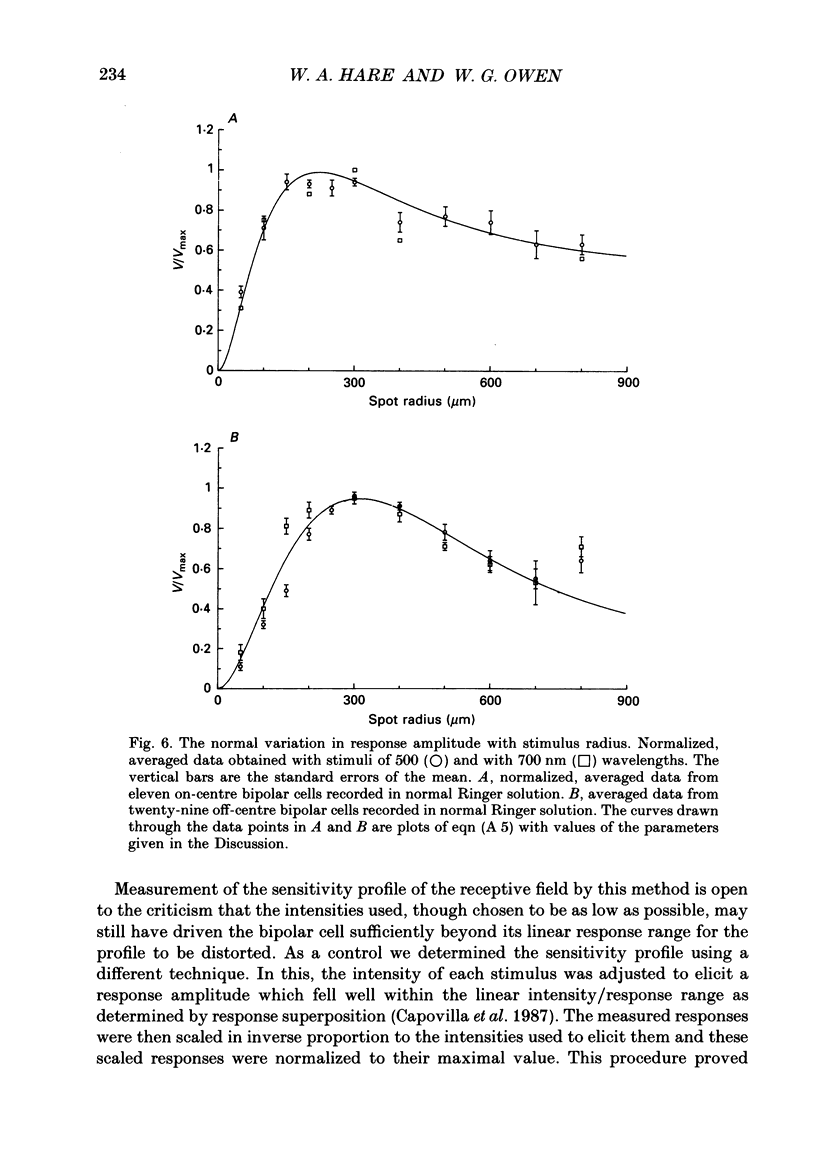
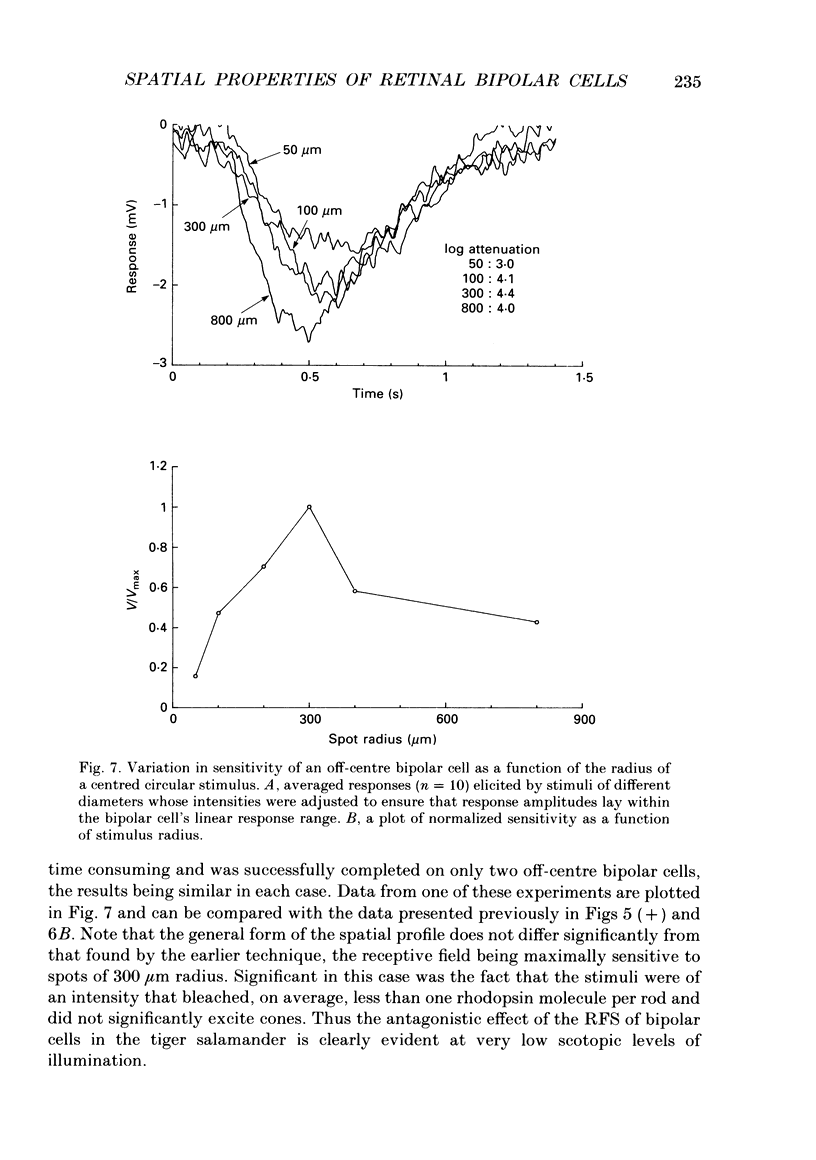
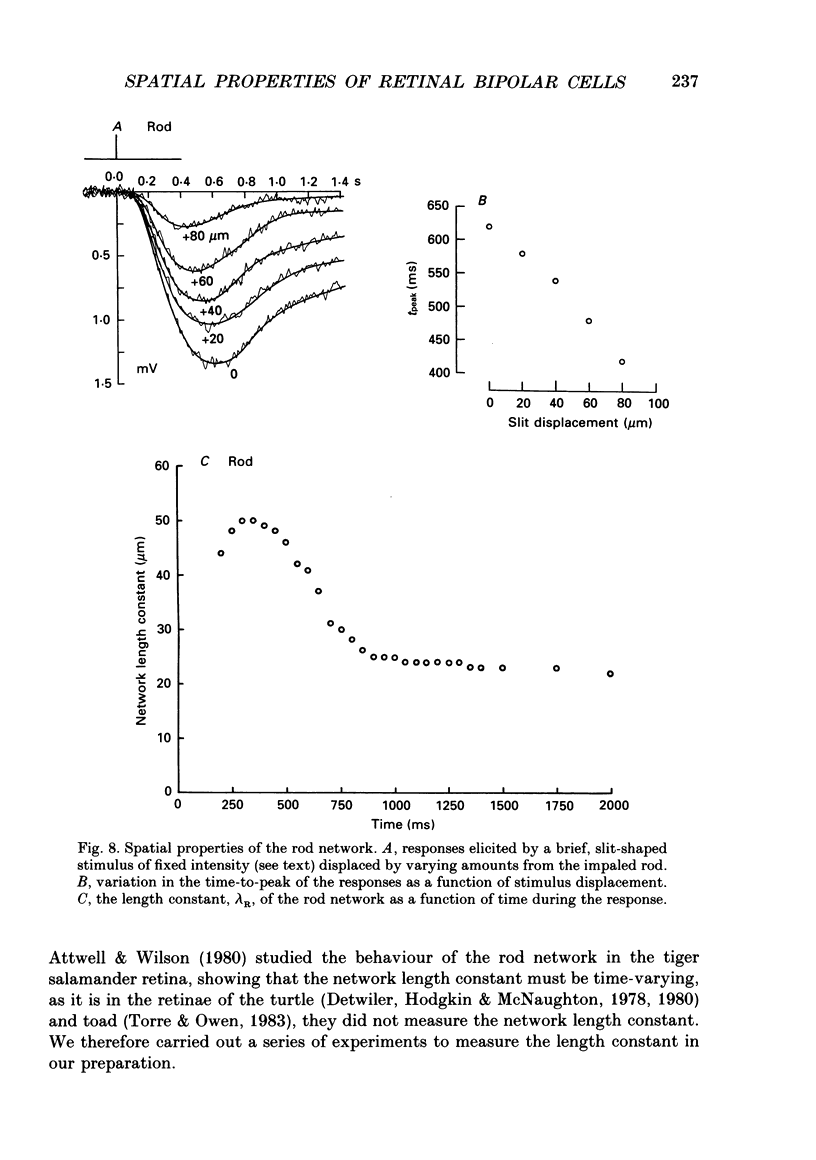
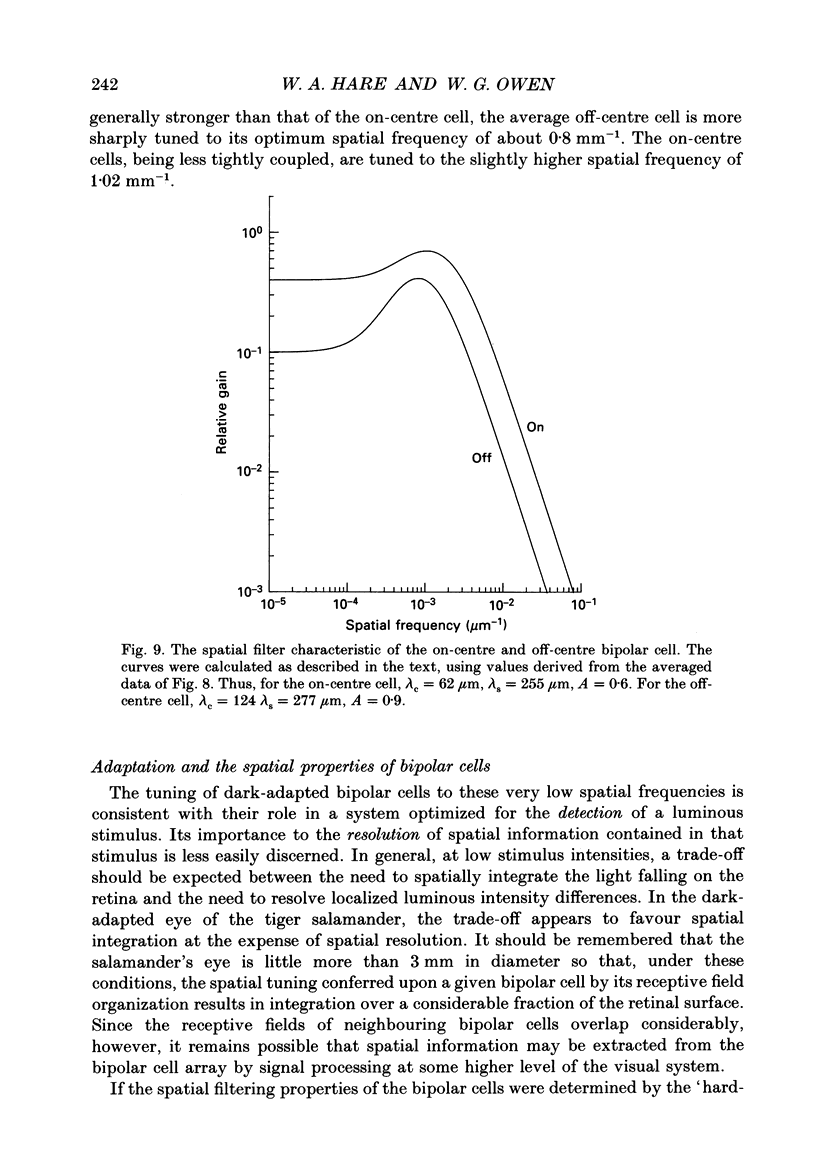
Abstract
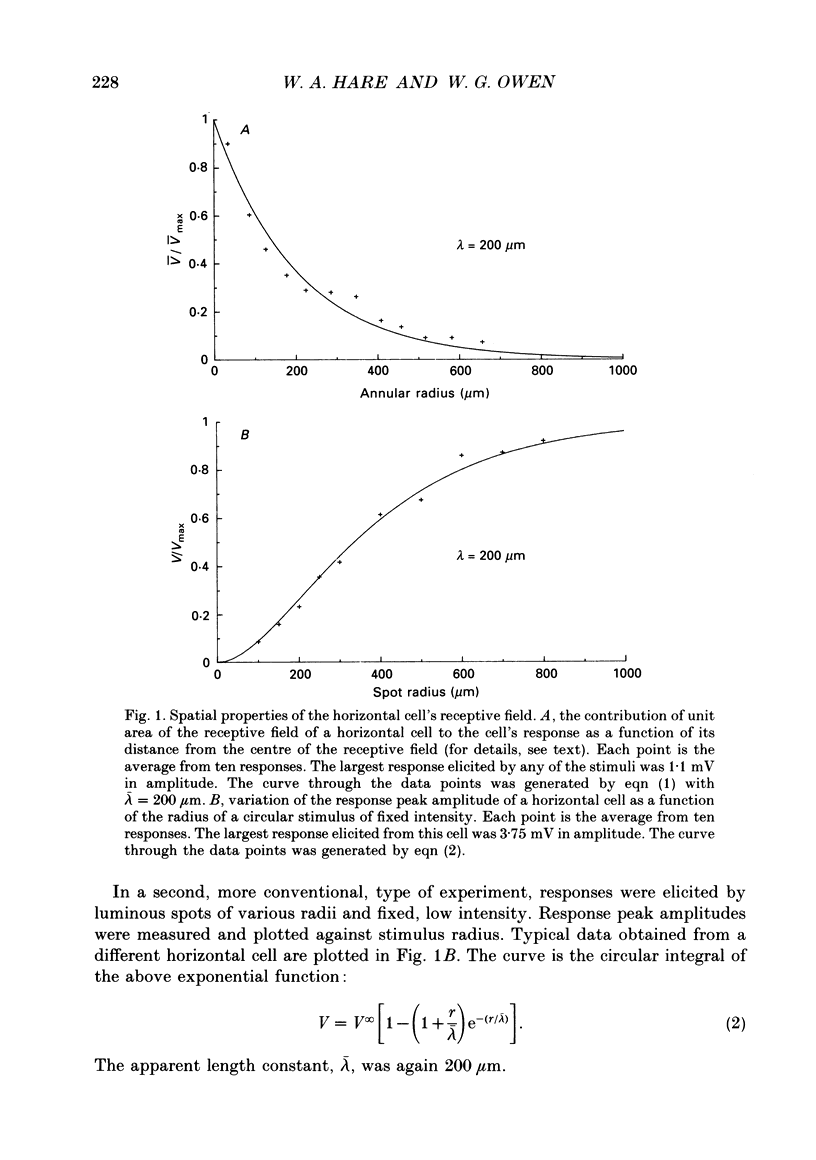
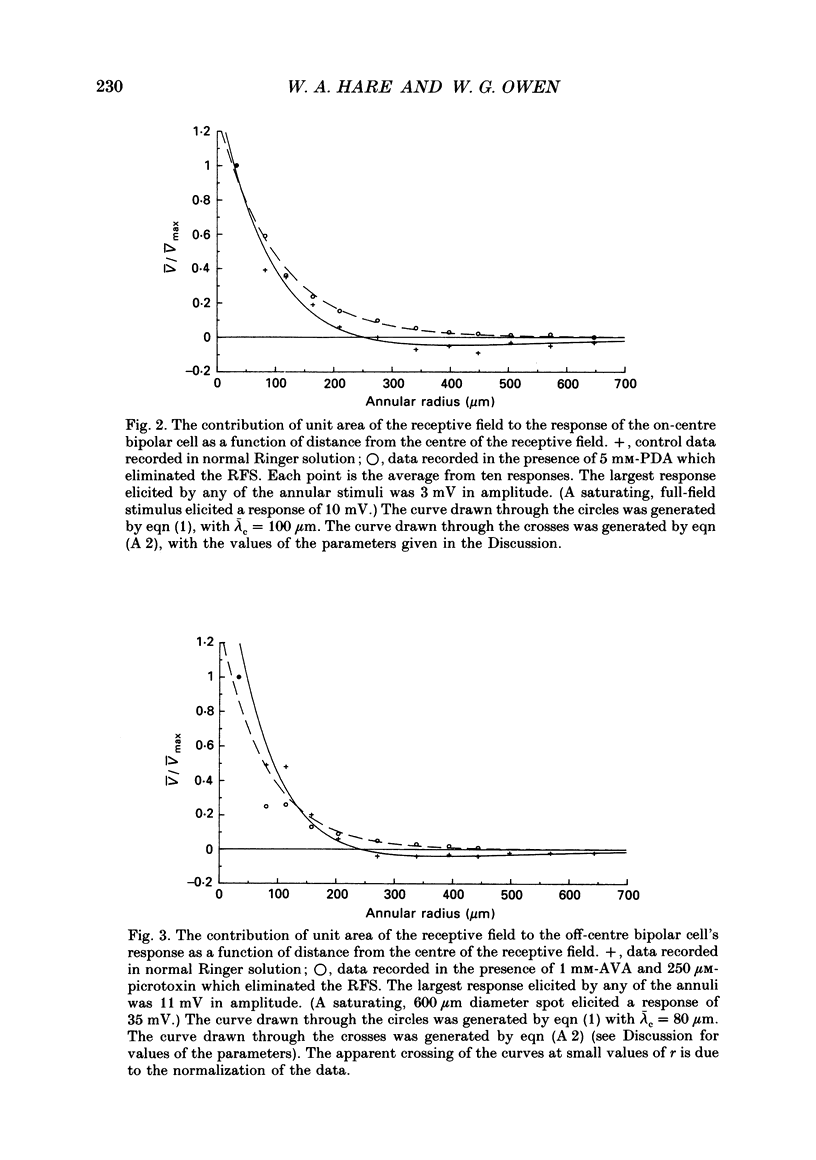
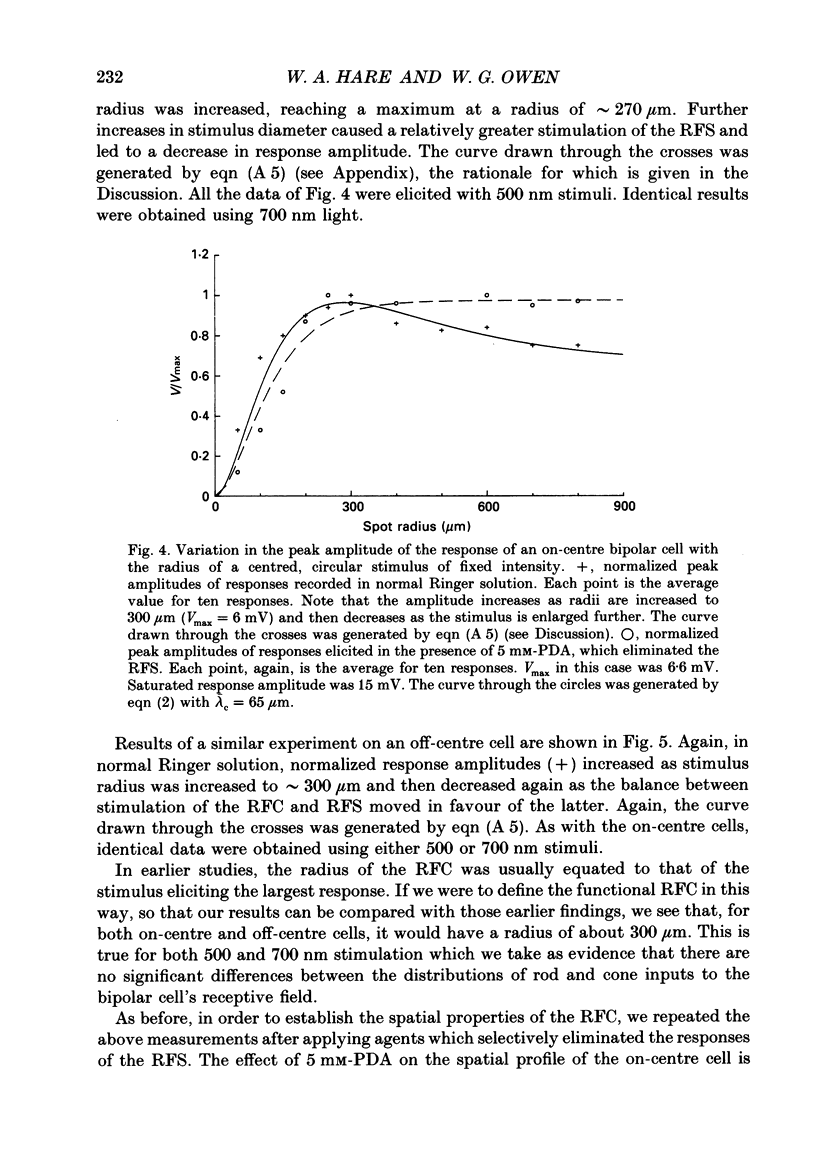
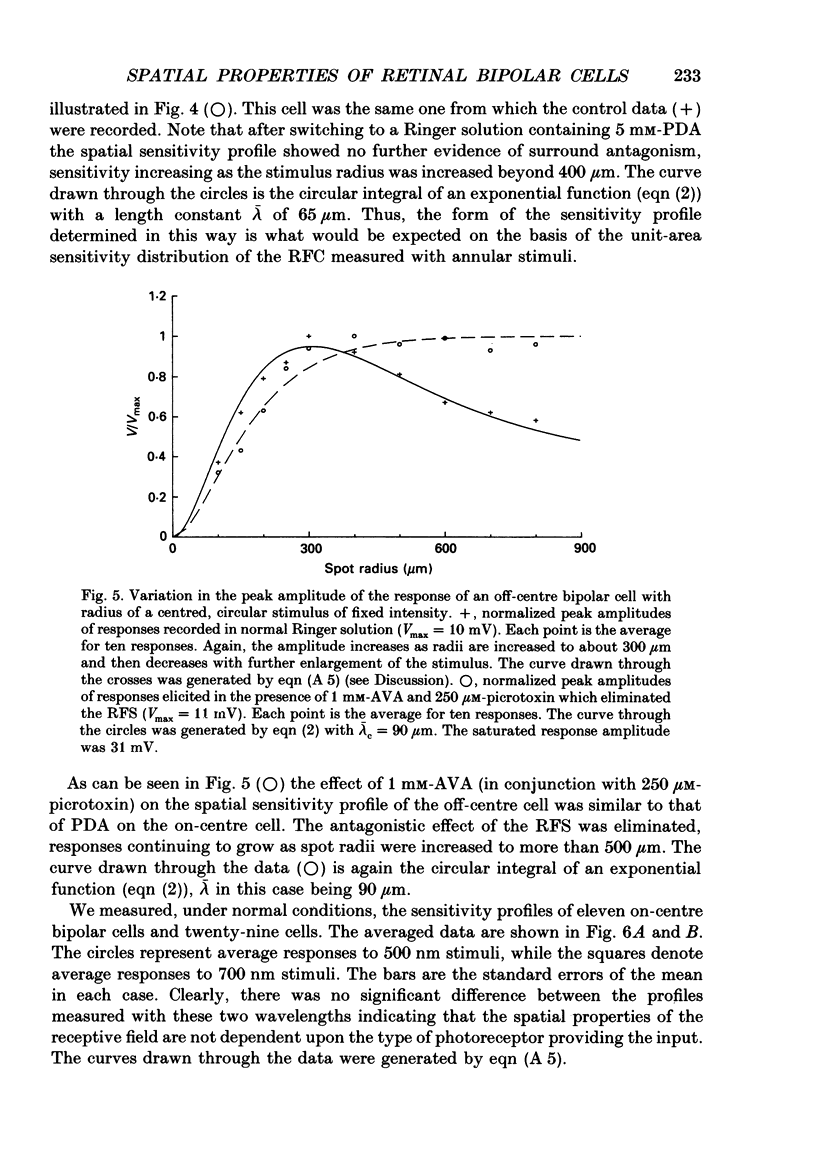
1. The spatial properties of rods, horizontal cells and bipolar cells were studied by intracellular recording in the isolated, perfused retina of the tiger salamander, Ambystoma tigrinum. Low stimulus intensities were used in order to keep cell responses close to, or within, their linear intensity/response range. 2. Spatial properties of bipolar cell receptive fields, measured while perfusing with normal Ringer solution, were compared with those measured during exposure to agents that eliminated the bipolar cells' receptive field surround (RFS). In this way, the spatial properties of the receptive field centre (RFC) and those of the RFS could be characterized independently. 3. To a good approximation, the contribution to the horizontal cell's response of unit area of its receptive field declined exponentially with distance from the centre of the receptive field. The (apparent) length constant describing this decay was 200 microns. The one-dimensional length constant of the horizontal cell syncytium was thus 248 microns. The variation of response amplitude with the radius of a centred circular stimulus was consistent with this finding. 4. This was true also of the RFCs of bipolar cells. The one-dimensional length constant of the RFC of off-centre bipolar cells averaged 124 microns. That of the RFC of on-centre cells averaged 62 microns though values were more variable, the RFCs of some on-centre cells being comparable to those of off-centre cells. These values were independent of the class of photoreceptor driving the bipolar cell. 5. The large size of the RFCs of off-centre cells and many on-centre cells cannot by explained by light scatter within the retina or by voltage spread within the rod syncytium. We proposed that off-centre cells are tightly coupled in a syncytium. On-centre cells, on average, are less tightly coupled. 6. The spatial properties of the bipolar cell's RFS were consistent with the notion that the RFS represents a convolution of the horizontal cell's receptive field and the bipolar cell's RFC. 7. The spatial properties of bipolar cell receptive fields were reconstructed from the measured properties of their RFCs and the measured properties of horizontal cell receptive fields. Under the conditions of our experiments, the bipolar cell's response could be described by a linear difference between a component generated by the RFC and a component generated by the RFS. 8. The spatial filtering characteristics of the bipolar cells were calculated from our data.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F., Copenhagen D. R. An analysis of transmission from cones to hyperpolarizing bipolar cells in the retina of the turtle. J Physiol. 1983 Jul;340:569–597. doi: 10.1113/jphysiol.1983.sp014781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Werblin F. S., Wilson M., Wu S. M. A sign-reversing pathway from rods to double and single cones in the retina of the tiger salamander. J Physiol. 1983 Mar;336:313–333. doi: 10.1113/jphysiol.1983.sp014583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Wilson M. Behaviour of the rod network in the tiger salamander retina mediated by membrane properties of individual rods. J Physiol. 1980 Dec;309:287–315. doi: 10.1113/jphysiol.1980.sp013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Hare W. A., Owen W. G. Voltage gain of signal transfer from retinal rods to bipolar cells in the tiger salamander. J Physiol. 1987 Oct;391:125–140. doi: 10.1113/jphysiol.1987.sp016730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler P. B., Hodgkin A. L., McNaughton P. A. A surprising property of electrical spread in the network of rods in the turtle's retina. Nature. 1978 Aug 10;274(5671):562–565. doi: 10.1038/274562a0. [DOI] [PubMed] [Google Scholar]
- Detwiler P. B., Hodgkin A. L., McNaughton P. A. Temporal and spatial characteristics of the voltage response of rods in the retina of the snapping turtle. J Physiol. 1980 Mar;300:213–250. doi: 10.1113/jphysiol.1980.sp013159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare W. A., Lowe J. S., Owen G. Morphology of physiologically identified bipolar cells in the retina of the tiger salamander, Ambystoma tigrinum. J Comp Neurol. 1986 Oct 1;252(1):130–138. doi: 10.1002/cne.902520108. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Double color-opponent receptive fields of carp bipolar cells. Vision Res. 1983;23(4):381–388. doi: 10.1016/0042-6989(83)90085-8. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Effects of gamma-aminobutyric acid on isolated cone photoreceptors of the turtle retina. J Physiol. 1986 Apr;373:443–461. doi: 10.1113/jphysiol.1986.sp016057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueppers F., Mills J. Trypsin inhibition by mouse serum: sexual dimorphism controlled by testosterone. Science. 1983 Jan 14;219(4581):182–184. doi: 10.1126/science.6849130. [DOI] [PubMed] [Google Scholar]
- Lasansky A. Contacts between receptors and electrophysiologically identified neurones in the retina of the larval tiger salamander. J Physiol. 1978 Dec;285:531–542. doi: 10.1113/jphysiol.1978.sp012587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Lateral contacts and interactions of horizontal cell dendrites in the retina of the larval tiger salamander. J Physiol. 1980 Apr;301:59–68. doi: 10.1113/jphysiol.1980.sp013188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. The generation and spread of S-potentials in fish (Cyprinidae). J Physiol. 1967 Sep;192(2):437–461. doi: 10.1113/jphysiol.1967.sp008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen W. G., Torre V. High-pass filtering of small signals by retinal rods. Ionic studies. Biophys J. 1983 Mar;41(3):325–339. doi: 10.1016/S0006-3495(83)84444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A., Simon E. J. Properties of centre-hyperpolarizing, red-sensitive bipolar cells in the turtle retina. J Physiol. 1975 Jun;248(2):317–334. doi: 10.1113/jphysiol.1975.sp010976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Kujiraoka T. Characteristics of bipolar-bipolar coupling in the carp retina. J Gen Physiol. 1988 Feb;91(2):275–287. doi: 10.1085/jgp.91.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Kujiraoka T. Physiological and morphological identification of two types of on-center bipolar cells in the carp retina. J Comp Neurol. 1982 Feb 20;205(2):161–170. doi: 10.1002/cne.902050207. [DOI] [PubMed] [Google Scholar]
- Skrzypek J., Werblin F. Lateral interactions in absence of feedback to cones. J Neurophysiol. 1983 Apr;49(4):1007–1016. doi: 10.1152/jn.1983.49.4.1007. [DOI] [PubMed] [Google Scholar]
- Torre V., Owen W. G. High-pass filtering of small signals by the rod network in the retina of the toad, Bufo marinus. Biophys J. 1983 Mar;41(3):305–324. doi: 10.1016/S0006-3495(83)84443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda J. Membrane resistance changes underlying the bipolar cell response in the carp retina. Vision Res. 1973 Feb;13(2):283–294. doi: 10.1016/0042-6989(73)90107-7. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Response of retinal cells to moving spots: intracellular recording in Necturus maculosus. J Neurophysiol. 1970 May;33(3):342–350. doi: 10.1152/jn.1970.33.3.342. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. T. Synaptic orgnization of the inner plexiform layer in the retina of the tiger salamander. J Neurocytol. 1974 Mar;3(1):1–33. doi: 10.1007/BF01111929. [DOI] [PubMed] [Google Scholar]


