Abstract
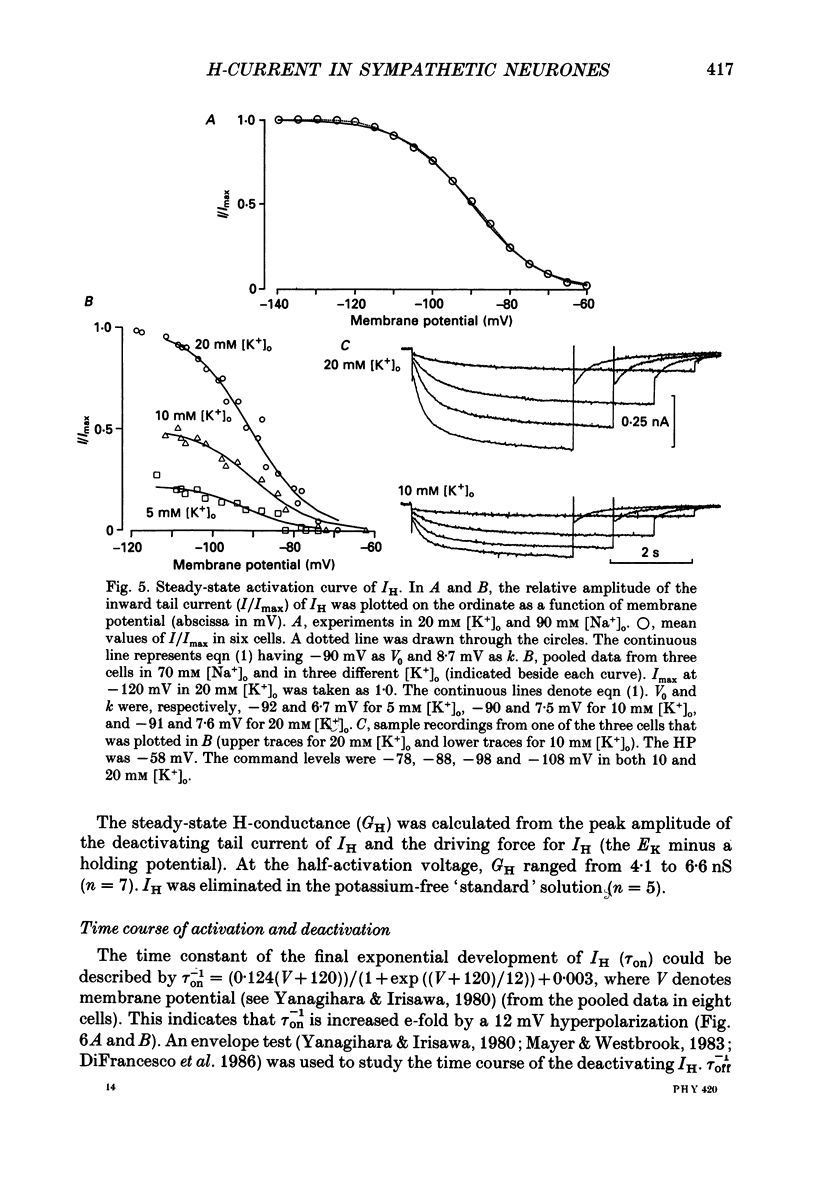
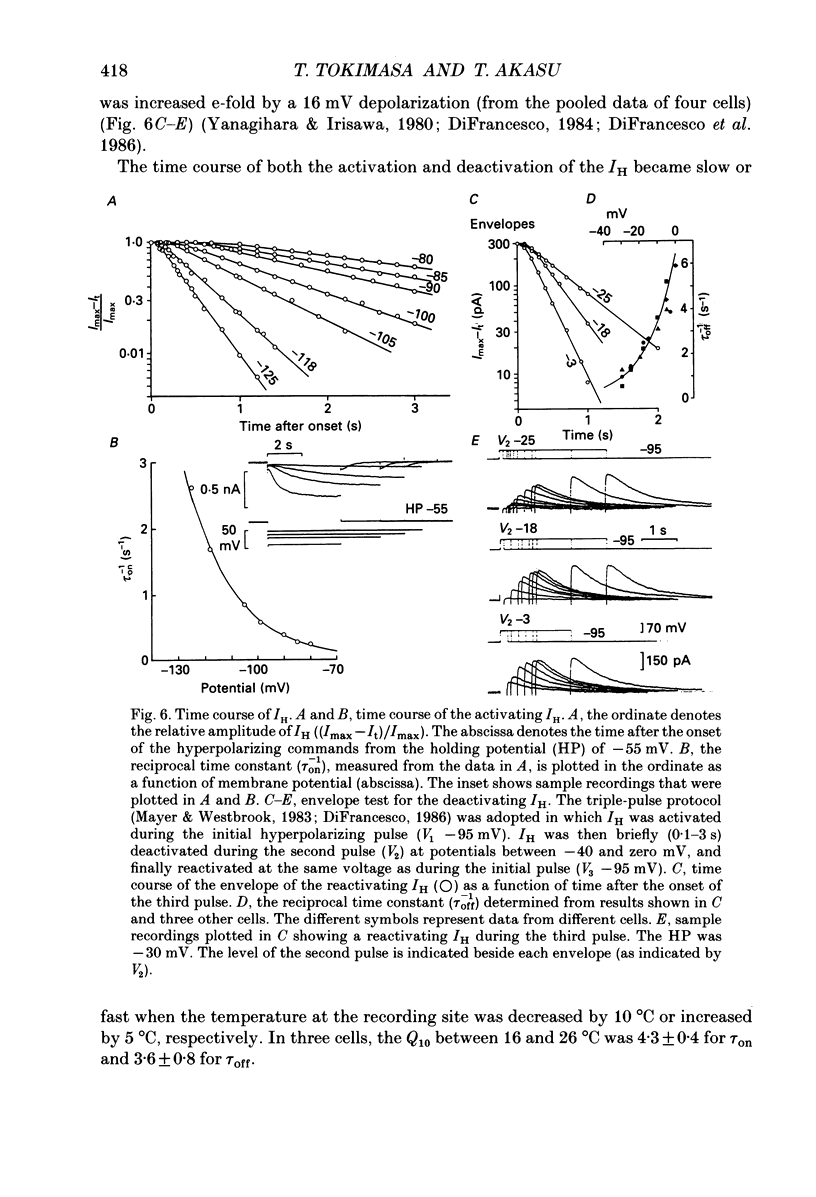
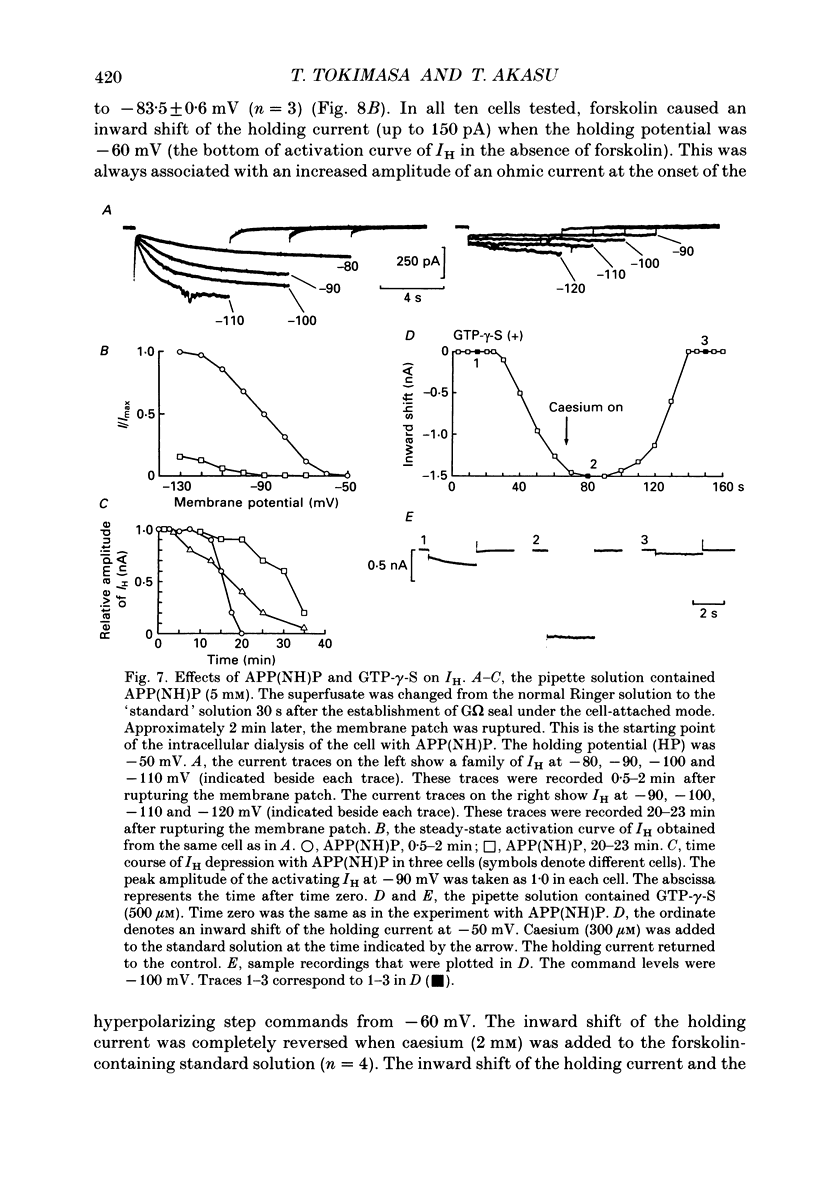
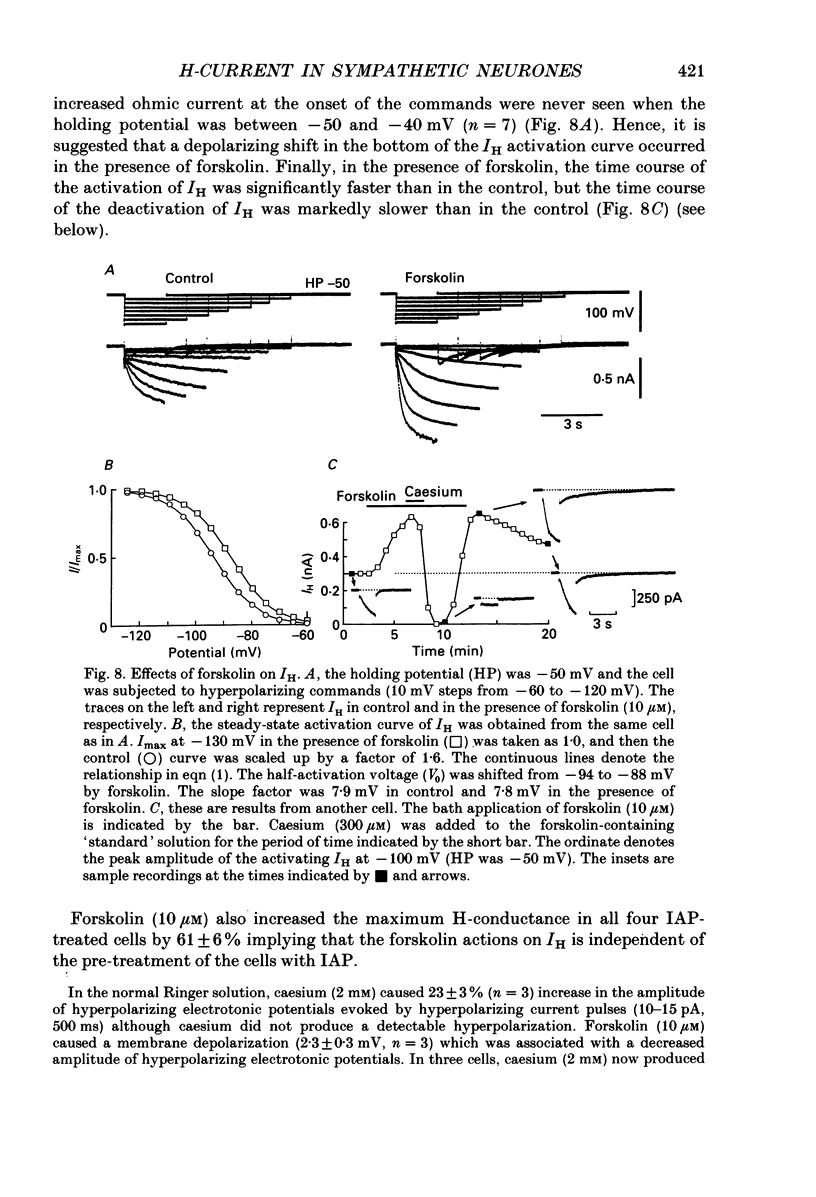
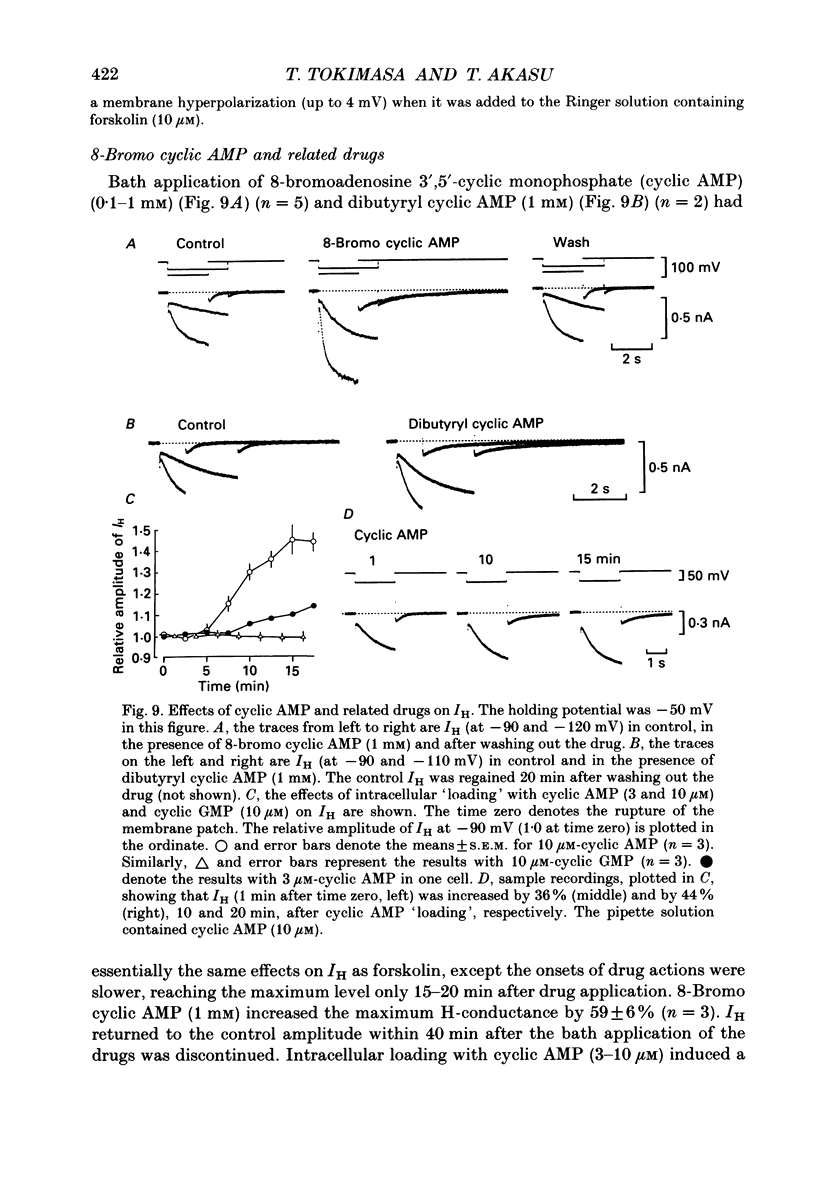
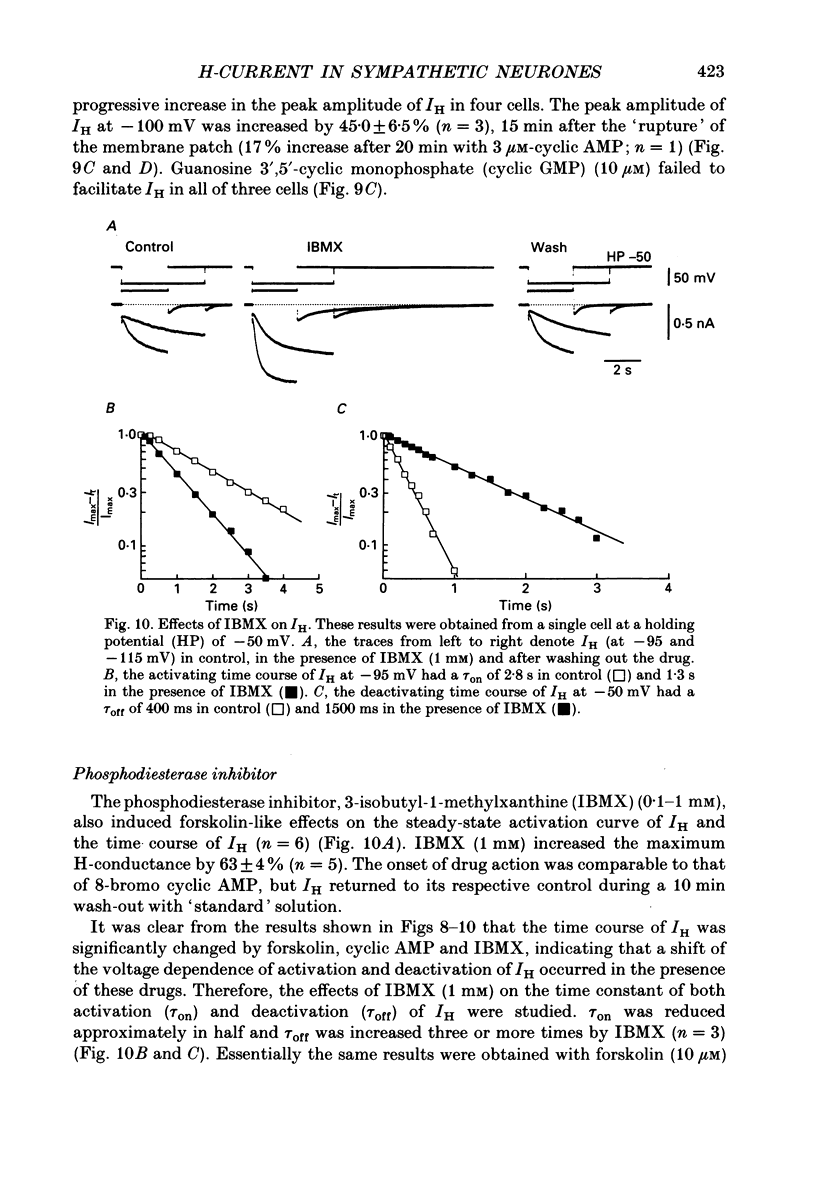
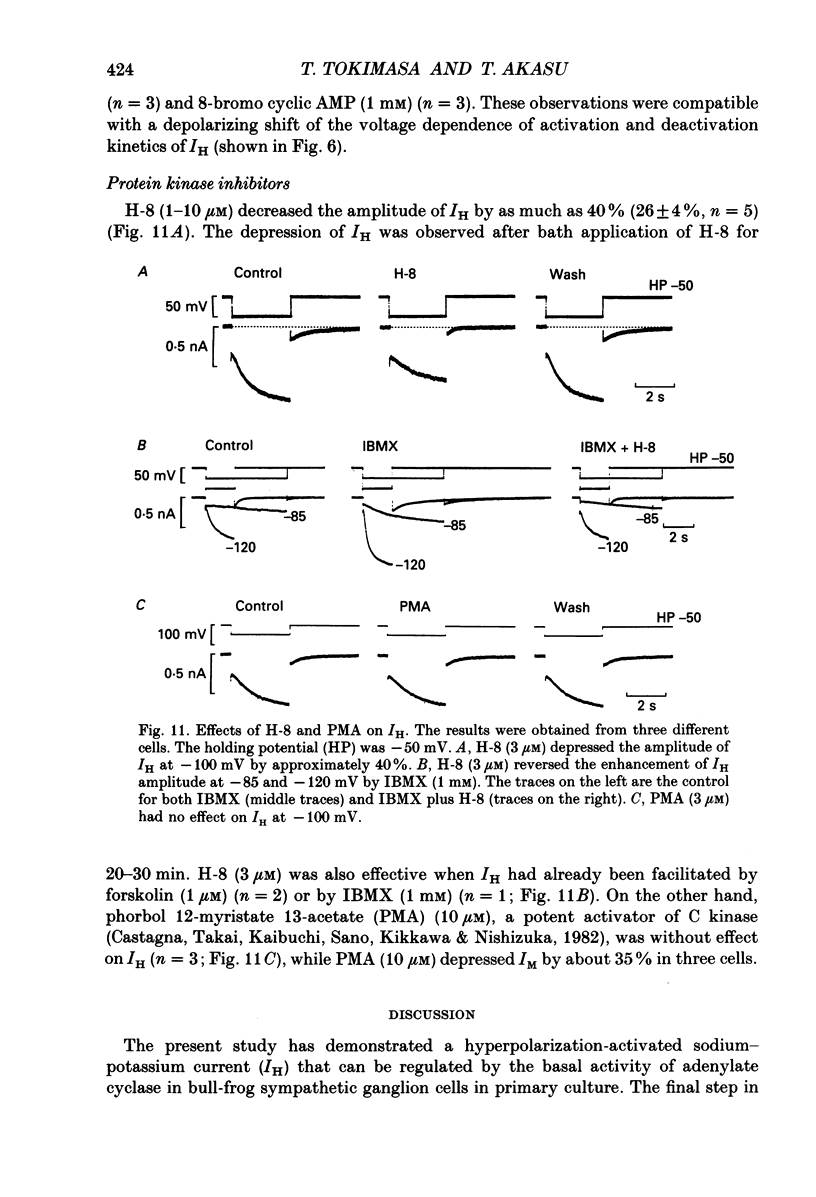
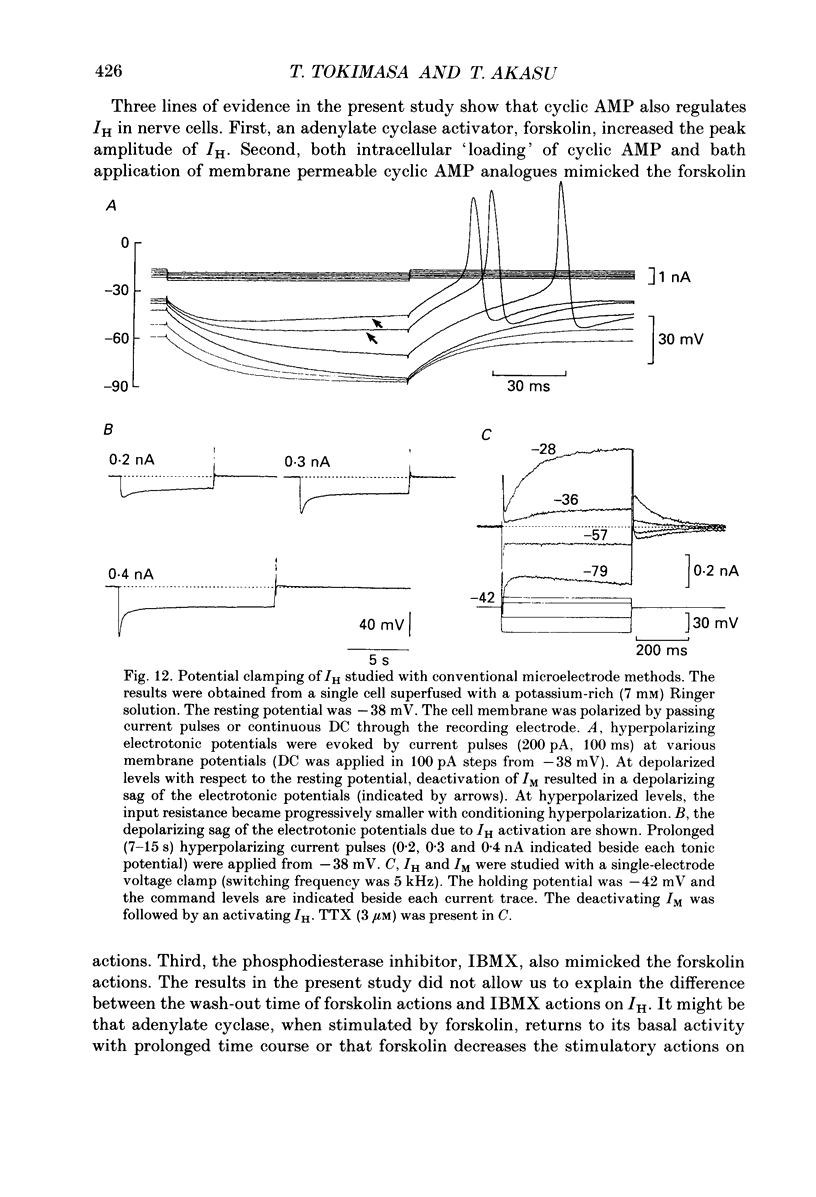
1. Bull-frog sympathetic neurones in primary culture were voltage clamped in the whole-cell configuration. The pipette solution contained ATP (5 mM). 2. A hyperpolarization-activated sodium-potassium current (H-current: IH) was separated from other membrane currents in a nominally calcium-free solution containing cobalt (2 mM), magnesium (4 mM), barium (2 mM), tetraethylammonium (20 mM), tetrodotoxin (3 microM), apamin (30 nM) and 4-aminopyridine (1 mM). IH was selectively blocked by caesium (10-300 microM). 3. The steady-state activation of IH occurred between -60 and -130 mV. The H-conductance was 4.1-6.6 nS at the half-activation voltage of -90 mV. With the concentrations of potassium and sodium ions in the superfusate at 20 and 70 mM, respectively, the reversal potential of IH was about -20 mV. IH was activated with a time constant of 2.8 s at -90 mV and 22 degrees C. The Q10 between 16 and 26 degrees C was 4.3. 4. A non-hydrolysable ATP analogue in the pipette solution did not support IH activation. Intracellular 'loading' of GTP-gamma-S (30-500 microM) led to a progressive activation of IH. 5. Forskolin (10 microM) increased the maximum conductance of IH by 70%. This was associated with a depolarizing shift in the half-activation voltage (5-10 mV) and in the voltage dependence of the activation/deactivation time constant of IH. 6. Essentially the same results as with forskolin were obtained by intracellular 'loading' with cyclic AMP (3-10 microM) or bath application of 8-bromo cyclic AMP (0.1-1 mM), dibutyryl cyclic AMP (1 mM) and 3-isobutyl-1-methylxanthine (0.1-1 mM). 7. The protein kinase inhibitor H-8 (1-10 microM) decreased the peak amplitude of IH. Phorbol 12-myristate 13-acetate (10 microM), a protein kinase C activator, was without effect. 8. It is concluded that a voltage-dependent cation current can be regulated by the basal activity of adenylate cyclase, presumably through protein kinase A, in vertebrate sympathetic neurones.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T. Adrenaline depolarization in paravertebral sympathetic neurones of bullfrogs. Pflugers Arch. 1988 Jan;411(1):80–87. doi: 10.1007/BF00581650. [DOI] [PubMed] [Google Scholar]
- Akasu T. Adrenaline inhibits muscarinic transmission in bullfrog sympathetic ganglia. Pflugers Arch. 1989 Apr;413(6):616–621. doi: 10.1007/BF00581811. [DOI] [PubMed] [Google Scholar]
- Akasu T., Hirai K., Koketsu K. Modulatory actions of ATP on membrane potentials of bullfrog sympathetic ganglion cells. Brain Res. 1983 Jan 10;258(2):313–317. doi: 10.1016/0006-8993(83)91157-5. [DOI] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N., Crill W. E. Voltage-sensitive outward currents in cat motoneurones. J Physiol. 1980 Jul;304:251–276. doi: 10.1113/jphysiol.1980.sp013323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Denbigh J. S., Lang R. J. Inward rectification in freshly isolated single smooth muscle cells of the rabbit jejunum. J Physiol. 1987 Feb;383:461–476. doi: 10.1113/jphysiol.1987.sp016421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. M-currents: an update. Trends Neurosci. 1988 Jul;11(7):294–299. doi: 10.1016/0166-2236(88)90089-6. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Crepel F., Penit-Soria J. Inward rectification and low threshold calcium conductance in rat cerebellar Purkinje cells. An in vitro study. J Physiol. 1986 Mar;372:1–23. doi: 10.1113/jphysiol.1986.sp015993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A new interpretation of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:359–376. doi: 10.1113/jphysiol.1981.sp013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. Characterization of the pace-maker current kinetics in calf Purkinje fibres. J Physiol. 1984 Mar;348:341–367. doi: 10.1113/jphysiol.1984.sp015114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Acetylcholine inhibits activation of the cardiac hyperpolarizing-activated current, if. Pflugers Arch. 1987 Sep;410(1-2):139–142. doi: 10.1007/BF00581906. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Inhibition of the hyperpolarization-activated current (if) induced by acetylcholine in rabbit sino-atrial node myocytes. J Physiol. 1988 Nov;405:477–491. doi: 10.1113/jphysiol.1988.sp017343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Muscarinic control of the hyperpolarization-activated current (if) in rabbit sino-atrial node myocytes. J Physiol. 1988 Nov;405:493–510. doi: 10.1113/jphysiol.1988.sp017344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J., Horn J. P. A reclassification of B and C neurones in the ninth and tenth paravertebral sympathetic ganglia of the bullfrog. J Physiol. 1983 Jan;334:255–269. doi: 10.1113/jphysiol.1983.sp014493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Stimulation of phosphatidylinositol (PI) turnover may mediate the muscarinic suppression of the M-current in hippocampal pyramidal cells. Neurosci Lett. 1988 Feb 15;85(1):89–94. doi: 10.1016/0304-3940(88)90434-x. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith W. H. Membrane properties of cell types within guinea pig basal forebrain nuclei in vitro. J Neurophysiol. 1988 May;59(5):1590–1612. doi: 10.1152/jn.1988.59.5.1590. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Jones S. W. Sodium currents in dissociated bull-frog sympathetic neurones. J Physiol. 1987 Aug;389:605–627. doi: 10.1113/jphysiol.1987.sp016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Sejnowski T. J. Peptidergic and muscarinic excitation at amphibian sympathetic synapses. J Physiol. 1983 Aug;341:257–278. doi: 10.1113/jphysiol.1983.sp014805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L. Selective block of inward but not outward rectification in rat sensory neurones infected with herpes simplex virus. J Physiol. 1986 Jun;375:327–338. doi: 10.1113/jphysiol.1986.sp016119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffinger P. J., Leibowitz M. D., Subers E. M., Nathanson N. M., Almers W., Hille B. Agonists that suppress M-current elicit phosphoinositide turnover and Ca2+ transients, but these events do not explain M-current suppression. Neuron. 1988 Aug;1(6):477–484. doi: 10.1016/0896-6273(88)90178-x. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. Muscarine and t-LHRH suppress M-current by activating an IAP-insensitive G-protein. J Neurosci. 1988 Sep;8(9):3343–3353. doi: 10.1523/JNEUROSCI.08-09-03343.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims S. M., Singer J. J., Walsh J. V., Jr Antagonistic adrenergic-muscarinic regulation of M current in smooth muscle cells. Science. 1988 Jan 8;239(4836):190–193. doi: 10.1126/science.2827305. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Kuba K. The Ca2+-sensitive K+-currents underlying the slow afterhyperpolarization of bullfrog sympathetic neurones. Pflugers Arch. 1987 Oct;410(3):234–242. doi: 10.1007/BF00580271. [DOI] [PubMed] [Google Scholar]
- Tokimasa T. Calcium-dependent hyperpolarizations in bullfrog sympathetic neurons. Neuroscience. 1984 Jul;12(3):929–937. doi: 10.1016/0306-4522(84)90180-5. [DOI] [PubMed] [Google Scholar]
- Tokimasa T. Intracellular Ca2+-ions inactivate K+-current in bullfrog sympathetic neurons. Brain Res. 1985 Jul 1;337(2):386–391. doi: 10.1016/0006-8993(85)90081-2. [DOI] [PubMed] [Google Scholar]
- Tokimasa T. Spontaneous muscarinic suppression of the Ca-activated K-current in bullfrog sympathetic neurons. Brain Res. 1985 Sep 30;344(1):134–141. doi: 10.1016/0006-8993(85)91197-7. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Giles W., Greengard P. Cyclic AMP mediates the effects of adrenaline on cardiac purkinje fibres. Nat New Biol. 1972 Dec 6;240(101):181–183. doi: 10.1038/newbio240181a0. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Minota S., Kuba K. Regulation of two ion channels by a common muscarinic receptor-transduction system in a vertebrate neuron. Neurosci Lett. 1987 Oct 16;81(1-2):139–145. doi: 10.1016/0304-3940(87)90354-5. [DOI] [PubMed] [Google Scholar]
- Williams J. T., Colmers W. F., Pan Z. Z. Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurons in vitro. J Neurosci. 1988 Sep;8(9):3499–3506. doi: 10.1523/JNEUROSCI.08-09-03499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. T., North R. A., Tokimasa T. Inward rectification of resting and opiate-activated potassium currents in rat locus coeruleus neurons. J Neurosci. 1988 Nov;8(11):4299–4306. doi: 10.1523/JNEUROSCI.08-11-04299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara K., Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflugers Arch. 1980 May;385(1):11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]


