Abstract
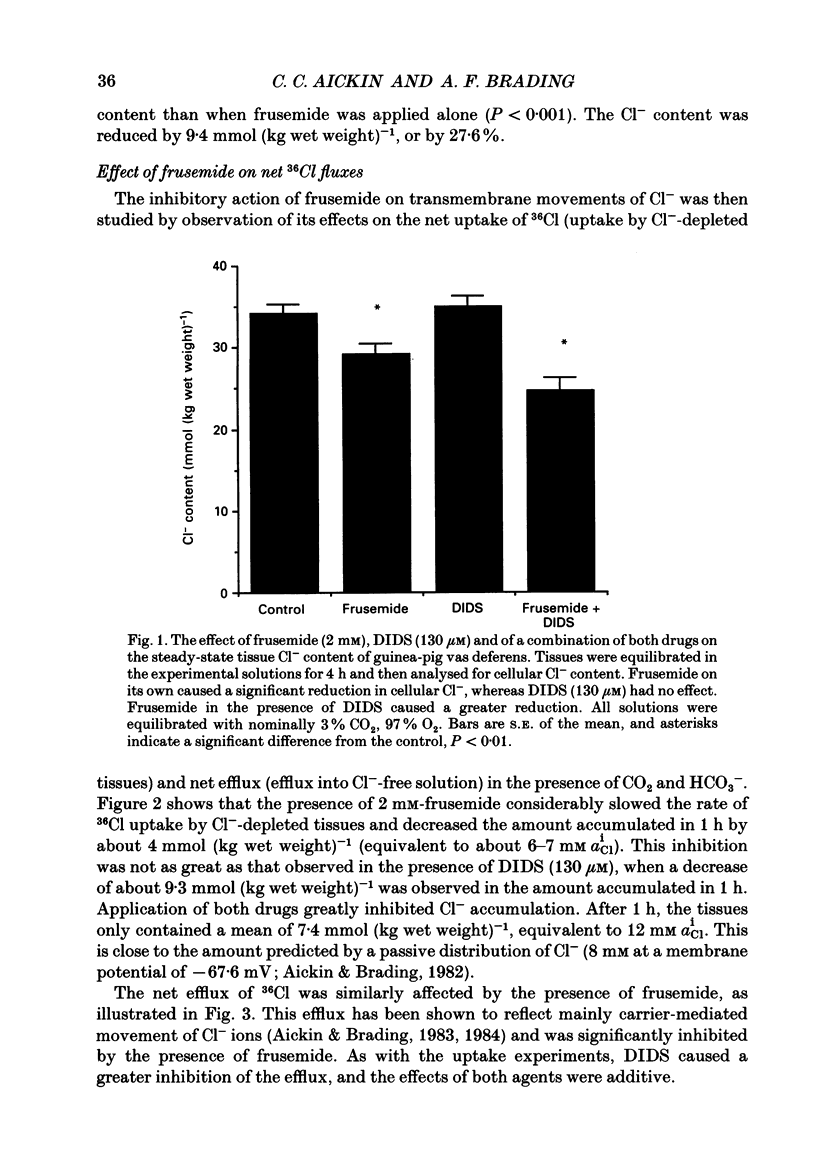
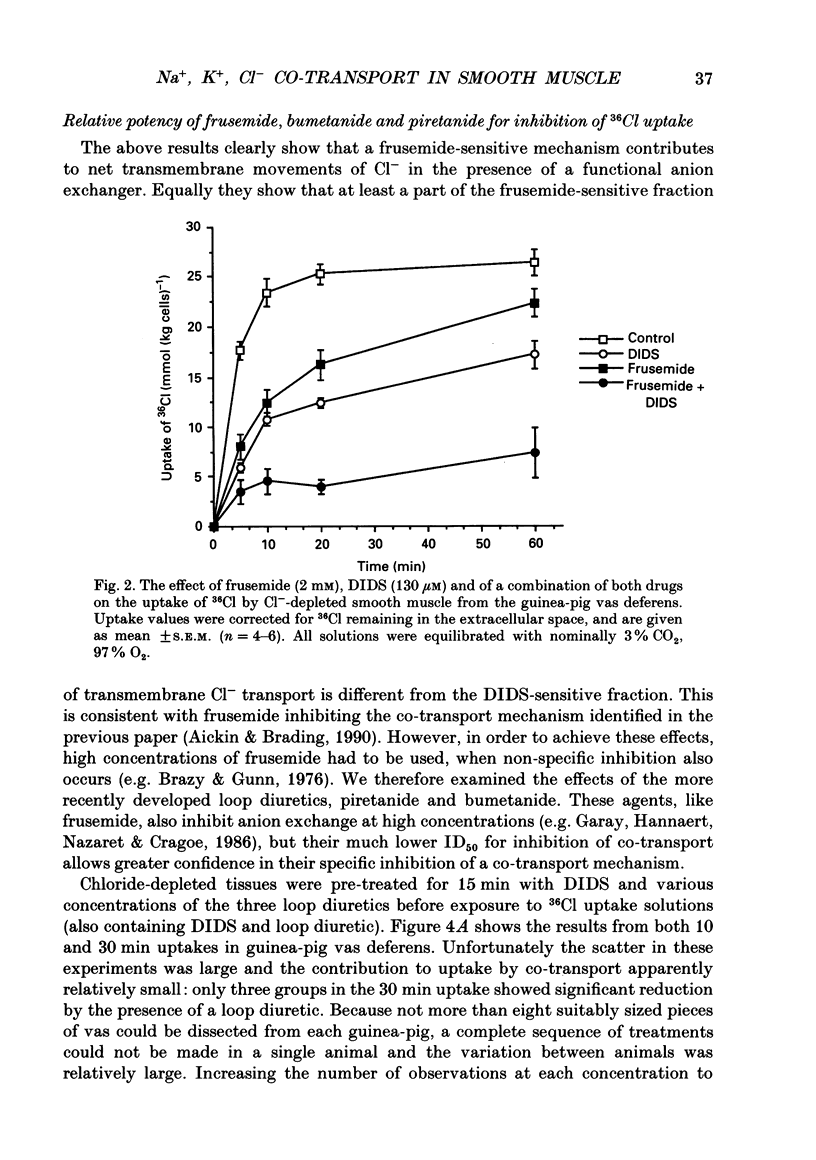
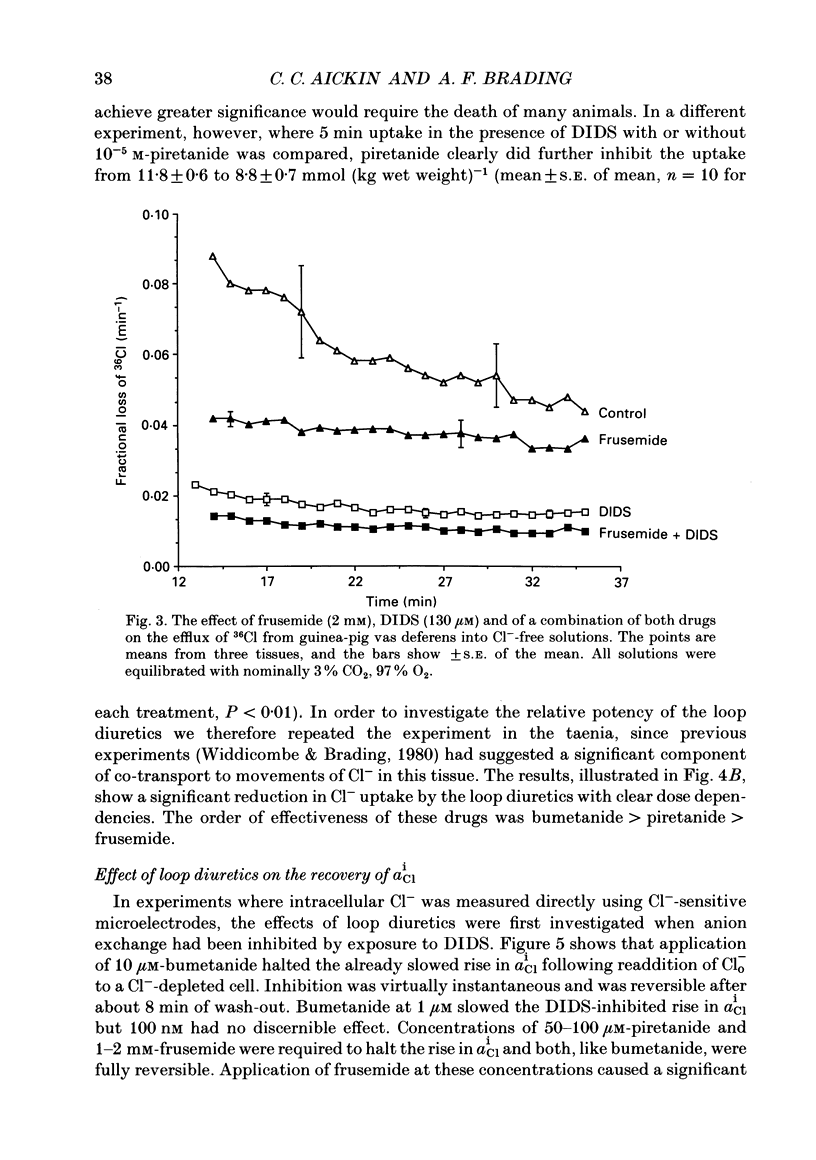
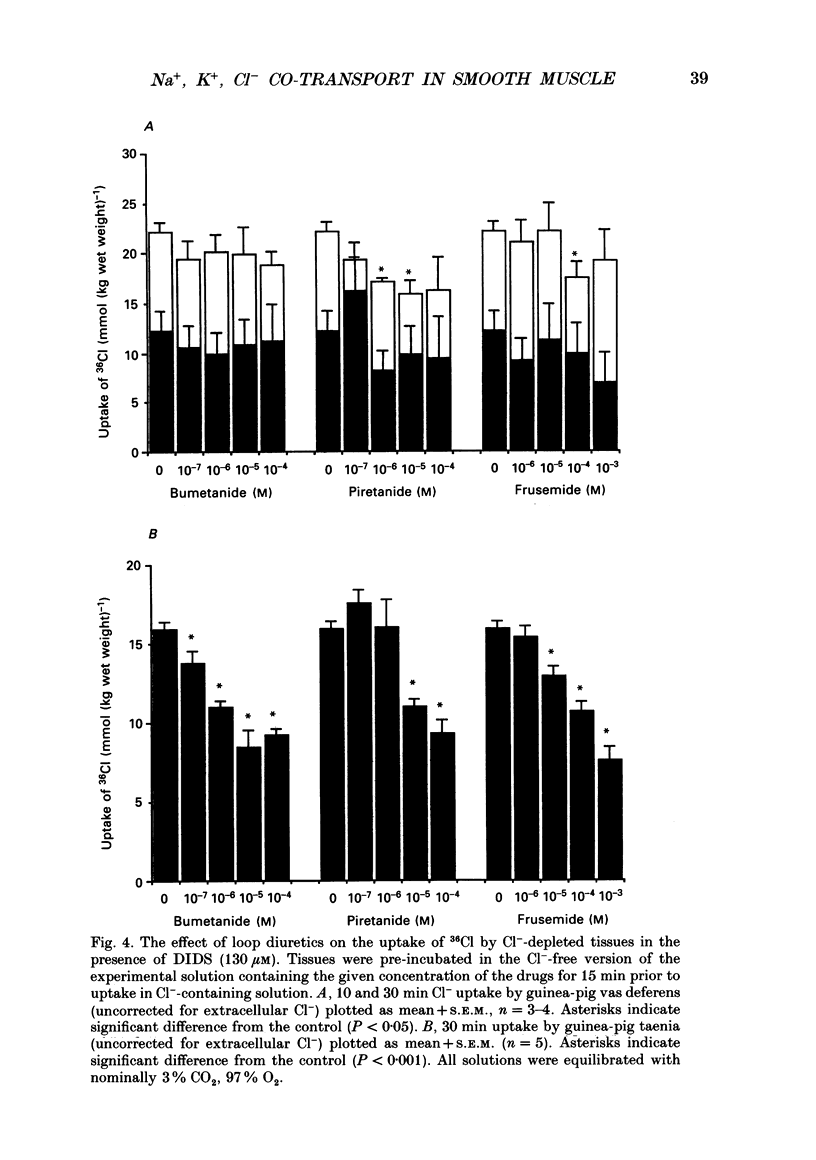
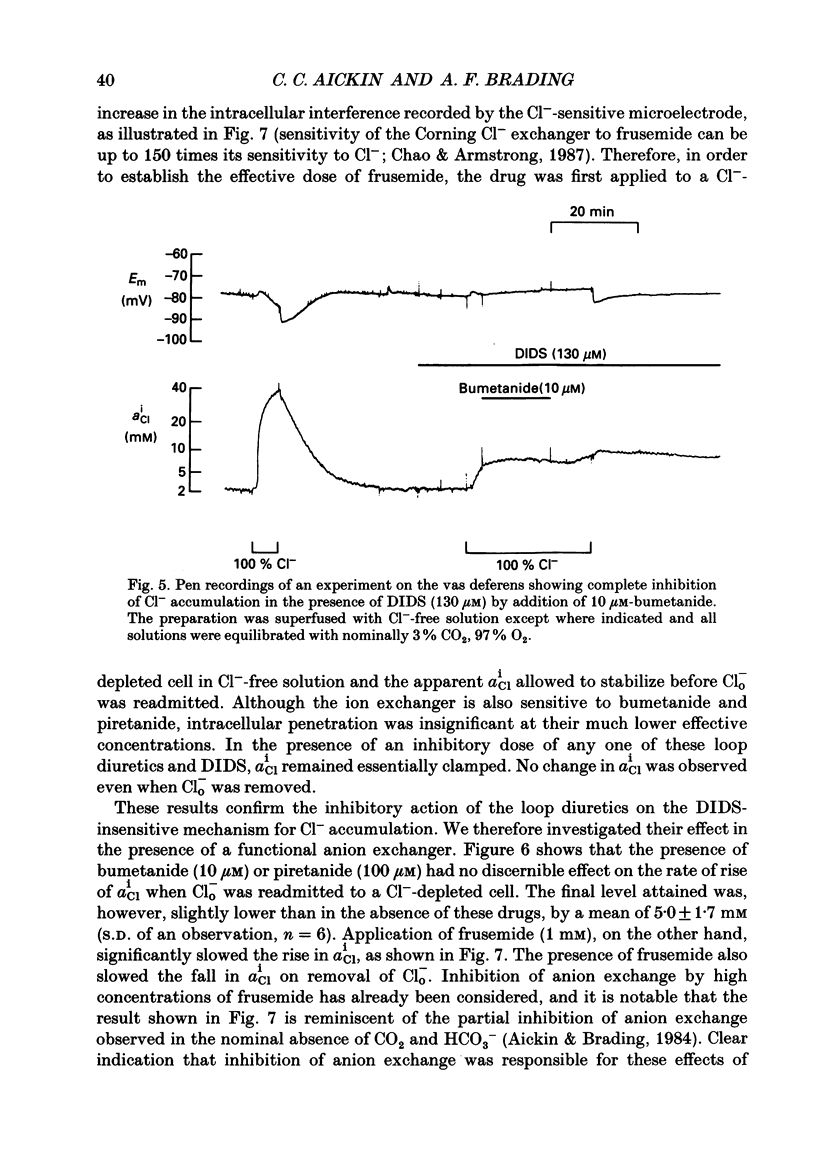
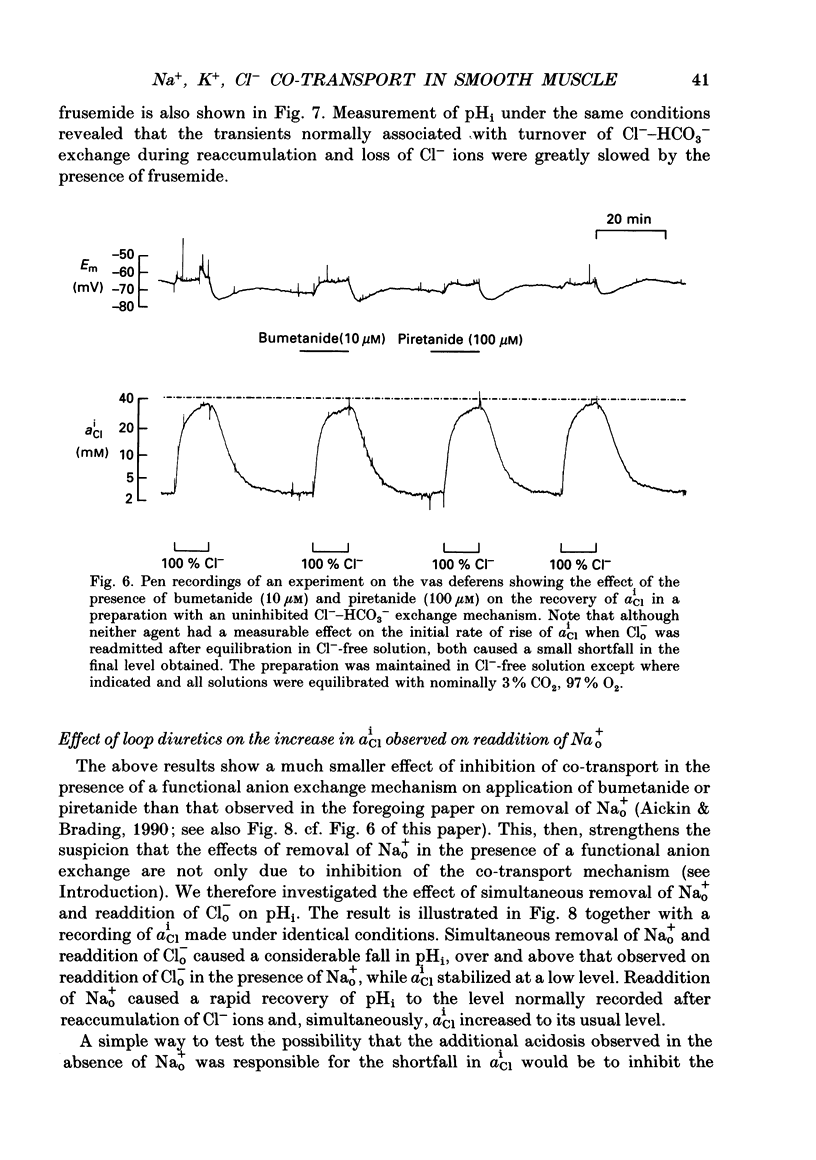
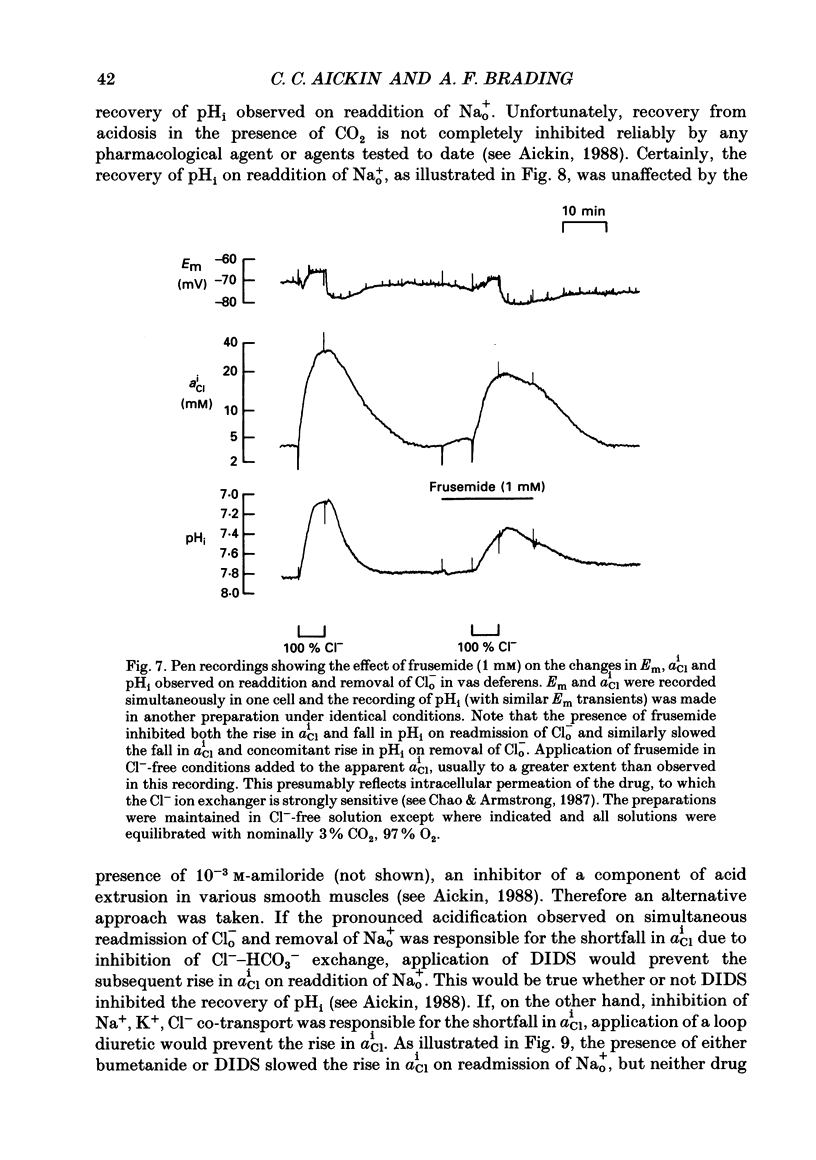
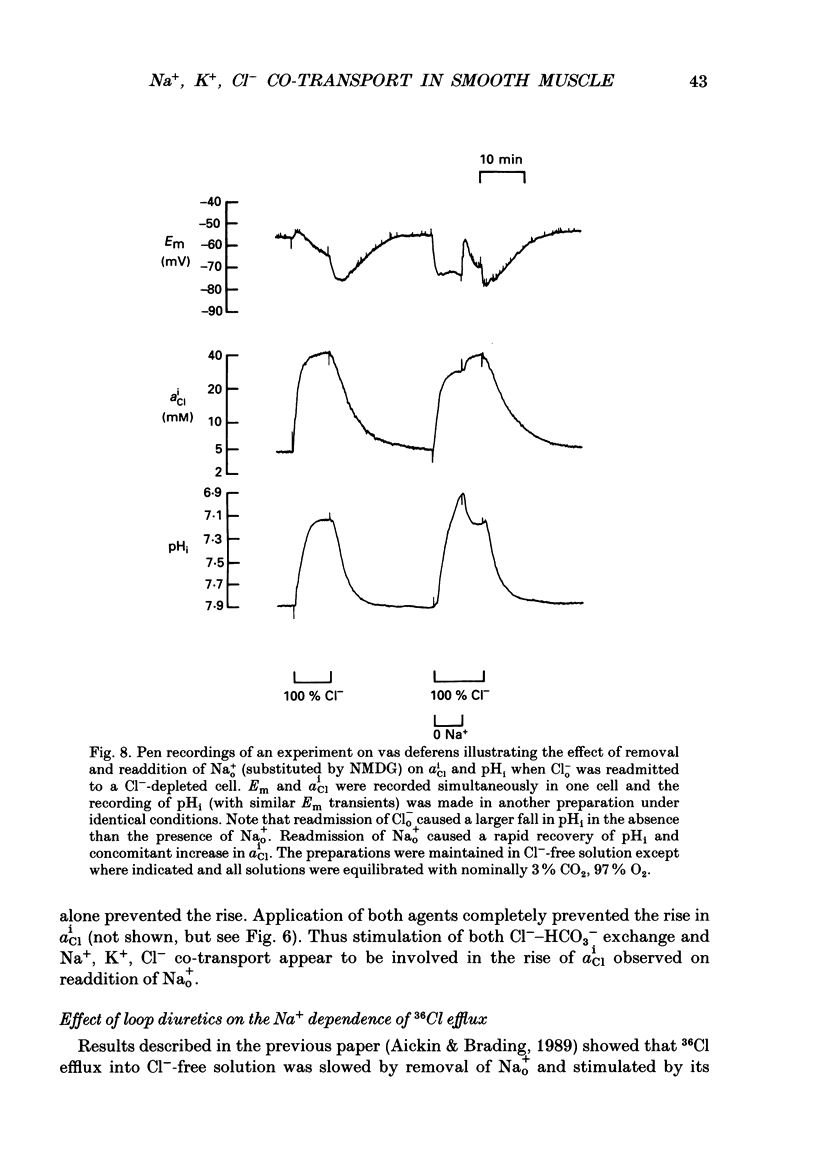
1. The role of Na+, K+, Cl- co-transport, identified in the previous paper (Aickin & Brading, 1990), has been characterized further by investigation of the effects of loop diuretics on Cl- movements in the smooth muscle cells of guinea-pig vas deferens measured by 36Cl fluxes and Cl(-)-sensitive microelectrodes. Some flux experiments were also repeated in the taenia from the guinea-pig caecum. 2. Frusemide (2 mM) reduced the steady-state Cl- content, slowed 36Cl loss into Cl(-)-free solution and both slowed and reduced Cl- accumulation by Cl(-)-depleted cells of the vas deferens. When anion exchange was inhibited by the presence of DIDS, (4,4'-diisothiocyanostilbene-2,2'-disulphonic acid), frusemide further slowed the loss of Cl- into Cl(-)-free solution, further reduced Cl- accumulation such that Cl- uptake amounted to a level consistent with a passive distribution and halted the rise in the intracellular Cl- activity (aiCl) at levels above about 10 mM. 3. Application of the higher-affinity loop diuretics bumetanide and piretanide in vas deferns had no significant effect on 36Cl efflux into Cl(-)-free solution or on the initial rate of rise of aiCl but reduced the final level attained. In the presence of DIDS, however, both agents further slowed efflux into Cl(-)-free solution, and halted the rise in aiCl at levels above about 10 mM. Measurement of greatly slowed intracellular pH transients on removal and readdition of external Cl- (Clo-) in the presence of frusemide suggests that the larger effects of this drug are mediated by inhibition of anion exchange as well as of co-transport. 4. The relative potency of the loop diuretics, investigated in the presence of DIDS was: bumetanide greater than piretanide greater than frusemide. This sequence was found in both vas deferens, using direct measurement of aiCl, and taenia, using 36Cl uptake. 5. Comparison of data from the vas and taenia showed that 36Cl efflux into Cl(-)-free, HCO3(-)-free solution was about twice as fast in the taenia, and that bumetanide or piretanide reduced this efflux to about the same rate as that observed in the vas with or without the loop diuretic. DIDS caused a similar absolute reduction of efflux in both preparations. 6. Stimulation of 36Cl efflux on readdition, and inhibition on removal of Nao+ in the presence of DIDS, was much greater in the taenia than in vas and in both preparations was blocked by bumetanide or piretanide.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
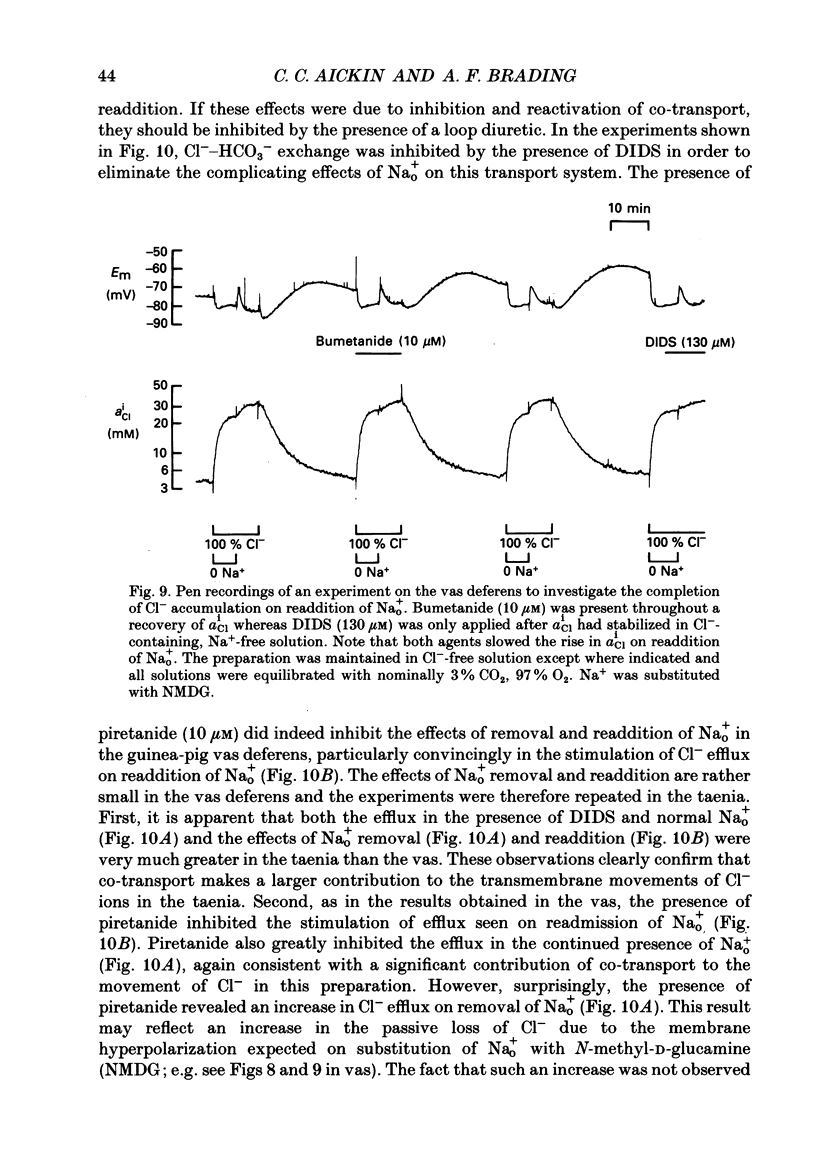
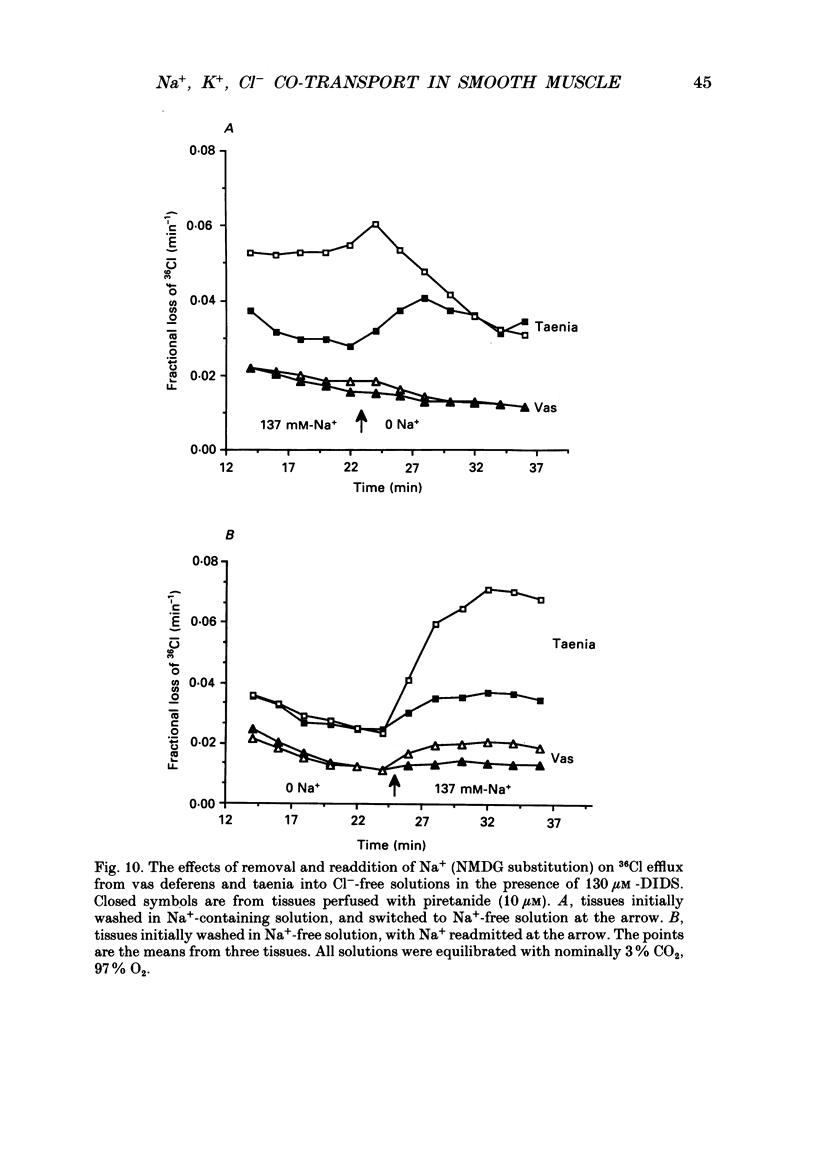
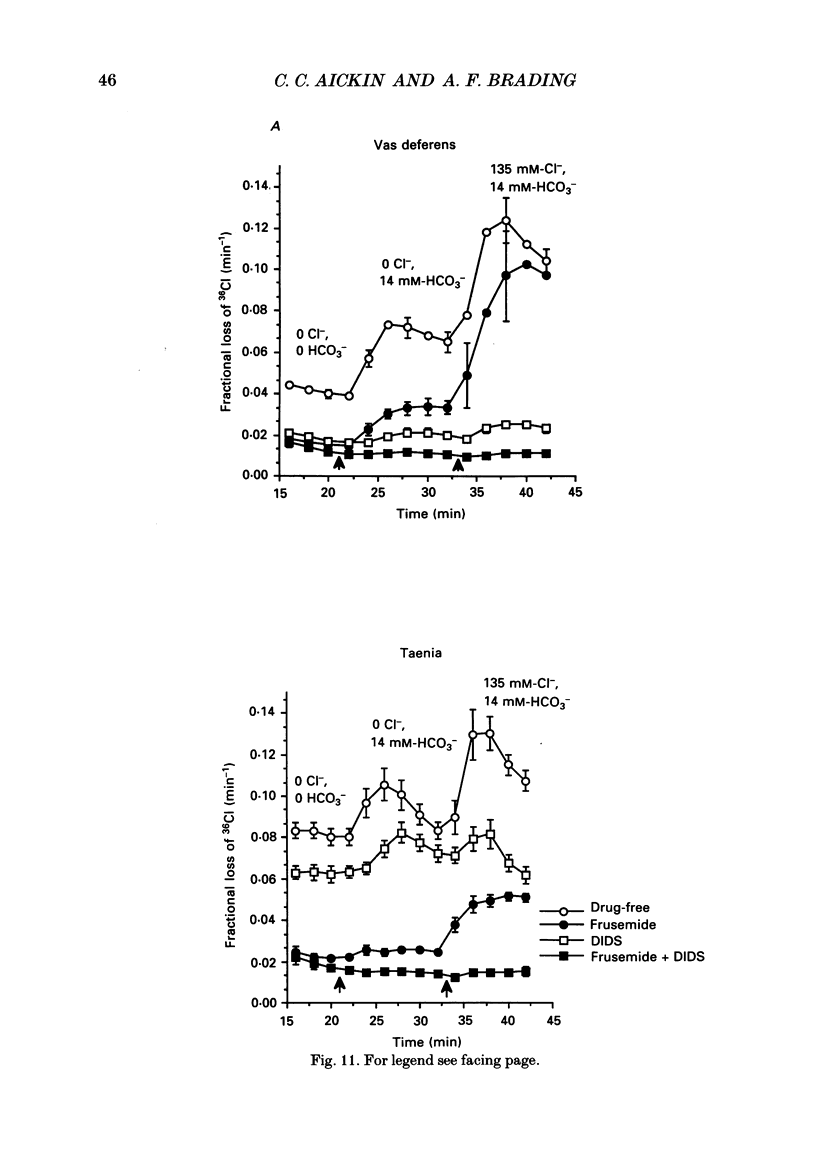
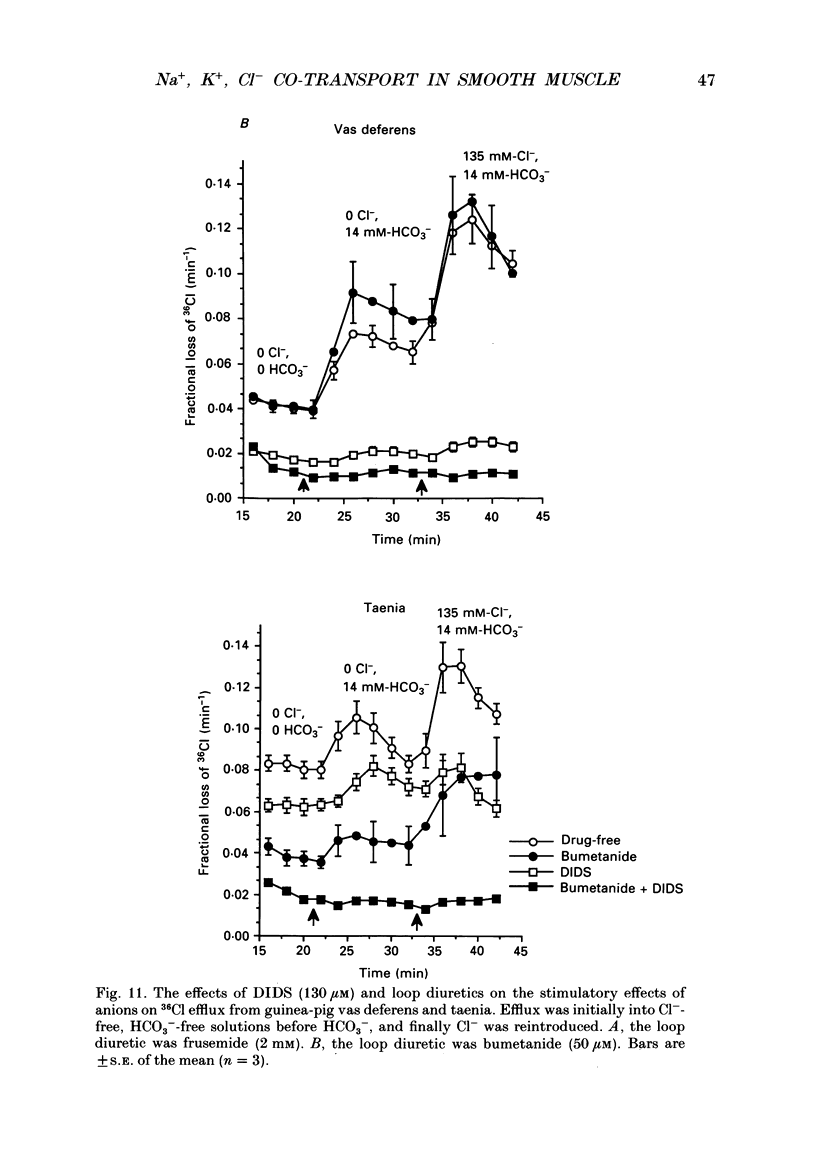
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalkjaer C., Cragoe E. J., Jr Intracellular pH regulation in resting and contracting segments of rat mesenteric resistance vessels. J Physiol. 1988 Aug;402:391–410. doi: 10.1113/jphysiol.1988.sp017211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F. Advances in the understanding of transmembrane ionic gradients and permeabilities in smooth muscle obtained by using ion-selective micro-electrodes. Experientia. 1985 Jul 15;41(7):879–887. doi: 10.1007/BF01970005. [DOI] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F. Effect of Na+ and K+ on Cl- distribution in guinea-pig vas deferens smooth muscle: evidence for Na+, K+, Cl- co-transport. J Physiol. 1990 Feb;421:13–32. doi: 10.1113/jphysiol.1990.sp017931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F. Measurement of intracellular chloride in guinea-pig vas deferens by ion analysis, 36chloride efflux and micro-electrodes. J Physiol. 1982 May;326:139–154. doi: 10.1113/jphysiol.1982.sp014182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F. The role of chloride-bicarbonate exchange in the regulation of intracellular chloride in guinea-pig vas deferens. J Physiol. 1984 Apr;349:587–606. doi: 10.1113/jphysiol.1984.sp015175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F. Towards an estimate of chloride permeability in the smooth muscle of guinea-pig vas deferens. J Physiol. 1983 Mar;336:179–197. doi: 10.1113/jphysiol.1983.sp014575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C. Movement of acid equivalents across the mammalian smooth muscle cell membrane. Ciba Found Symp. 1988;139:3–22. doi: 10.1002/9780470513699.ch2. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Jones A. W. Distribution and kinetics of CoEDTA in smooth muscle, and its use as an extracellular marker. J Physiol. 1969 Feb;200(2):387–401. doi: 10.1113/jphysiol.1969.sp008700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Widdicombe J. H. The use of lanthanum to estimate the numbers of extracellular cation-exchanging sites in the guinea-pig's taenia coli, and its effects on transmembrane monovalent ion movements. J Physiol. 1977 Apr;266(2):255–273. doi: 10.1113/jphysiol.1977.sp011767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazy P. C., Gunn R. B. Furosemide inhibition of chloride transport in human red blood cells. J Gen Physiol. 1976 Dec;68(6):583–599. doi: 10.1085/jgp.68.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. Calculation of the membrane potential in smooth muscle cells of the guinea-pig's taenia coli by the Goldman equation. J Physiol. 1969 Nov;205(1):193–208. doi: 10.1113/jphysiol.1969.sp008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. Ion content and ion fluxes in the smooth muscle cells of the longitudinal layer of the guinea-pig's vas deferens. Pflugers Arch. 1969;313(2):95–105. doi: 10.1007/BF00586238. [DOI] [PubMed] [Google Scholar]
- Chao A. C., Armstrong W. M. Cl(-)-selective microelectrodes: sensitivity to anionic Cl- transport inhibitors. Am J Physiol. 1987 Aug;253(2 Pt 1):C343–C347. doi: 10.1152/ajpcell.1987.253.2.C343. [DOI] [PubMed] [Google Scholar]
- Ellory J. C., Dunham P. B., Logue P. J., Stewart G. W. Anion-dependent cation transport in erythrocytes. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):483–495. doi: 10.1098/rstb.1982.0146. [DOI] [PubMed] [Google Scholar]
- Garay R. P., Hannaert P. A., Nazaret C., Cragoe E. J., Jr The significance of the relative effects of loop diuretics and anti-brain edema agents on the Na+,K+,Cl- cotransport system and the Cl-/NaCO3- anion exchanger. Naunyn Schmiedebergs Arch Pharmacol. 1986 Oct;334(2):202–209. doi: 10.1007/BF00505823. [DOI] [PubMed] [Google Scholar]
- Heintze K., Petersen K. U., Olles P., Saverymuttu S. H., Wood J. R. Effects of bicarbonate on fluid and electrolyte transport by the guinea pig gallbladder: a bicarbonate-chloride exchange. J Membr Biol. 1979 Mar 28;45(1-2):43–59. doi: 10.1007/BF01869294. [DOI] [PubMed] [Google Scholar]
- Korbmacher C., Helbig H., Stahl F., Wiederholt M. Evidence for Na/H exchange and Cl/HCO3 exchange in A10 vascular smooth muscle cells. Pflugers Arch. 1988 Jul;412(1-2):29–36. doi: 10.1007/BF00583728. [DOI] [PubMed] [Google Scholar]
- Kreye V. A., Bauer P. K., Villhauer I. Evidence for furosemide-sensitive active chloride transport in vascular smooth muscle. Eur J Pharmacol. 1981 Jul 17;73(1):91–95. doi: 10.1016/0014-2999(81)90150-3. [DOI] [PubMed] [Google Scholar]
- Oashi H. An estimate of the proportion of the resting membrane conductance of the smooth muscle of guinea-pog taenia coli attributable to chloride. J Physiol. 1970 Sep;210(2):405–419. doi: 10.1113/jphysiol.1970.sp009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen N. E. Regulation of Na/K/Cl cotransport in vascular smooth muscle cells. Biochem Biophys Res Commun. 1984 Dec 14;125(2):500–508. doi: 10.1016/0006-291x(84)90568-0. [DOI] [PubMed] [Google Scholar]
- Reuss L., Lewis S. A., Wills N. K., Helman S. I., Cox T. C., Boron W. F., Siebens A. W., Guggino W. B., Giebisch G., Schultz S. G. Ion transport processes in basolateral membranes of epithelia. Fed Proc. 1984 Jul;43(10):2488–2502. [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Chloride activity and its control in skeletal and cardiac muscle. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):537–548. doi: 10.1098/rstb.1982.0150. [DOI] [PubMed] [Google Scholar]
- Villamil M. F., Ponce J., Amorena C., Müller A. Effect of furosemide on the ionic composition of the arterial wall. TIT J Life Sci. 1979;9(1-2):9–14. [PubMed] [Google Scholar]
- Warnock D. G., Greger R., Dunham P. B., Benjamin M. A., Frizzell R. A., Field M., Spring K. R., Ives H. E., Aronson P. S., Seifter J. Ion transport processes in apical membranes of epithelia. Fed Proc. 1984 Jul;43(10):2473–2487. [PubMed] [Google Scholar]
- Weissberg P. L., Little P. J., Cragoe E. J., Jr, Bobik A. Na-H antiport in cultured rat aortic smooth muscle: its role in cytoplasmic pH regulation. Am J Physiol. 1987 Aug;253(2 Pt 1):C193–C198. doi: 10.1152/ajpcell.1987.253.2.C193. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H., Brading A. F. A possible role of linked Na and Cl movement in active Cl uptake in smooth muscle. Pflugers Arch. 1980 Jul;386(1):35–37. doi: 10.1007/BF00584184. [DOI] [PubMed] [Google Scholar]


