Abstract
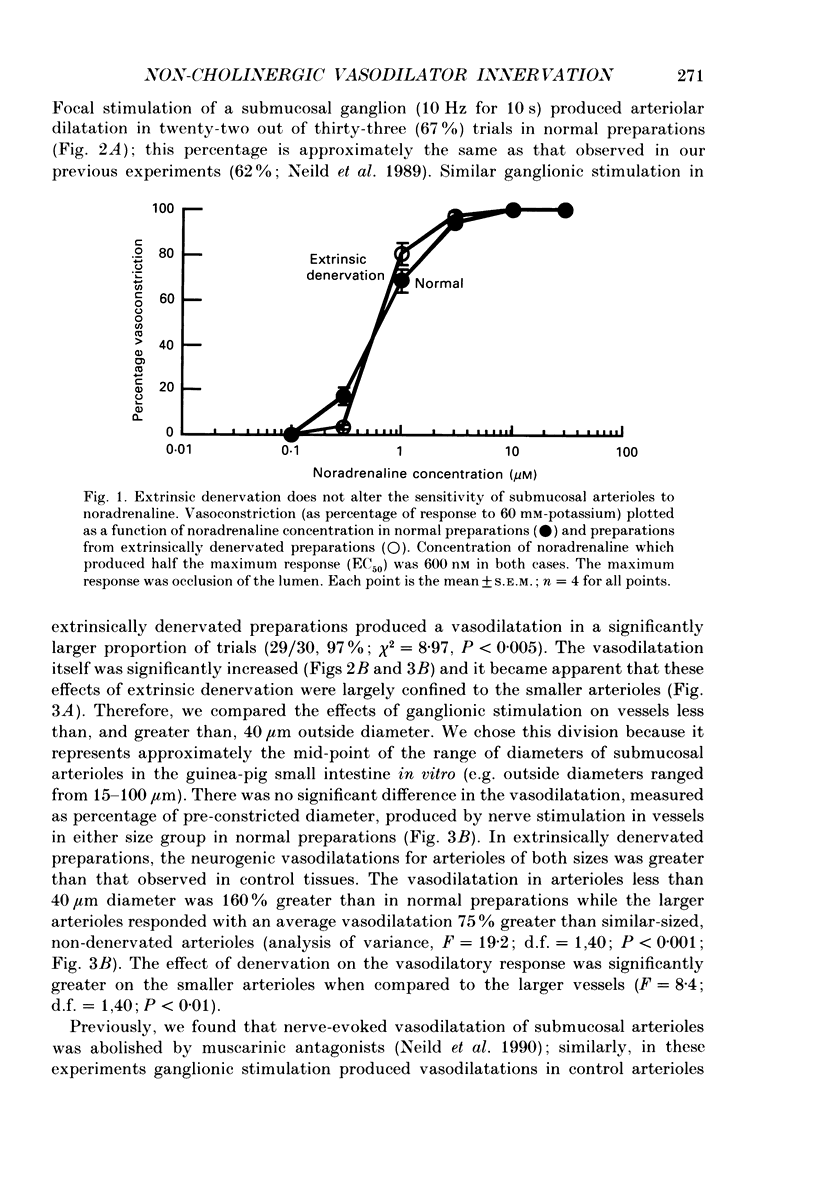
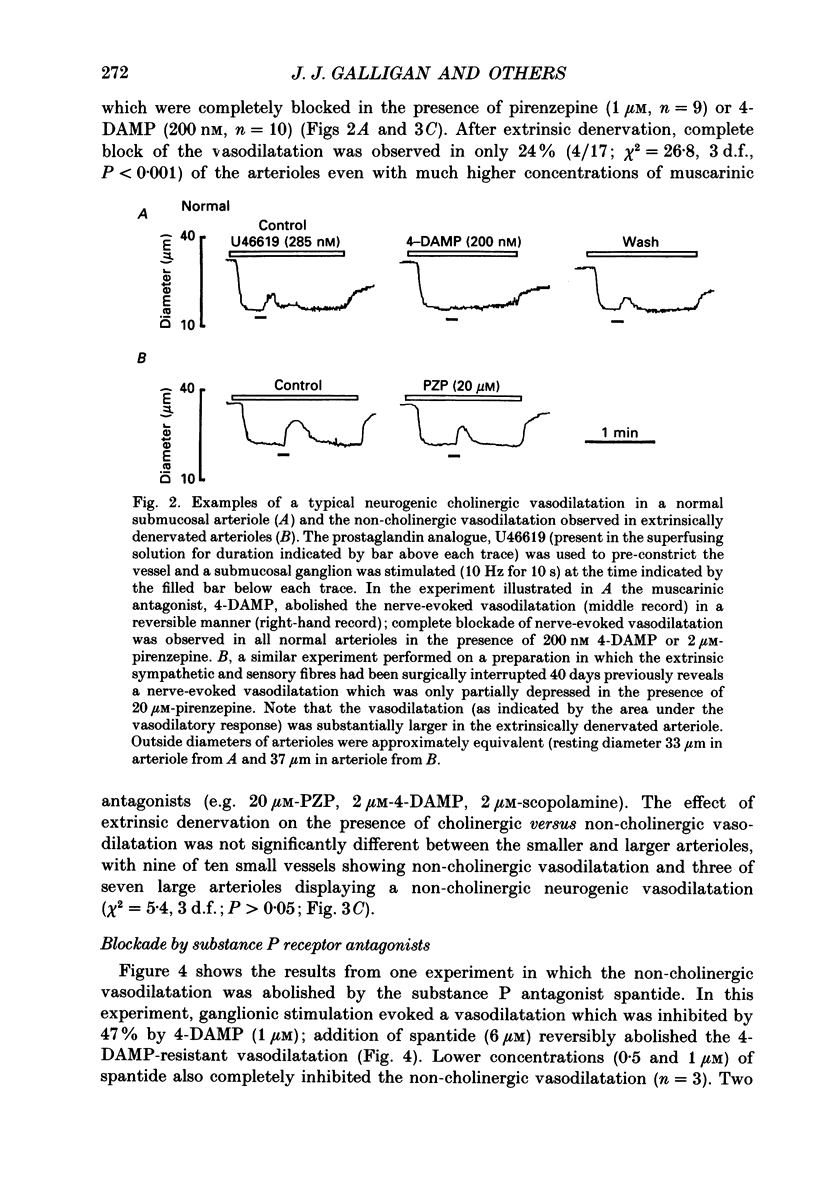
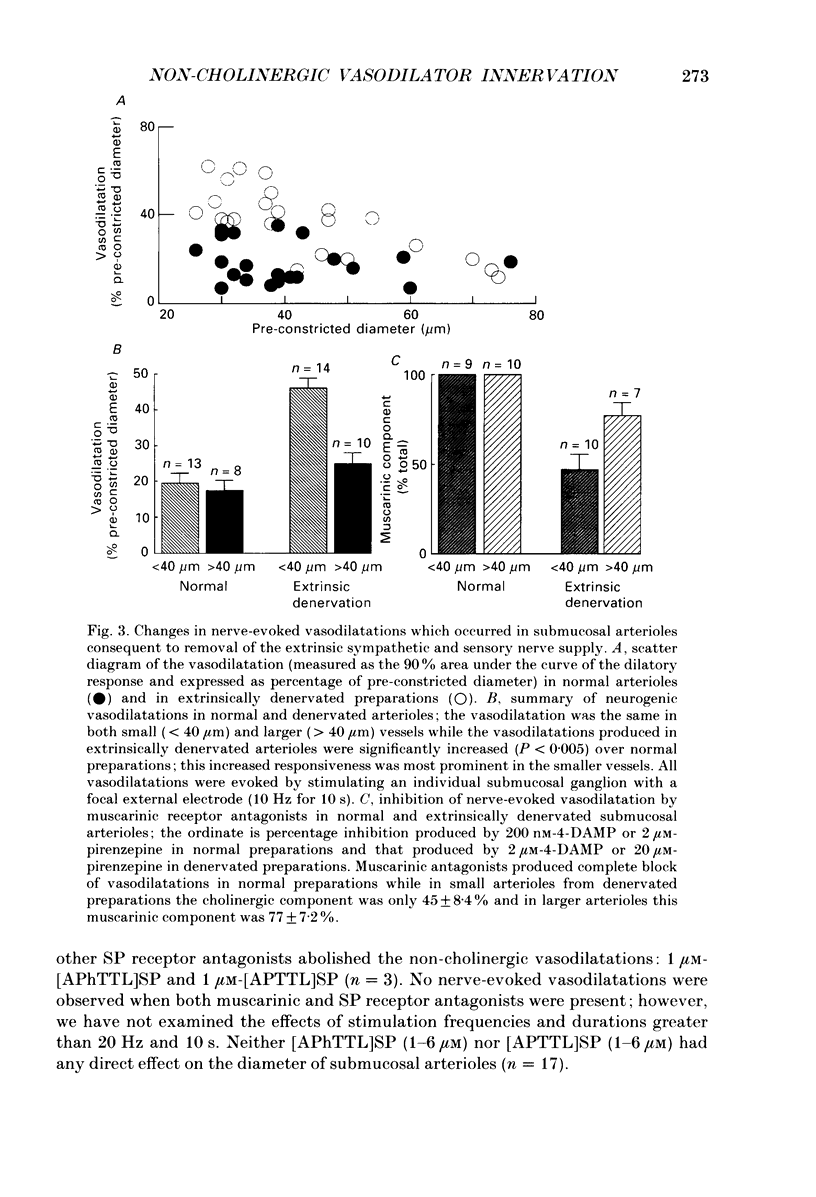
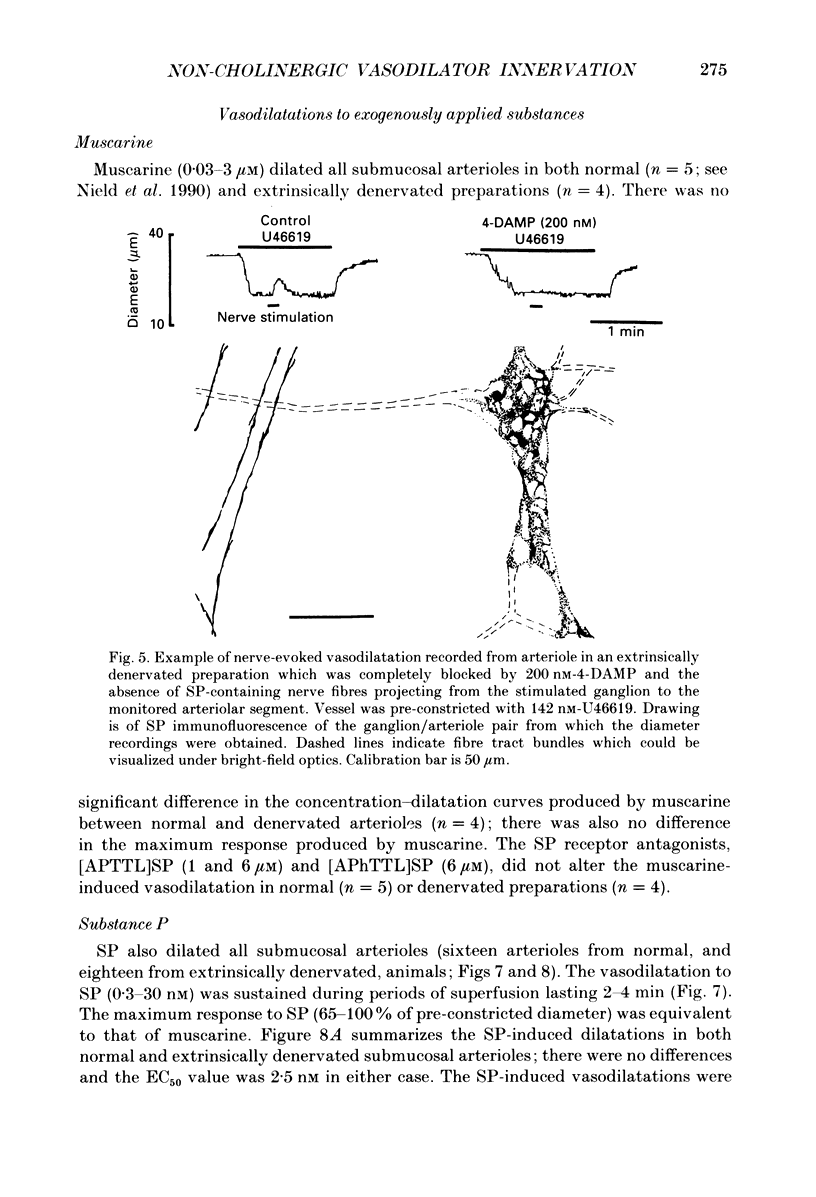
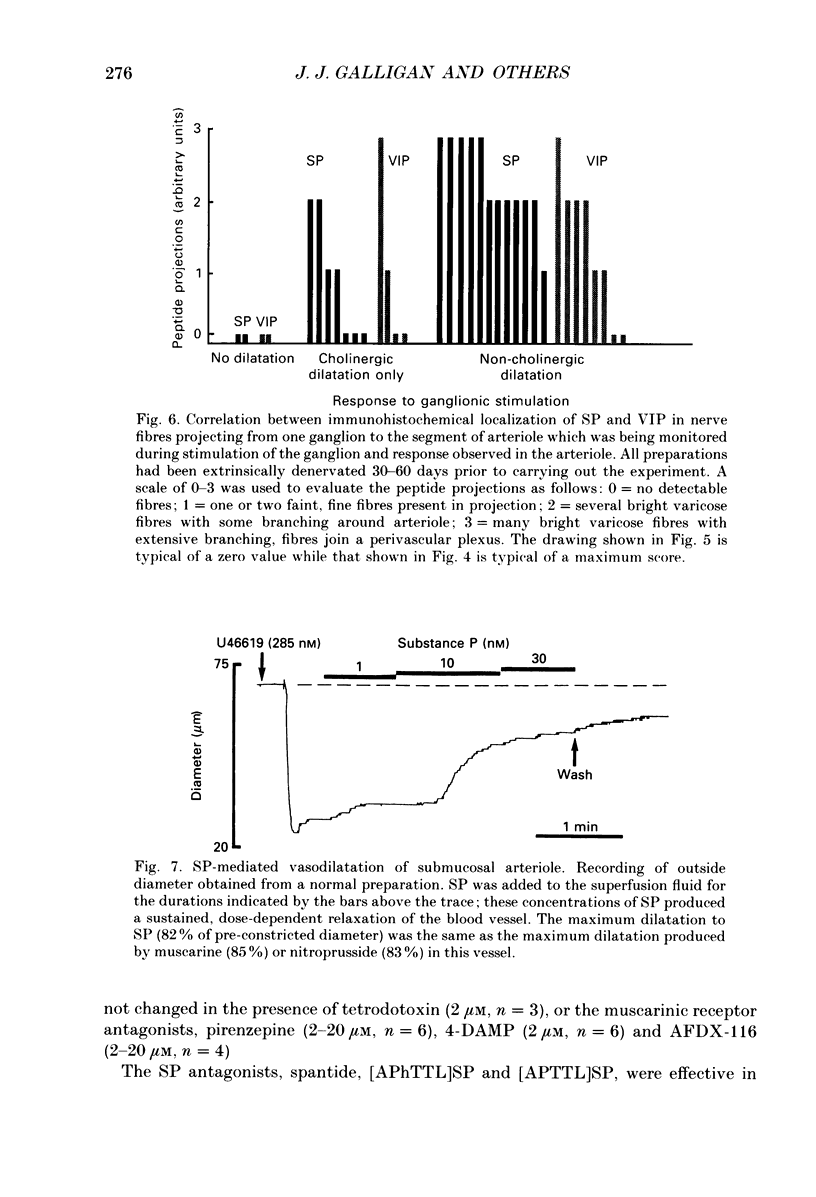
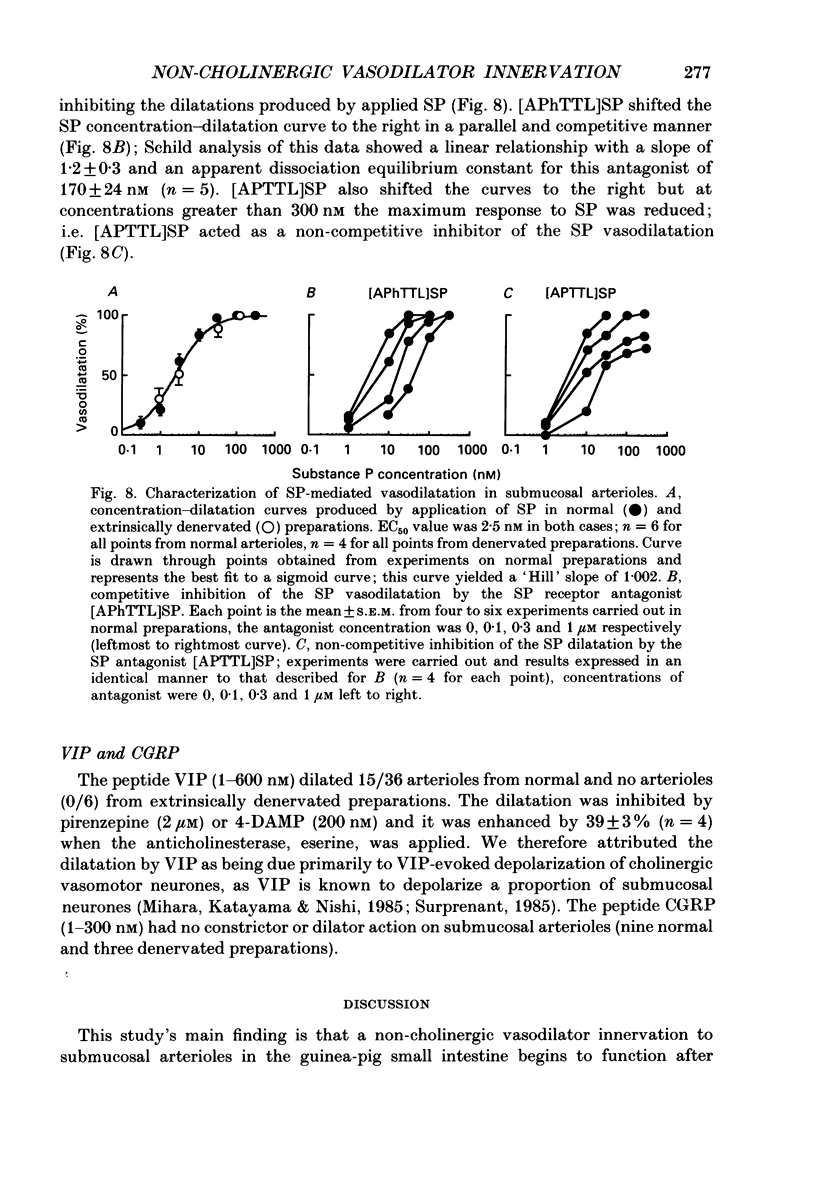
1. Arteriolar diameter was measured using an optical method in preparations of guinea-pig submucosal plexus in vitro. Electrical stimulation of one or more neurones in ganglia of the submucosal plexus causes a cholinergic vasodilatation in normal animals. The vasomotor innervation to the arterioles was studied in guinea-pigs in which the extrinsic nerves to the intestine had been removed. Tissues were processed for immunohistochemistry after the in vitro experiments. 2. Extrinsic denervation resulted in complete loss of catecholamine fluorescence, NPY (neuropeptide Y) and CGRP (calcitonin gene-related peptide) immunofluorescence around the blood vessels and no neurogenic vasoconstriction was observed up to 60 days post-denervation. Vasodilatation in response to ganglionic stimulation was increased; smaller arterioles (outside diameter less than 40 microns) showed a greater enhancement of neurogenic vasodilatation than larger arterioles. 3. Nerve-evoked vasodilatations were only partially inhibited by muscarinic antagonists at 30-60 days after extrinsic denervations. 4. The non-cholinergic neurogenic vasodilatation was abolished by the substance P antagonists, spantide, [D-Arg1, D-Pro2, D-Trp7.9, Leu11]substance P and [D-Arg1, D-Phe5, D-Trp7.9, Leu11]substance P. These antagonists did not alter the cholinergic vasodilatation in normal or extrinsically denervated arterioles. 5. Exogenous substance P dilated all submucosal arterioles; the concentration which produced half-maximal vasodilatations was 2.5 mM in both normal and extrinsically denervated arterioles. Substance P antagonists inhibited the vasodilatation caused by substance P at concentrations similar to those needed to block nerve-mediated vasodilatation. 6. There was a strong correlation between the finding of non-cholinergic vasodilatation in response to ganglionic stimulation, and the presence of substance P-immunoreactive fibres running from ganglion to arteriole. This correlation did not exist for VIP (vasoactive intestinal peptide). 7. These results suggest that intrinsic intestinal substance P-containing nerve fibres supply submucosal arterioles after sympathetic efferents and sensory afferents are removed. Stimulation of these nerves releases substance P to produce arteriolar dilatation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barthó L., Holzer P. Search for a physiological role of substance P in gastrointestinal motility. Neuroscience. 1985 Sep;16(1):1–32. doi: 10.1016/0306-4522(85)90043-0. [DOI] [PubMed] [Google Scholar]
- Bayliss W. M., Starling E. H. The movements and innervation of the small intestine. J Physiol. 1899 May 11;24(2):99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan J. A., Brayden J. E. Nonadrenergic neural vasodilator mechanisms. Circ Res. 1987 Mar;60(3):309–326. doi: 10.1161/01.res.60.3.309. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B. Somatostatin is present in a subpopulation of noradrenergic nerve fibres supplying the intestine. Neuroscience. 1984 Nov;13(3):911–919. doi: 10.1016/0306-4522(84)90105-2. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B. The origins, pathways and terminations of neurons with VIP-like immunoreactivity in the guinea-pig small intestine. Neuroscience. 1983 Apr;8(4):665–676. doi: 10.1016/0306-4522(83)90002-7. [DOI] [PubMed] [Google Scholar]
- Cunnane T. C., Stjärne L. Secretion of transmitter from individual varicosities of guinea-pig and mouse vas deferens: all-or-none and extremely intermittent. Neuroscience. 1982;7(11):2565–2576. doi: 10.1016/0306-4522(82)90085-9. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M. Distribution of intrinsic nerve cell bodies and axons which take up aromatic amines and their precursors in the small intestine of the guinea-pig. Cell Tissue Res. 1978 Apr 28;188(3):527–543. doi: 10.1007/BF00219790. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Keast J. R. Choline acetyltransferase- and peptide immunoreactivity of submucous neurons in the small intestine of the guinea-pig. Cell Tissue Res. 1984;237(2):329–336. doi: 10.1007/BF00217152. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M. The use of glyoxylic acid for the fluorescence histochemical demonstration of peripheral stores of noradrenaline and 5-hydroxytryptamine in whole mounts. Histochemistry. 1975;41(4):335–352. doi: 10.1007/BF00490076. [DOI] [PubMed] [Google Scholar]
- Galligan J. J., Costa M., Furness J. B. Changes in surviving nerve fibers associated with submucosal arteries following extrinsic denervation of the small intestine. Cell Tissue Res. 1988 Sep;253(3):647–656. doi: 10.1007/BF00219756. [DOI] [PubMed] [Google Scholar]
- Gibbins I. L., Furness J. B., Costa M., MacIntyre I., Hillyard C. J., Girgis S. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neurosci Lett. 1985 Jun 12;57(2):125–130. doi: 10.1016/0304-3940(85)90050-3. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Edwards F. R. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989 Apr;69(2):546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Some properties of spontaneous excitatory junction potentials recorded from arterioles of guinea-pigs. J Physiol. 1980 Jun;303:43–60. doi: 10.1113/jphysiol.1980.sp013269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara S., Katayama Y., Nishi S. Slow postsynaptic potentials in neurones of submucous plexus of guinea-pig caecum and their mimicry by noradrenaline and various peptides. Neuroscience. 1985 Dec;16(4):1057–1068. doi: 10.1016/0306-4522(85)90116-2. [DOI] [PubMed] [Google Scholar]
- Neild T. O. Measurement of arteriole diameter changes by analysis of television images. Blood Vessels. 1989;26(1):48–52. [PubMed] [Google Scholar]
- Neild T. O., Shen K. Z., Surprenant A. Vasodilatation of arterioles by acetylcholine released from single neurones in the guinea-pig submucosal plexus. J Physiol. 1990 Jan;420:247–265. doi: 10.1113/jphysiol.1990.sp017910. [DOI] [PMC free article] [PubMed] [Google Scholar]


