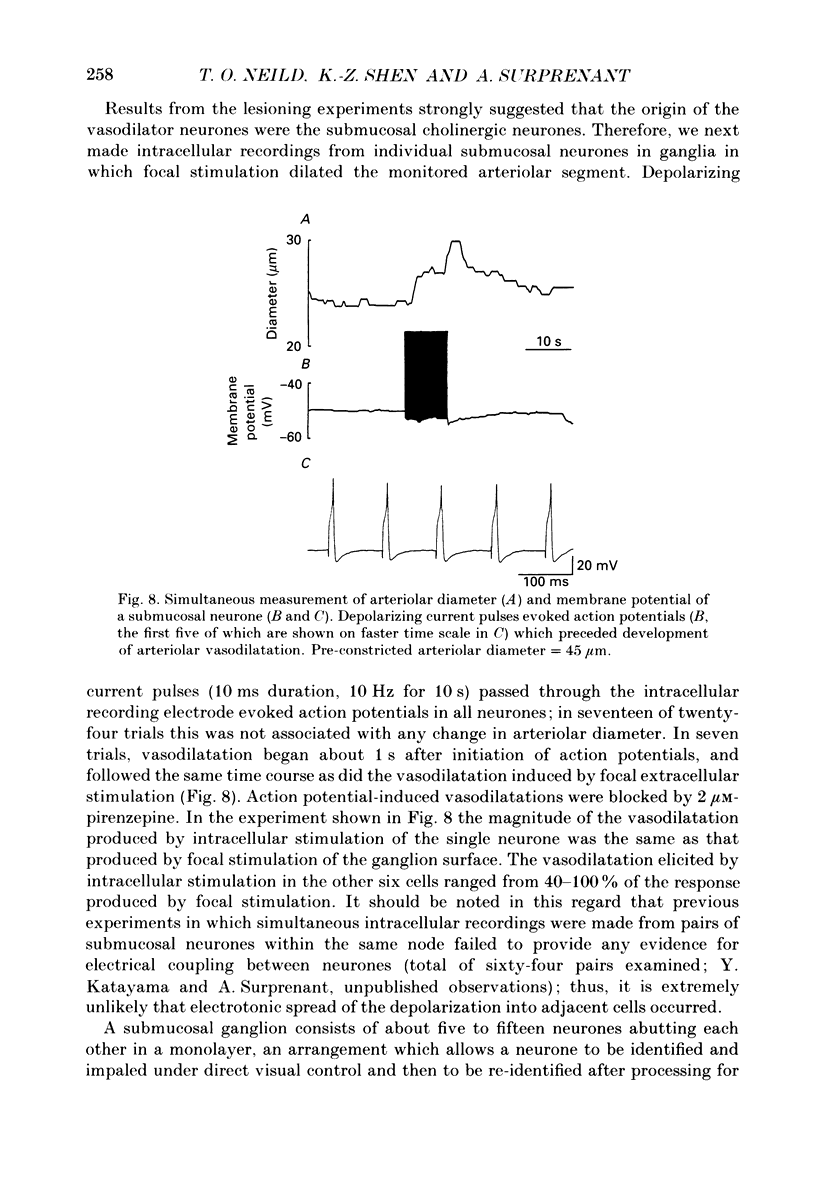
The nervous control of arterioles in the guinea-pig submucosal plexus was studied. Outside diameters of arterioles were recorded using a video-monitoring system. Changes in arteriolar diameter in response to electrical stimulation of single neurones or ganglia in the plexus were measured. 2. When the arteriole was pre-constricted with the prostaglandin analogue U46619 or with phenylephrine, electrical stimulation (2-20 Hz, 10 s) of a ganglion dilated the blood vessel. This vasodilatation was abolished by tetrodotoxin or by cutting the fine nerve strands running between the ganglion and the arteriole. 3. The vasodilatations caused by ganglionic stimulation were blocked by the muscarinic antagonists atropine, pirenzepine, (11[[2-[(diethylamino)methyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H- pyrido[2,3-b][1,4]benzodiazepine-6-)-one (AFDX-116), 4-diphenylacetoxy-N-methyl-piperidine methiodide (4-DAMP) and hexahydrosilodifenidol (HSDF). IC50 values for the inhibition of nerve-evoked vasodilatation by pirenzepine, AFDX-116 and HSDF were 500 nM, 4 microM and 25 nM respectively. Physostigmine (1 microM) increased the dilatation by 90%. 4. Muscarine dilated all submucosal arterioles; the concentration causing half-maximum effects was 200 nM. Muscarinic vasodilatations were inhibited by pirenzepine, AFDX-116, and HSDF in a competitive manner; dissociation equilibrium constants determined by Schild analyses were 125 nM, 1.3 microM and 4 nM respectively. 5. Gossypol, an irreversible inhibitor of the production of endothelium-derived relaxing factor (EDRF), did not reduce the vasodilatation produced by either ganglionic stimulation or muscarine in submucosal arterioles. 6. Intracellular recordings were made from submucosal neurones and action potentials were elicited by depolarizing current pulses (10 ms duration, 10 Hz/10 s). In seven neurones vasodilatation was associated with intracellularly evoked action potentials; this vasodilatation was blocked by pirenzepine. Cell bodies of reidentified vasodilator neurones were subsequently shown to contain immunoreactive choline acetyltransferase. 7. These results show that cholinergic neurones in the submucosal plexus project to submucosal arterioles and that they release acetylcholine onto muscarinic receptors to produce vasodilatation. The muscarinic receptor activated by nerve-released acetylcholine is the M3 subtype and its location appears to be on the vascular smooth muscle rather than the endothelium.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid U., Dudel C., Förstermann U. Selective inhibition by gossypol of endothelium-dependent relaxations augments relaxations to glyceryl trinitrate in rabbit coeliac artery. Br J Pharmacol. 1987 Sep;92(1):237–240. doi: 10.1111/j.1476-5381.1987.tb11317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus J. A., Campbell G. R., Cocks T. M., Manderson J. A. Vasodilatation by acetylcholine is endothelium-dependent: a study by sonomicrometry in canine femoral artery in vivo. J Physiol. 1983 Nov;344:209–222. doi: 10.1113/jphysiol.1983.sp014934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C. Transmission from vasoconstrictor and vasodilator nerves to single smooth muscle cells of the guinea-pig uterine artery. J Physiol. 1969 Dec;205(3):695–708. doi: 10.1113/jphysiol.1969.sp008991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan J. A., Brayden J. E. Nonadrenergic neural vasodilator mechanisms. Circ Res. 1987 Mar;60(3):309–326. doi: 10.1161/01.res.60.3.309. [DOI] [PubMed] [Google Scholar]
- Bevan J. A., Buga G. M., Jope C. A., Jope R. S., Moritoki H. Further evidence for a muscarinic component to the neural vasodilator innervation of cerebral and cranial extracerebral arteries of the cat. Circ Res. 1982 Oct;51(4):421–429. doi: 10.1161/01.res.51.4.421. [DOI] [PubMed] [Google Scholar]
- Biber B., Fara J., Lundgren O. Intestinal vasodilatation in response to transmural electrical field stimulation. Acta Physiol Scand. 1973 Feb;87(2):277–282. doi: 10.1111/j.1748-1716.1973.tb05391.x. [DOI] [PubMed] [Google Scholar]
- Biber B., Lundgren O., Svanvik J. Studies on the intestinal vasodilatation observed after mechanical stimulation of the mucosa of the gut. Acta Physiol Scand. 1971 Jun;82(2):177–190. doi: 10.1111/j.1748-1716.1971.tb04957.x. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Bonner T. I. The molecular basis of muscarinic receptor diversity. Trends Neurosci. 1989 Apr;12(4):148–151. doi: 10.1016/0166-2236(89)90054-4. [DOI] [PubMed] [Google Scholar]
- Bornstein J. C., Furness J. B., Costa M. Sources of excitatory synaptic inputs to neurochemically identified submucous neurons of guinea-pig small intestine. J Auton Nerv Syst. 1987 Jan;18(1):83–91. doi: 10.1016/0165-1838(87)90137-8. [DOI] [PubMed] [Google Scholar]
- Brayden J. E., Large W. A. Electrophysiological analysis of neurogenic vasodilatation in the isolated lingual artery of the rabbit. Br J Pharmacol. 1986 Sep;89(1):163–171. doi: 10.1111/j.1476-5381.1986.tb11132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Burn J. H. Sympathetic vaso-dilatation in the skin and the intestine of the dog. J Physiol. 1936 Aug 19;87(3):254–274. doi: 10.1113/jphysiol.1936.sp003404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. A., Shepherd J. T., Vanhoutte P. M. Neurogenic cholinergic prejunctional inhibition of sympathetic beta adrenergic relaxation in the canine coronary artery. J Pharmacol Exp Ther. 1984 May;229(2):417–421. [PubMed] [Google Scholar]
- Cooke H. J. Neurobiology of the intestinal mucosa. Gastroenterology. 1986 Apr;90(4):1057–1081. doi: 10.1016/0016-5085(86)90889-9. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B. The origins, pathways and terminations of neurons with VIP-like immunoreactivity in the guinea-pig small intestine. Neuroscience. 1983 Apr;8(4):665–676. doi: 10.1016/0306-4522(83)90002-7. [DOI] [PubMed] [Google Scholar]
- Dale H. H. On the action of ergotoxine; with special reference to the existence of sympathetic vasodilators. J Physiol. 1913 Jun 19;46(3):291–300. doi: 10.1113/jphysiol.1913.sp001592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckles S. P. Evidence for a functional cholinergic innervation of cerebral arteries. J Pharmacol Exp Ther. 1981 Jun;217(3):544–548. [PubMed] [Google Scholar]
- Eckenstein F. P., Baughman R. W., Quinn J. An anatomical study of cholinergic innervation in rat cerebral cortex. Neuroscience. 1988 May;25(2):457–474. doi: 10.1016/0306-4522(88)90251-5. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Carvalho M. H., Khan M. T., Matsunaga K. Evidence for endothelium-dependent vasodilation of resistance vessels by acetylcholine. Blood Vessels. 1987;24(3):145–149. doi: 10.1159/000158689. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M. Distribution of intrinsic nerve cell bodies and axons which take up aromatic amines and their precursors in the small intestine of the guinea-pig. Cell Tissue Res. 1978 Apr 28;188(3):527–543. doi: 10.1007/BF00219790. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Eckenstein F. Neurones localized with antibodies against choline acetyltransferase in the enteric nervous system. Neurosci Lett. 1983 Sep 30;40(2):105–109. doi: 10.1016/0304-3940(83)90287-2. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Keast J. R. Choline acetyltransferase- and peptide immunoreactivity of submucous neurons in the small intestine of the guinea-pig. Cell Tissue Res. 1984;237(2):329–336. doi: 10.1007/BF00217152. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M. The use of glyoxylic acid for the fluorescence histochemical demonstration of peripheral stores of noradrenaline and 5-hydroxytryptamine in whole mounts. Histochemistry. 1975;41(4):335–352. doi: 10.1007/BF00490076. [DOI] [PubMed] [Google Scholar]
- Galligan J. J., Costa M., Furness J. B. Changes in surviving nerve fibers associated with submucosal arteries following extrinsic denervation of the small intestine. Cell Tissue Res. 1988 Sep;253(3):647–656. doi: 10.1007/BF00219756. [DOI] [PubMed] [Google Scholar]
- Galligan J. J., Jiang M. M., Shen K. Z., Surprenant A. Substance P mediates neurogenic vasodilatation in extrinsically denervated guinea-pig submucosal arterioles. J Physiol. 1990 Jan;420:267–280. doi: 10.1113/jphysiol.1990.sp017911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel K. A. Intestinal nerves and ion transport: stimuli, reflexes, and responses. Am J Physiol. 1985 Mar;248(3 Pt 1):G261–G271. doi: 10.1152/ajpgi.1985.248.3.G261. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S., Chaudhuri G. Pharmacological evidence that endothelium-derived relaxing factor is nitric oxide: use of pyrogallol and superoxide dismutase to study endothelium-dependent and nitric oxide-elicited vascular smooth muscle relaxation. J Pharmacol Exp Ther. 1988 Jan;244(1):181–189. [PubMed] [Google Scholar]
- Iwayama T., Furness J. B., Burnstock G. Dual adrenergic and cholinergic innervation of the cerebral arteries of the rat. An ultrastructural study. Circ Res. 1970 May;26(5):635–646. doi: 10.1161/01.res.26.5.635. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Anggård A., Fahrenkrug J., Hökfelt T., Mutt V. Vasoactive intestinal polypeptide in cholinergic neurons of exocrine glands: functional significance of coexisting transmitters for vasodilation and secretion. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1651–1655. doi: 10.1073/pnas.77.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M. Evidence for coexistence of vasoactive intestinal polypeptide (VIP) and acetylcholine in neurons of cat exocrine glands. Morphological, biochemical and functional studies. Acta Physiol Scand Suppl. 1981;496:1–57. [PubMed] [Google Scholar]
- Neild T. O. Actions of neuropeptide Y on innervated and denervated rat tail arteries. J Physiol. 1987 May;386:19–30. doi: 10.1113/jphysiol.1987.sp016519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild T. O. Measurement of arteriole diameter changes by analysis of television images. Blood Vessels. 1989;26(1):48–52. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Peralta E. G., Winslow J. W., Ashkenazi A., Smith D. H., Ramachandran J., Capon D. J. Structural basis of muscarinic acetylcholine receptor subtype diversity. Trends Pharmacol Sci. 1988 Feb;Suppl:6–11. [PubMed] [Google Scholar]
- Peralta E. G., Winslow J. W., Peterson G. L., Smith D. H., Ashkenazi A., Ramachandran J., Schimerlik M. I., Capon D. J. Primary structure and biochemical properties of an M2 muscarinic receptor. Science. 1987 May 1;236(4801):600–605. doi: 10.1126/science.3107123. [DOI] [PubMed] [Google Scholar]
- Samata K., Kimura T., Satoh S., Watanabe H. Chemical removal of the endothelium by saponin in the isolated dog femoral artery. Eur J Pharmacol. 1986 Aug 22;128(1-2):85–91. doi: 10.1016/0014-2999(86)90561-3. [DOI] [PubMed] [Google Scholar]
- Shirasaki Y., Su C. Endothelium removal augments vasodilation by sodium nitroprusside and sodium nitrite. Eur J Pharmacol. 1985 Aug 7;114(1):93–96. doi: 10.1016/0014-2999(85)90527-8. [DOI] [PubMed] [Google Scholar]
- Surprenant A. Slow excitatory synaptic potentials recorded from neurones of guinea-pig submucous plexus. J Physiol. 1984 Jun;351:343–361. doi: 10.1113/jphysiol.1984.sp015249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A., Williams J. T. Inhibitory synaptic potentials recorded from mammalian neurones prolonged by blockade of noradrenaline uptake. J Physiol. 1987 Jan;382:87–103. doi: 10.1113/jphysiol.1987.sp016357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper E. J. Local modulation of intestinal ion transport by enteric neurons. Am J Physiol. 1983 May;244(5):G457–G468. doi: 10.1152/ajpgi.1983.244.5.G457. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B., Halpern W. Endothelium-dependent and endothelium-independent vasodilation in resistance arteries from hypertensive rats. Hypertension. 1988 May;11(5):440–444. doi: 10.1161/01.hyp.11.5.440. [DOI] [PubMed] [Google Scholar]