Abstract
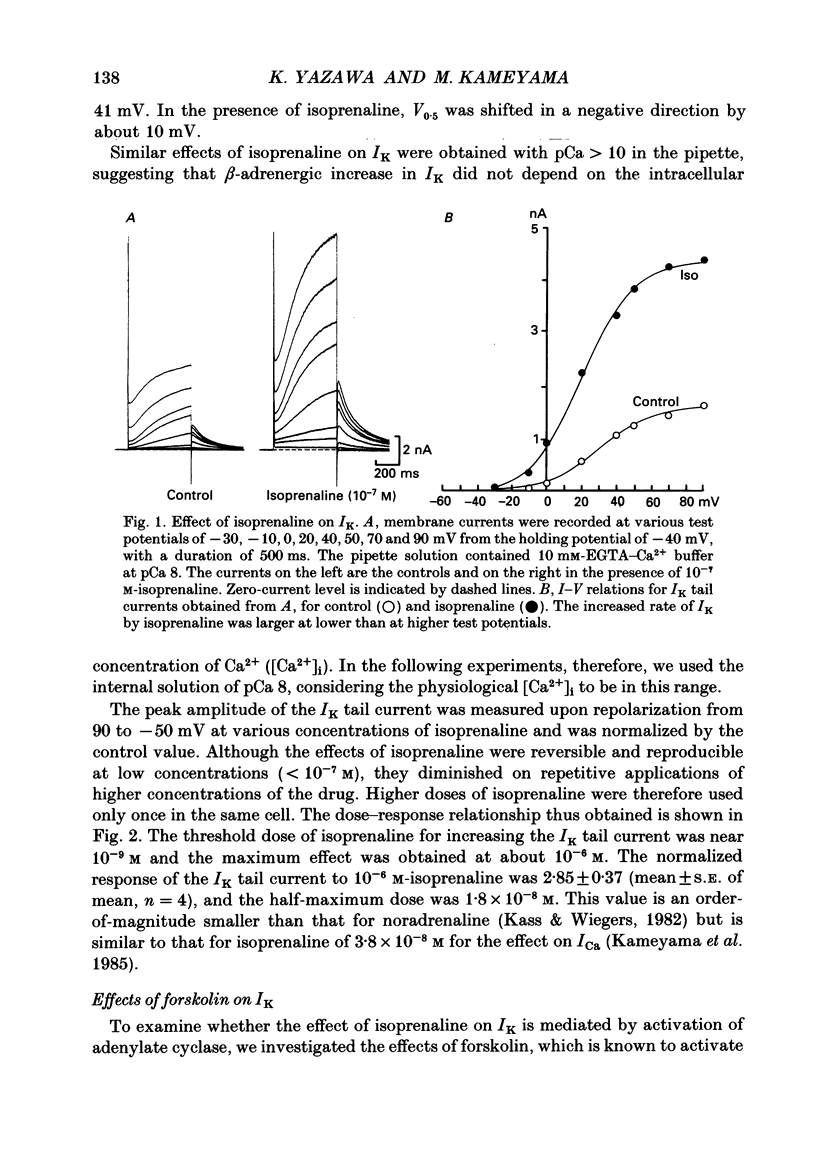
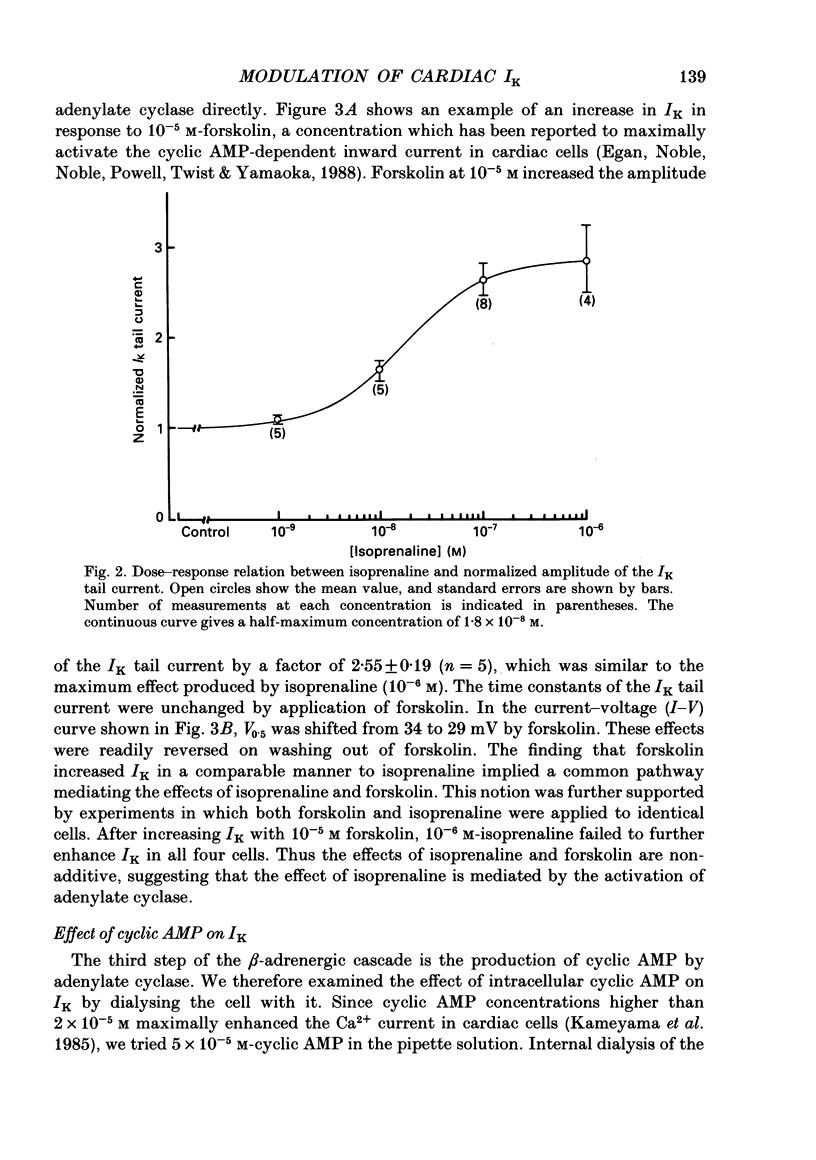
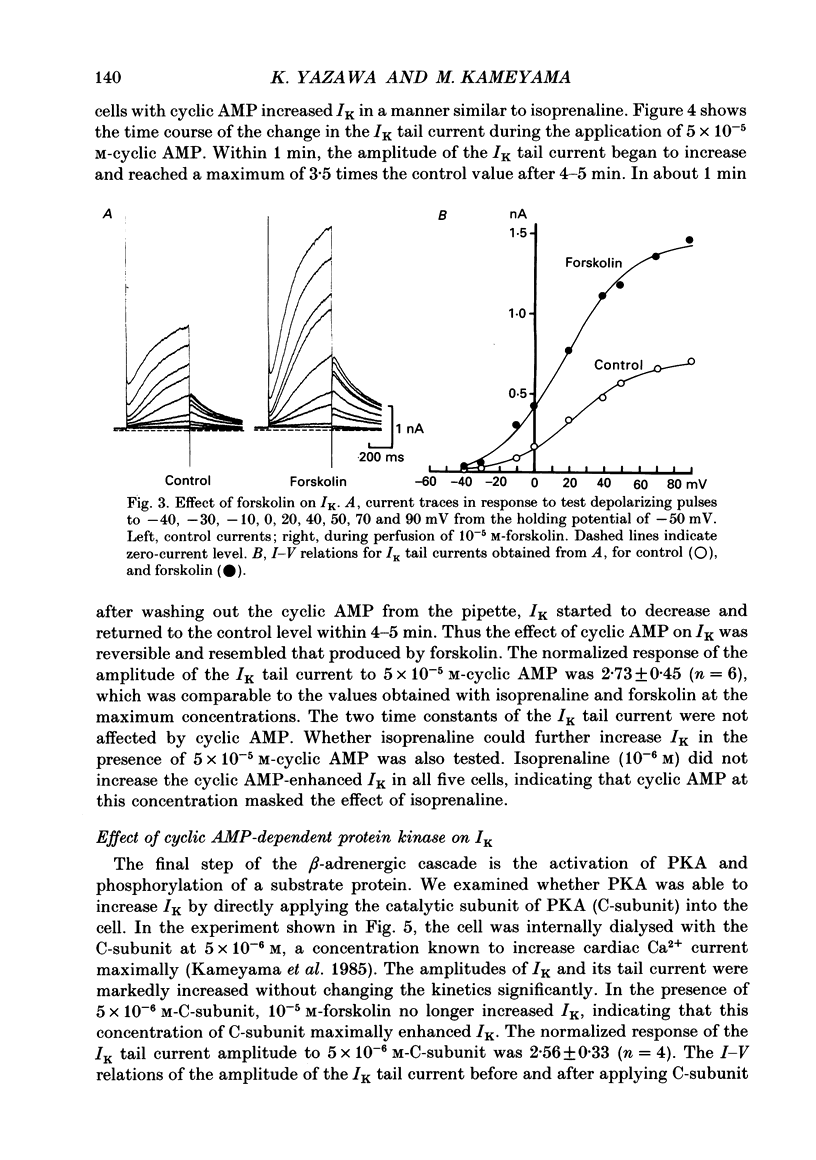
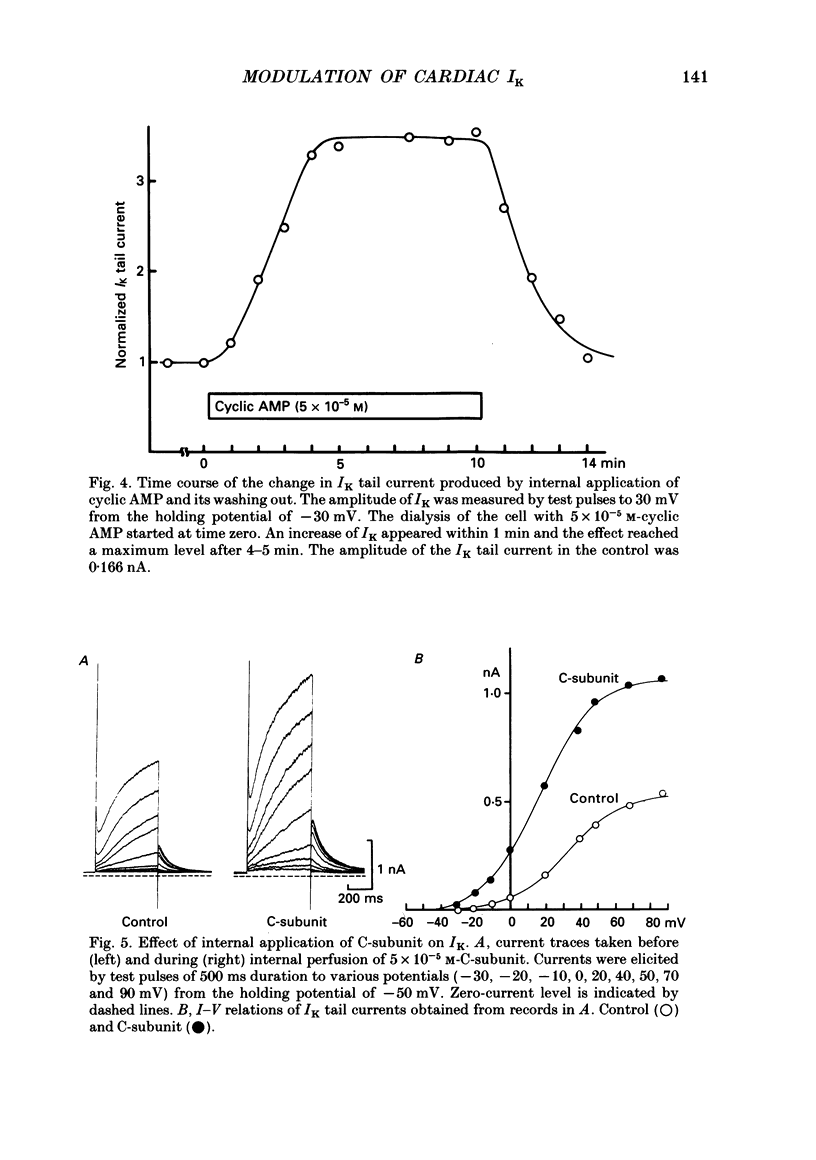
1. Receptor-mediated modulation of the delayed outward potassium current (IK) was investigated in guinea-pig single ventricular cells by using whole-cell voltage clamp and intracellular dialysis. 2. Isoprenaline increased IK in a dose-dependent manner with a half-maximum dose of 1.8 X 10(-8) M. Isoprenaline (10(-6) M) maximally increased IK by a factor of 2.85. This effect did not depend on the concentration of intracellular Ca2+ [( Ca2+]i). 3. External application of 10(-5) M-forskolin and internal application of 5 X 10(-5) M-cyclic AMP or 5 X 10(-6) M of the catalytic subunit of cyclic AMP-dependent protein kinase (PKA) also increased IK about 3-fold. The effect of isoprenaline on IK was masked by previous application of cyclic AMP. 4. All the above phosphorylating agents increased the amplitude of IK without a significant change in the current kinetics. 5. In the presence of 10(-5) M-forskolin, an additional application of 10(-8) M-12-O-tetradecanoylphorbol-13-acetate, an activator of protein kinase C (PKC), produced a further increase in IK, suggesting that the active sites of PKA and PKC on the IK channel are different. 6. Acetylcholine (10(-6) M) suppressed IK when the current was previously enhanced by 2 X 10(-8) M-isoprenaline, but had little effect in the absence of isoprenaline. 7. We conclude that beta-adrenergic modulation of IK is mediated by cyclic AMP-dependent phosphorylation but not by an increase in [Ca2+]i, that PKA and PKC enhance IK independently, and that acetylcholine antagonizes beta-adrenergic stimulation of IK most probably by inhibiting adenylate cyclase.
Full text
PDF



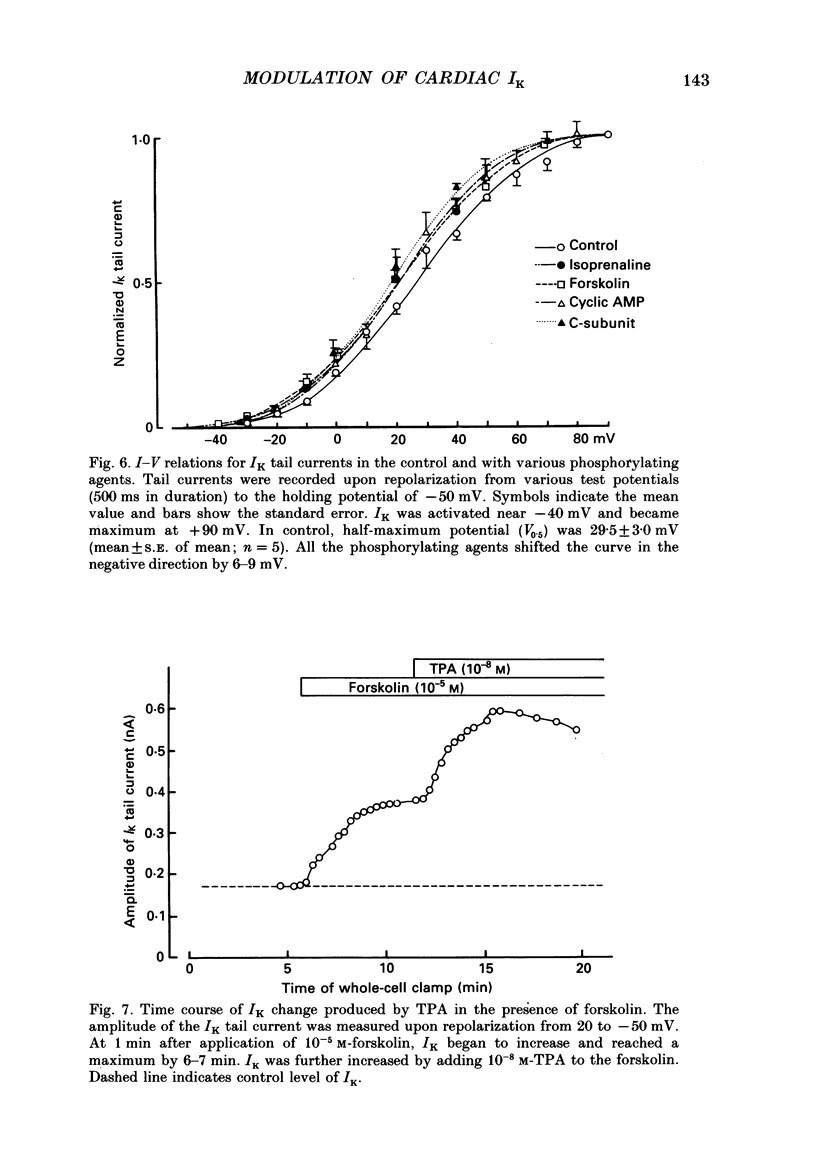
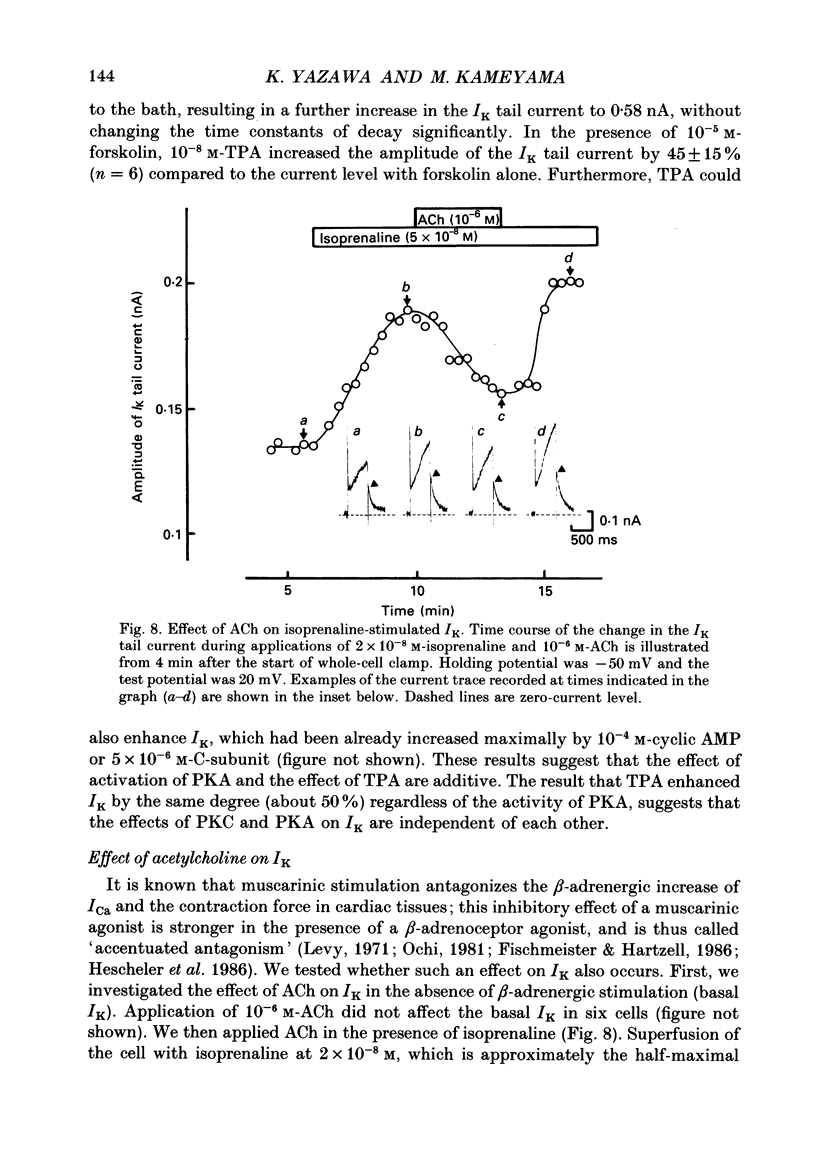
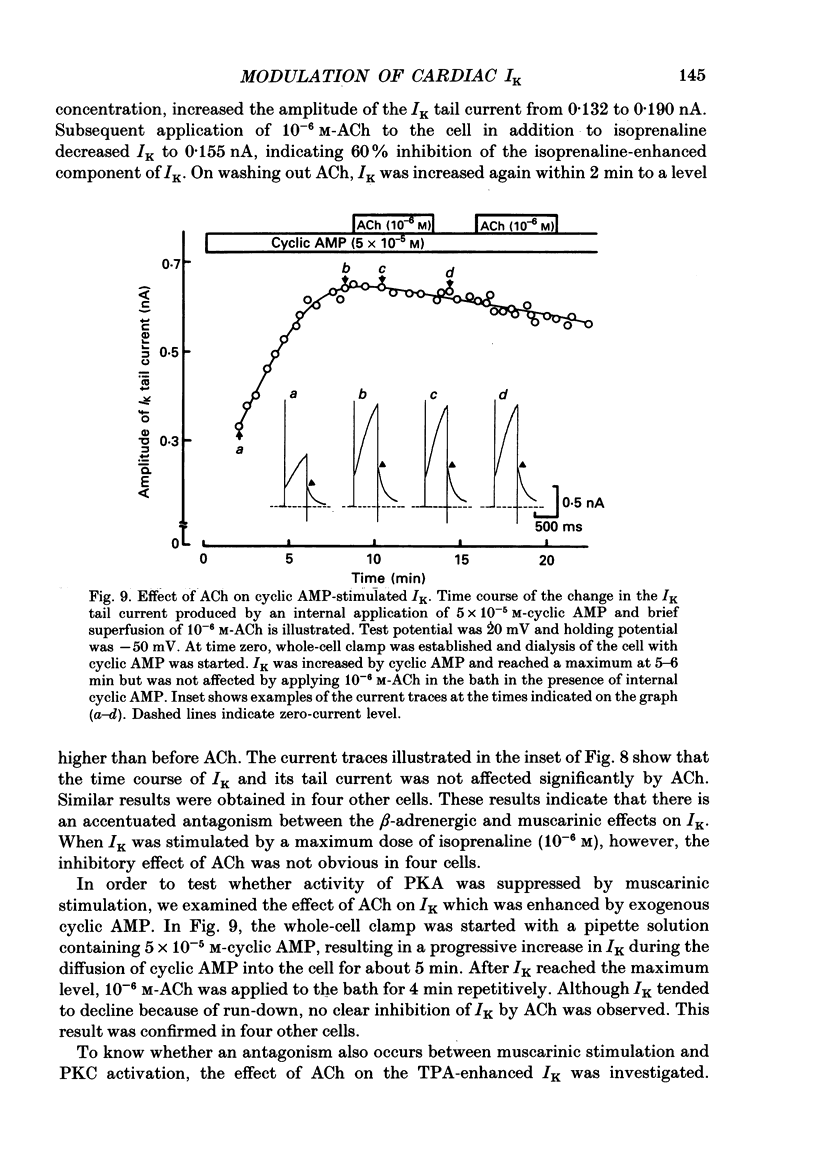


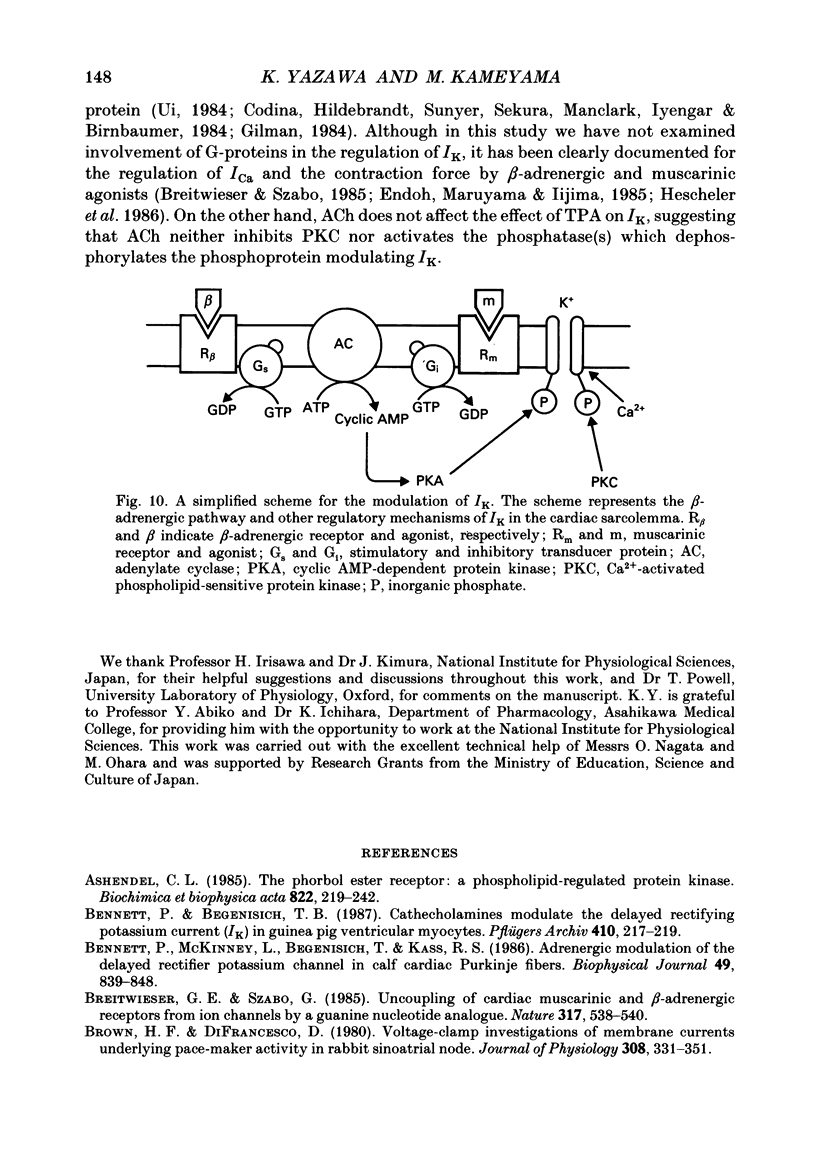


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashendel C. L. The phorbol ester receptor: a phospholipid-regulated protein kinase. Biochim Biophys Acta. 1985 Sep 9;822(2):219–242. doi: 10.1016/0304-4157(85)90009-7. [DOI] [PubMed] [Google Scholar]
- Bennett P. B., Begenisich T. B. Catecholamines modulate the delayed rectifying potassium current (IK) in guinea pig ventricular myocytes. Pflugers Arch. 1987 Sep;410(1-2):217–219. doi: 10.1007/BF00581919. [DOI] [PubMed] [Google Scholar]
- Bennett P., McKinney L., Begenisich T., Kass R. S. Adrenergic modulation of the delayed rectifier potassium channel in calf cardiac Purkinje fibers. Biophys J. 1986 Apr;49(4):839–848. doi: 10.1016/S0006-3495(86)83713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Brown H. F., Noble S. J. Proceedings: Effects of adrenaline on membrane currents underlying pacemaker activity in frog atrial muscle. J Physiol. 1974 Apr;238(1):51P–53P. [PubMed] [Google Scholar]
- Brown H., Difrancesco D. Voltage-clamp investigations of membrane currents underlying pace-maker activity in rabbit sino-atrial node. J Physiol. 1980 Nov;308:331–351. doi: 10.1113/jphysiol.1980.sp013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Buxton I. L., Brunton L. L. Alpha 1-adrenergic and muscarinic cholinergic stimulation of phosphoinositide hydrolysis in adult rat cardiomyocytes. Circ Res. 1985 Oct;57(4):532–537. doi: 10.1161/01.res.57.4.532. [DOI] [PubMed] [Google Scholar]
- Codina J., Hildebrandt J., Sunyer T., Sekura R. D., Manclark C. R., Iyengar R., Birnbaumer L. Mechanisms in the vectorial receptor-adenylate cyclase signal transduction. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:111–125. [PubMed] [Google Scholar]
- Drummond G. I., Severson D. L. Cyclic nucleotides and cardiac function. Circ Res. 1979 Feb;44(2):145–153. doi: 10.1161/01.res.44.2.145. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Twist V. W., Yamaoka K. On the mechanism of isoprenaline- and forskolin-induced depolarization of single guinea-pig ventricular myocytes. J Physiol. 1988 Jun;400:299–320. doi: 10.1113/jphysiol.1988.sp017121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehara T., Noma A., Ono K. Calcium-activated non-selective cation channel in ventricular cells isolated from adult guinea-pig hearts. J Physiol. 1988 Sep;403:117–133. doi: 10.1113/jphysiol.1988.sp017242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh M., Maruyama M., Iijima T. Attenuation of muscarinic cholinergic inhibition by islet-activating protein in the heart. Am J Physiol. 1985 Aug;249(2 Pt 2):H309–H320. doi: 10.1152/ajpheart.1985.249.2.H309. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol. 1986 Jul;376:183–202. doi: 10.1113/jphysiol.1986.sp016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W. R., Shibata E. F. Voltage clamp of bull-frog cardiac pace-maker cells: a quantitative analysis of potassium currents. J Physiol. 1985 Nov;368:265–292. doi: 10.1113/jphysiol.1985.sp015857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 1986 Aug;407(2):182–189. doi: 10.1007/BF00580674. [DOI] [PubMed] [Google Scholar]
- Iwasa Y., Hosey M. M. Phosphorylation of cardiac sarcolemma proteins by the calcium-activated phospholipid-dependent protein kinase. J Biol Chem. 1984 Jan 10;259(1):534–540. [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Hofmann F., Trautwein W. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Arch. 1986 Aug;407(2):123–128. doi: 10.1007/BF00580662. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Wiegers S. E. The ionic basis of concentration-related effects of noradrenaline on the action potential of calf cardiac purkinje fibres. J Physiol. 1982 Jan;322:541–558. doi: 10.1113/jphysiol.1982.sp014054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M. N. Sympathetic-parasympathetic interactions in the heart. Circ Res. 1971 Nov;29(5):437–445. doi: 10.1161/01.res.29.5.437. [DOI] [PubMed] [Google Scholar]
- Matsuura H., Ehara T., Imoto Y. An analysis of the delayed outward current in single ventricular cells of the guinea-pig. Pflugers Arch. 1987 Dec;410(6):596–603. doi: 10.1007/BF00581319. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Noma A., Kotake H., Irisawa H. Slow inward current and its role mediating the chronotropic effect of epinephrine in the rabbit sinoatrial node. Pflugers Arch. 1980 Oct;388(1):1–9. doi: 10.1007/BF00582621. [DOI] [PubMed] [Google Scholar]
- Otani H., Otani H., Das D. K. Alpha 1-adrenoceptor-mediated phosphoinositide breakdown and inotropic response in rat left ventricular papillary muscles. Circ Res. 1988 Jan;62(1):8–17. doi: 10.1161/01.res.62.1.8. [DOI] [PubMed] [Google Scholar]
- Pappano A. J., Carmeliet E. E. Epinephrine and the pacemaking mechanism at plateau potentials in sheep cardiac Purkinje fibers. Pflugers Arch. 1979 Oct;382(1):17–26. doi: 10.1007/BF00585899. [DOI] [PubMed] [Google Scholar]
- Quadbeck J., Reiter M. Cardiac action potential and inotropic effect of noradrenaline and calcium. Naunyn Schmiedebergs Arch Pharmacol. 1975;286(4):337–351. doi: 10.1007/BF00506649. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Taniguchi J., Kokubun S., Noma A., Irisawa H. Spontaneously active cells isolated from the sino-atrial and atrio-ventricular nodes of the rabbit heart. Jpn J Physiol. 1981;31(4):547–558. doi: 10.2170/jjphysiol.31.547. [DOI] [PubMed] [Google Scholar]
- Tohse N., Kameyama M., Irisawa H. Intracellular Ca2+ and protein kinase C modulate K+ current in guinea pig heart cells. Am J Physiol. 1987 Nov;253(5 Pt 2):H1321–H1324. doi: 10.1152/ajpheart.1987.253.5.H1321. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Cyclic AMP and contractile activity in heart. Adv Cyclic Nucleotide Res. 1977;8:363–420. [PubMed] [Google Scholar]
- Tsien R. W., Giles W., Greengard P. Cyclic AMP mediates the effects of adrenaline on cardiac purkinje fibres. Nat New Biol. 1972 Dec 6;240(101):181–183. doi: 10.1038/newbio240181a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Walsh K. B., Begenisich T. B., Kass R. S. Beta-adrenergic modulation in the heart. Independent regulation of K and Ca channels. Pflugers Arch. 1988 Feb;411(2):232–234. doi: 10.1007/BF00582323. [DOI] [PubMed] [Google Scholar]
- Walsh K. B., Kass R. S. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988 Oct 7;242(4875):67–69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]


