Abstract
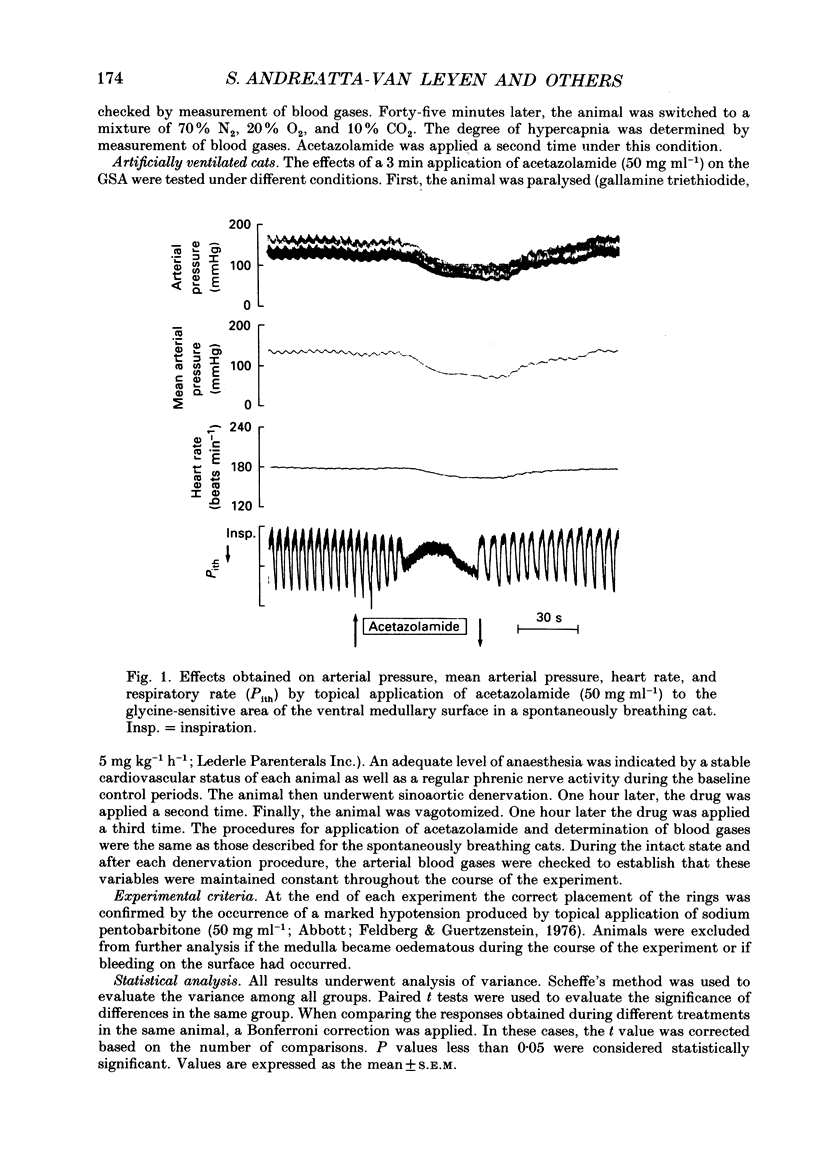
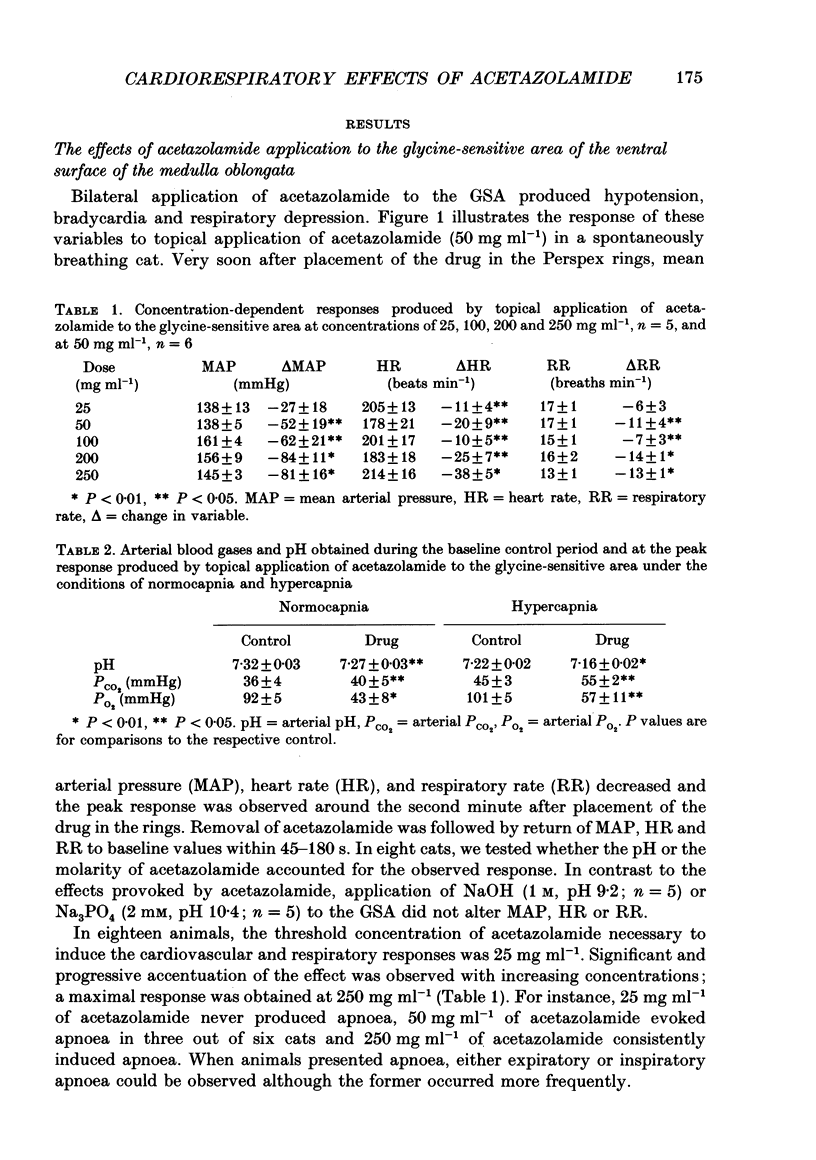
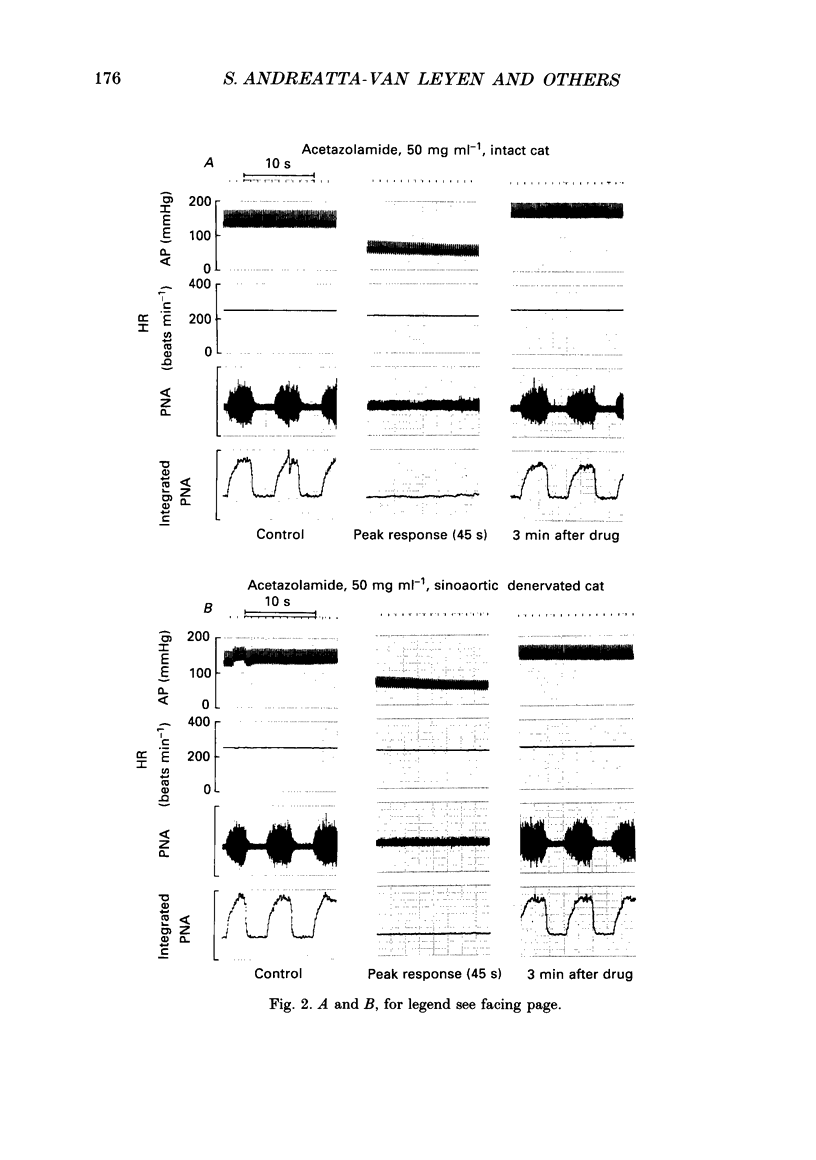
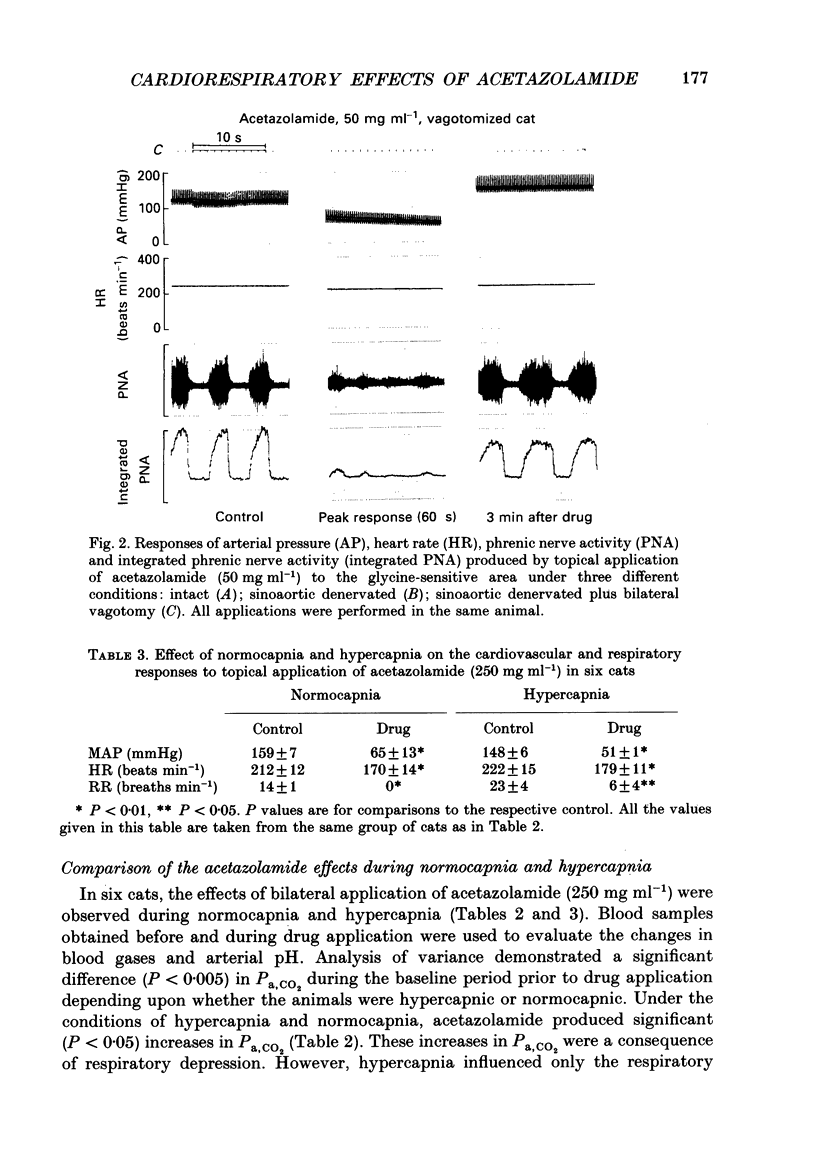
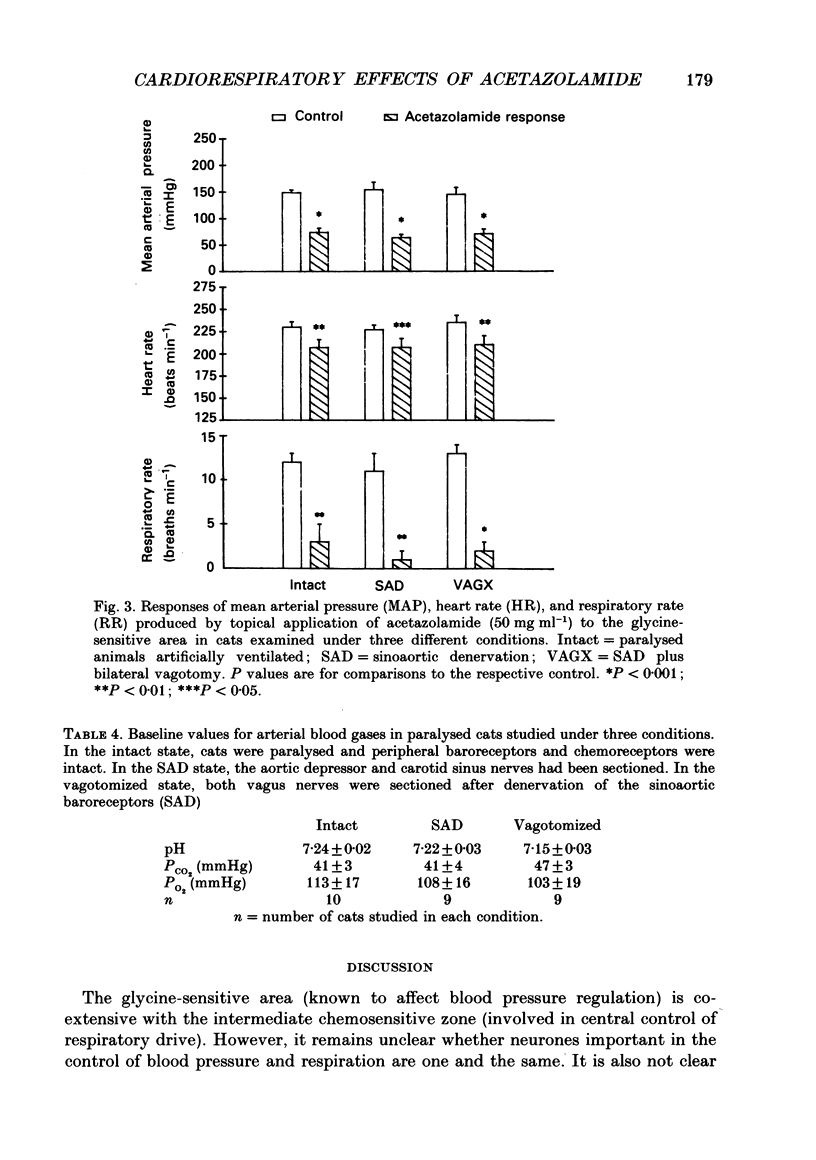
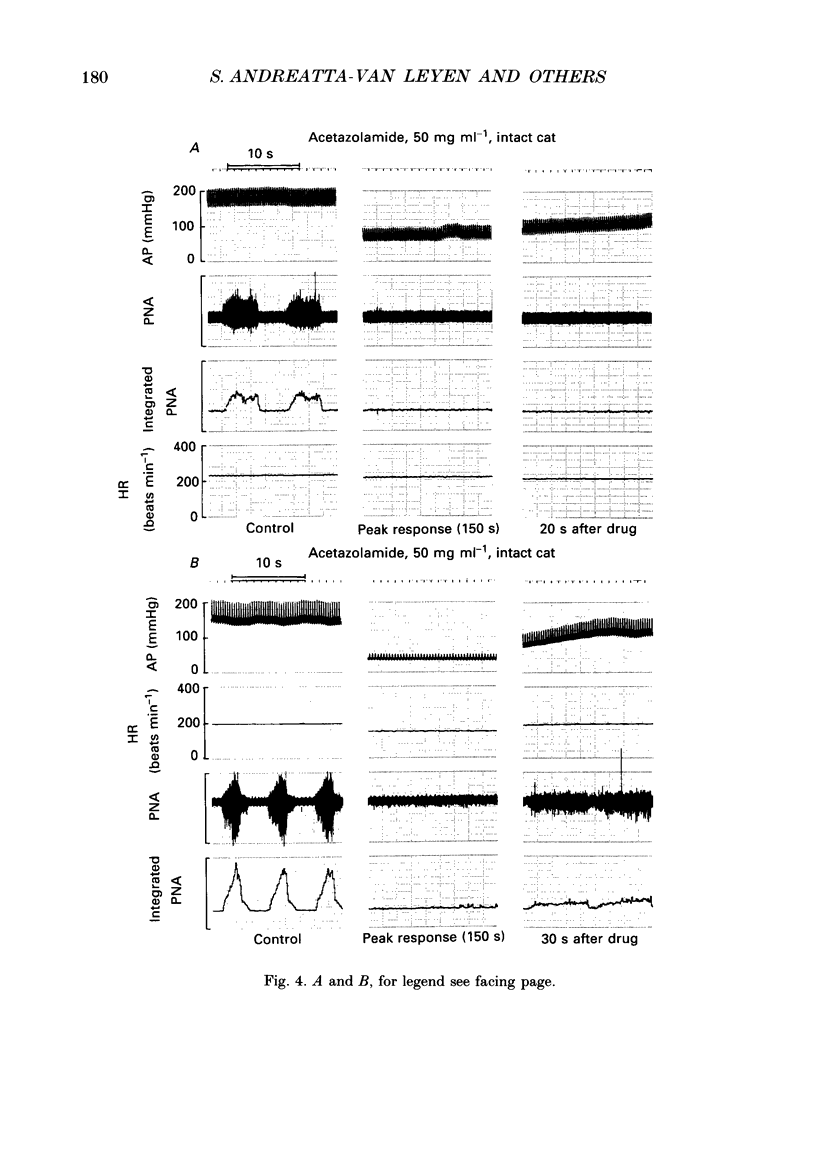
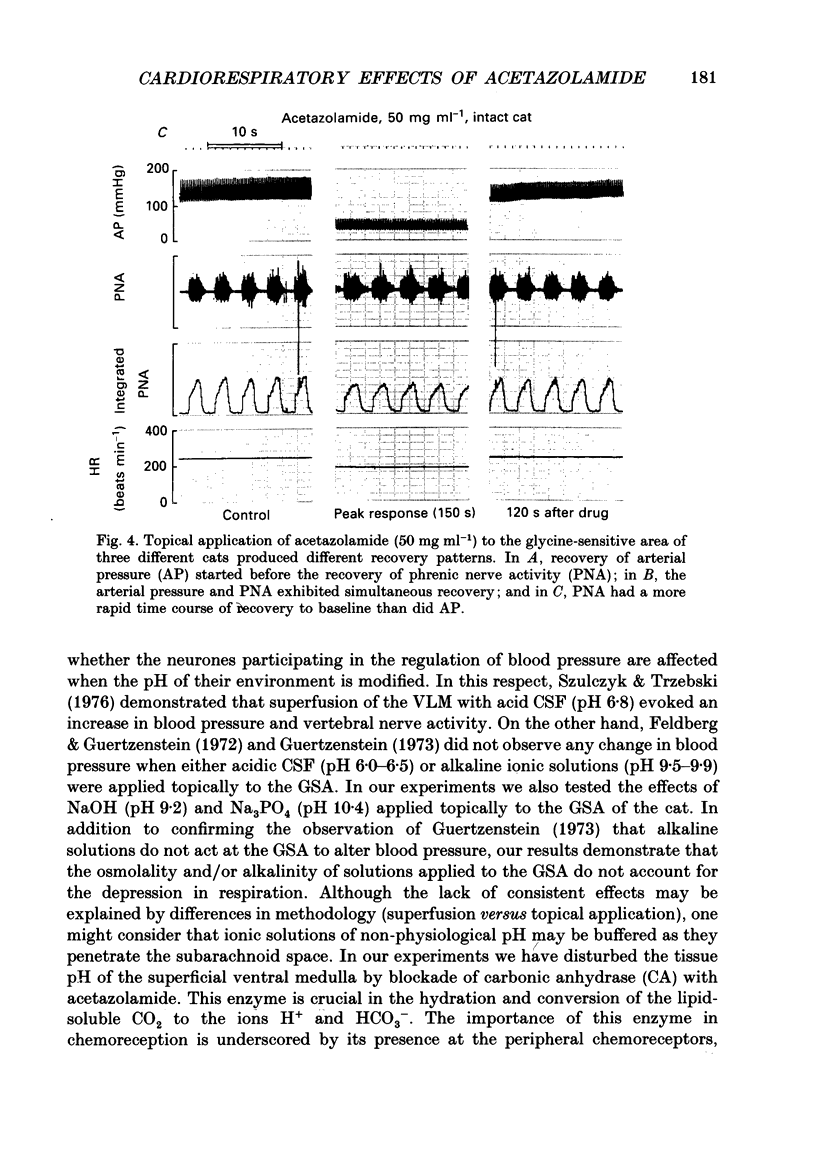
1. Inhibition of carbonic anhydrase by acetazolamide in alpha-chloralose-anaesthetized cats, in a region of the brain stem co-extensive with the glycine-sensitive area, intermediate chemosensitive area, and probably C1 catecholaminergic neurones produces hypotension, bradycardia and depression of the central respiratory drive. 2. These responses are concentration dependent, and can still be observed when the enzyme substrate (CO2) is elevated. Therefore, in both the hypercapnic and the normocapnic condition, similar responses in arterial blood pressure, heart rate and respiratory rate are observed when acetazolamide is topically applied to the glycine-sensitive area. 3. To investigate further the contribution of peripheral baro-, chemo- and cardiopulmonary receptors to these responses, acetazolamide was topically applied to the glycine-sensitive area under three different conditions: intact gallamine-paralysed (5 mg kg-1 h-1) and artificially ventilated (A), sinoaortic denervated (B), and sinoaortic denervated plus bilaterally vagotomized cats (C). Under all conditions, similar responses were observed. The fall in arterial blood pressure was 75 +/- 11 (A), 90 +/- 13 (B), and 75 +/- 9 mmHg (C). Changes in heart rate during acetazolamide application were -23 +/- 6, -20 +/- 8, and -26 +/- 6 beats min-1, respectively. The decreases in respiratory rate were 9 +/- 2 (A), 11 +/- 2 (B), and 11 +/- 2 breaths min-1 (C). 4. The data indicate that the responses to topical application of acetazolamide are mainly due to its central action at the glycine-sensitive area and are not influenced by peripheral baroreceptor and chemoreceptor inputs.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black A. M., Torrance R. W. Respiratory oscillations in chemoreceptor discharge in the control of breathing. Respir Physiol. 1971 Nov;13(2):221–237. doi: 10.1016/0034-5687(71)90092-2. [DOI] [PubMed] [Google Scholar]
- Dampney R. A., Goodchild A. K., Tan E. Vasopressor neurons in the rostral ventrolateral medulla of the rabbit. J Auton Nerv Syst. 1985 Nov;14(3):239–254. doi: 10.1016/0165-1838(85)90113-4. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L., Kiley J. P., Paydarfar D. Dynamics of medullary hydrogen ion and respiratory responses to square-wave change of arterial carbon dioxide in cats. J Physiol. 1987 Apr;385:627–642. doi: 10.1113/jphysiol.1987.sp016511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Guertzenstein P. G. A vasodepressor effect of pentobarbitone sodium. J Physiol. 1972 Jul;224(1):83–103. doi: 10.1113/jphysiol.1972.sp009882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Guertzenstein P. G. Blood pressure effects of leptazol applied to the ventral surface of the brain stem of cats. J Physiol. 1986 Mar;372:445–456. doi: 10.1113/jphysiol.1986.sp016019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Guertzenstein P. G., Rocha e Silva M., Jr Vasopressin release by nicotine: the site of action. Br J Pharmacol. 1975 Aug;54(4):463–474. doi: 10.1111/j.1476-5381.1975.tb07592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Guertzenstein P. G. Vasodepressor effects obtained by drugs acting on the ventral surface of the brain stem. J Physiol. 1976 Jun;258(2):337–355. doi: 10.1113/jphysiol.1976.sp011423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y., Loeschcke H. H. A cholinergic mechanism involved in the neuronal excitation by H+ in the respiratory chemosensitive structures of the ventral medulla oblongata of rats in vitro. Pflugers Arch. 1979 Mar 16;379(2):125–135. doi: 10.1007/BF00586938. [DOI] [PubMed] [Google Scholar]
- GIACOBINI E. A cytochemical study of the localization of carbonic anhydrase in the nervous system. J Neurochem. 1962 Mar-Apr;9:169–177. doi: 10.1111/j.1471-4159.1962.tb11859.x. [DOI] [PubMed] [Google Scholar]
- Gardner W. N. The pattern of breathing following step changes of alveolar partial pressures of carbon dioxide and oxygen in man. J Physiol. 1980 Mar;300:55–73. doi: 10.1113/jphysiol.1980.sp013151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertzenstein P. G. Blood pressure effects obtained by drugs applied to the ventral surface of the brain stem. J Physiol. 1973 Mar;229(2):395–408. doi: 10.1113/jphysiol.1973.sp010145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertzenstein P. G., Lopes O. U. Cardiovascular responses evoked from the nicotine-sensitive area on the ventral surface of the medulla oblongata in the cat. J Physiol. 1984 Feb;347:345–360. doi: 10.1113/jphysiol.1984.sp015069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertzenstein P. G., Silver A. Fall in blood pressure produced from discrete regions of the ventral surface of the medulla by glycine and lesions. J Physiol. 1974 Oct;242(2):489–503. doi: 10.1113/jphysiol.1974.sp010719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. A., Nye P. C., Torrance R. W. The location of carbonic anhydrase in relation to the blood-brain barrier at the medullary chemoreceptors of the cat. J Physiol. 1981 Nov;320:113–125. doi: 10.1113/jphysiol.1981.sp013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH A., WOODBURY D. M. Carbonic anhydrase inhibition and brain electrolyte composition. Am J Physiol. 1960 Feb;198:434–440. doi: 10.1152/ajplegacy.1960.198.2.434. [DOI] [PubMed] [Google Scholar]
- Loeschcke H. H. Central chemosensitivity and the reaction theory. J Physiol. 1982 Nov;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen R. M. Location of neurones with cardiovascular and respiratory function, at the ventral surface of the cat's medulla. Neuroscience. 1986 May;18(1):43–49. doi: 10.1016/0306-4522(86)90177-6. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E. Neural respiratory and circulatory interaction during chemoreceptor stimulation and cooling of ventral medulla in cats. J Physiol. 1986 Jan;370:217–231. doi: 10.1113/jphysiol.1986.sp015931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderstråle Y., Hanson M. Histochemical study of the distribution of carbonic anhydrase in the cat brain. Acta Physiol Scand. 1985 Aug;124(4):557–564. doi: 10.1111/j.1748-1716.1985.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Ross C. A., Ruggiero D. A., Joh T. H., Park D. H., Reis D. J. Rostral ventrolateral medulla: selective projections to the thoracic autonomic cell column from the region containing C1 adrenaline neurons. J Comp Neurol. 1984 Sep 10;228(2):168–185. doi: 10.1002/cne.902280204. [DOI] [PubMed] [Google Scholar]
- Sapirstein V. S., Strocchi P., Gilbert J. M. Properties and function of brain carbonic anhydrase. Ann N Y Acad Sci. 1984;429:481–493. doi: 10.1111/j.1749-6632.1984.tb12375.x. [DOI] [PubMed] [Google Scholar]
- Schlaefke M. E. Central chemosensitivity: a respiratory drive. Rev Physiol Biochem Pharmacol. 1981;90:171–244. doi: 10.1007/BFb0034080. [DOI] [PubMed] [Google Scholar]
- Szulczyk P., Trzebski A. The local effect of pH changes in the cerebrospinal fluid on the ventrolateral areas of medulla oblongata and on the spinal cord surface on the activity of cardiac and vertebral sympathetic nerves. Acta Physiol Pol. 1976 Jan-Feb;27(1):9–17. [PubMed] [Google Scholar]
- Travis D. M. Molecular CO 2 is inert on carotid chemoreceptor: demonstration by inhibition of carbonic anhydrase. J Pharmacol Exp Ther. 1971 Sep;178(3):529–540. [PubMed] [Google Scholar]


