Abstract
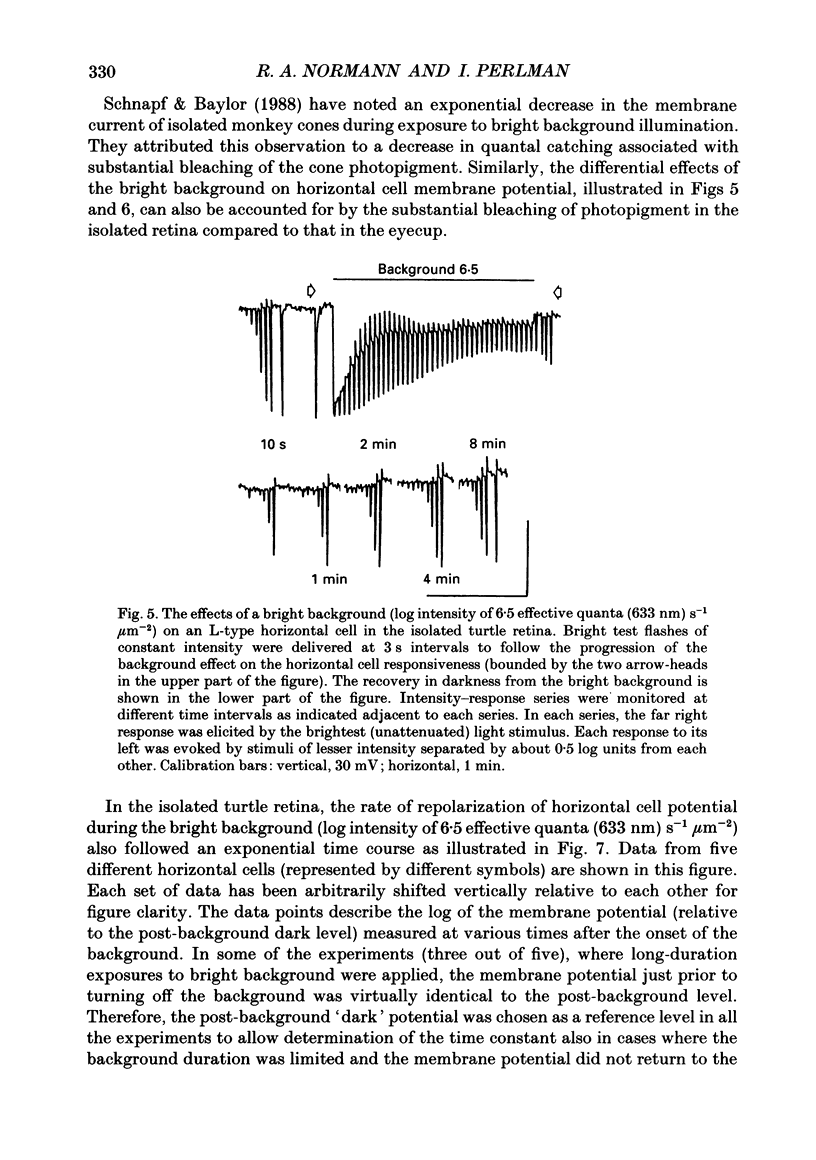
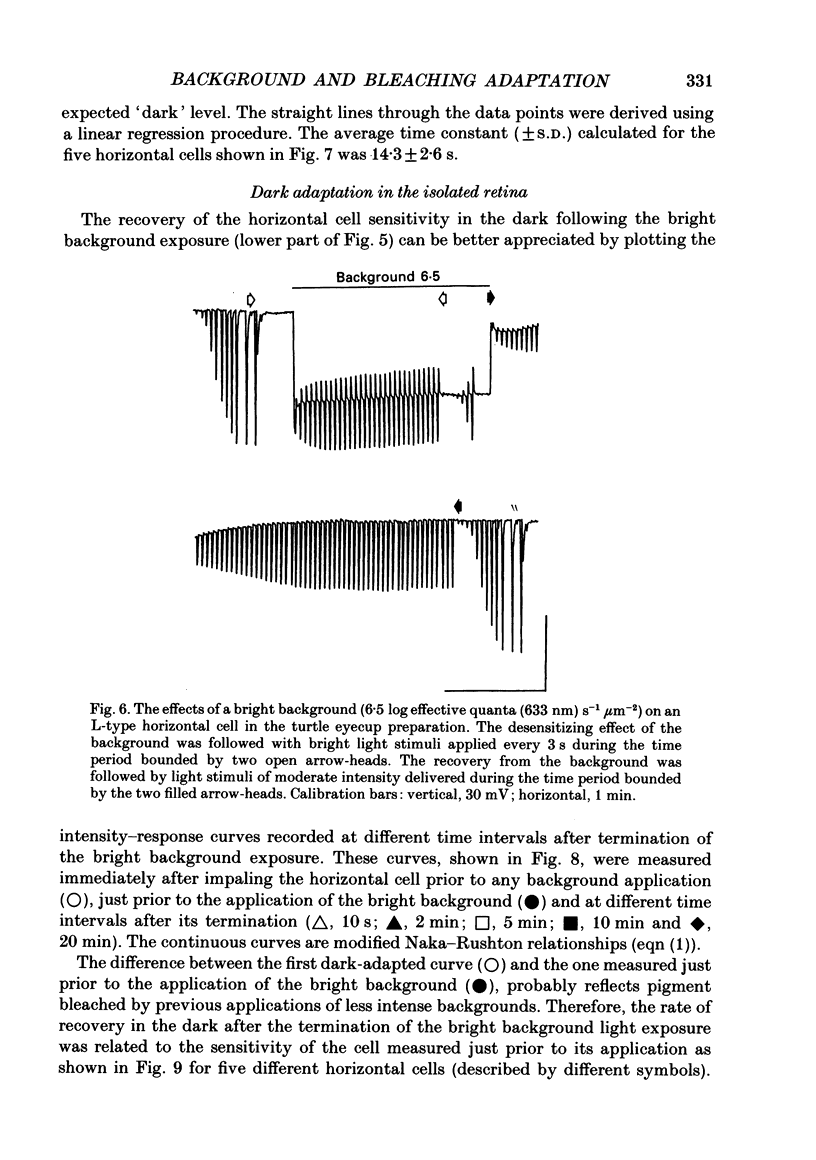
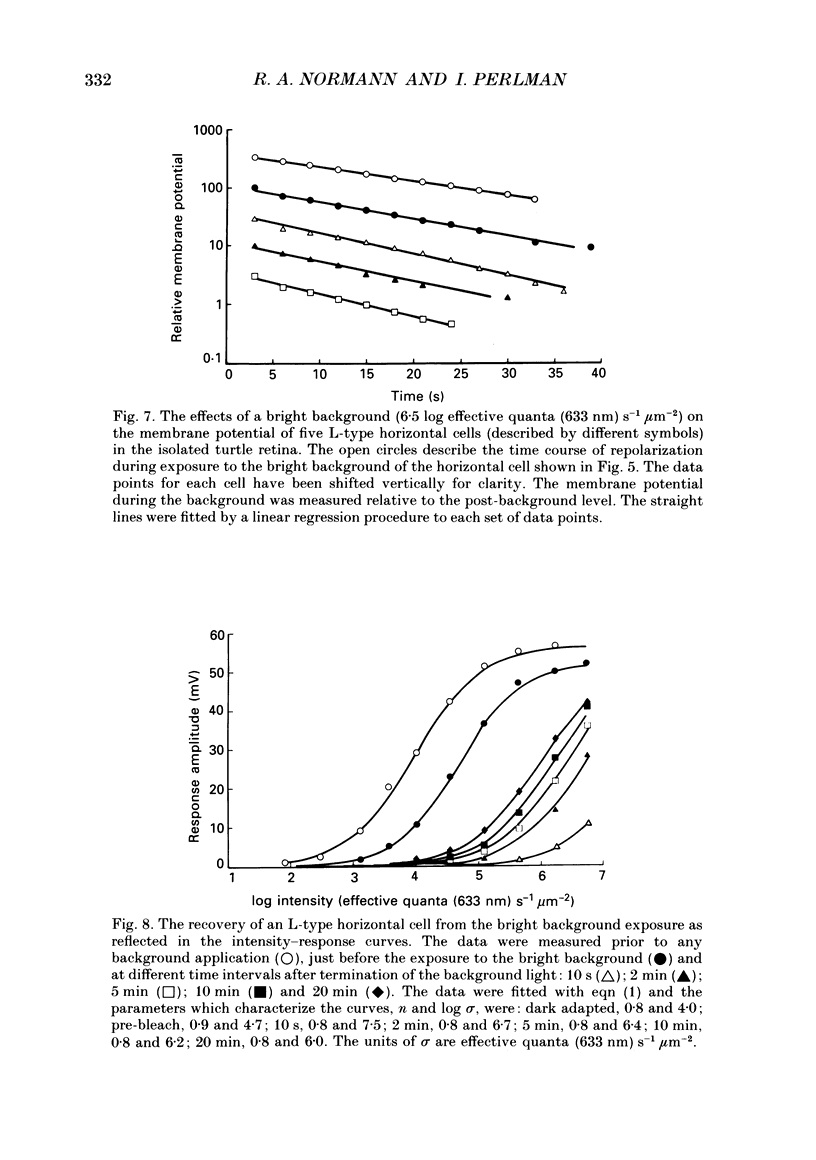
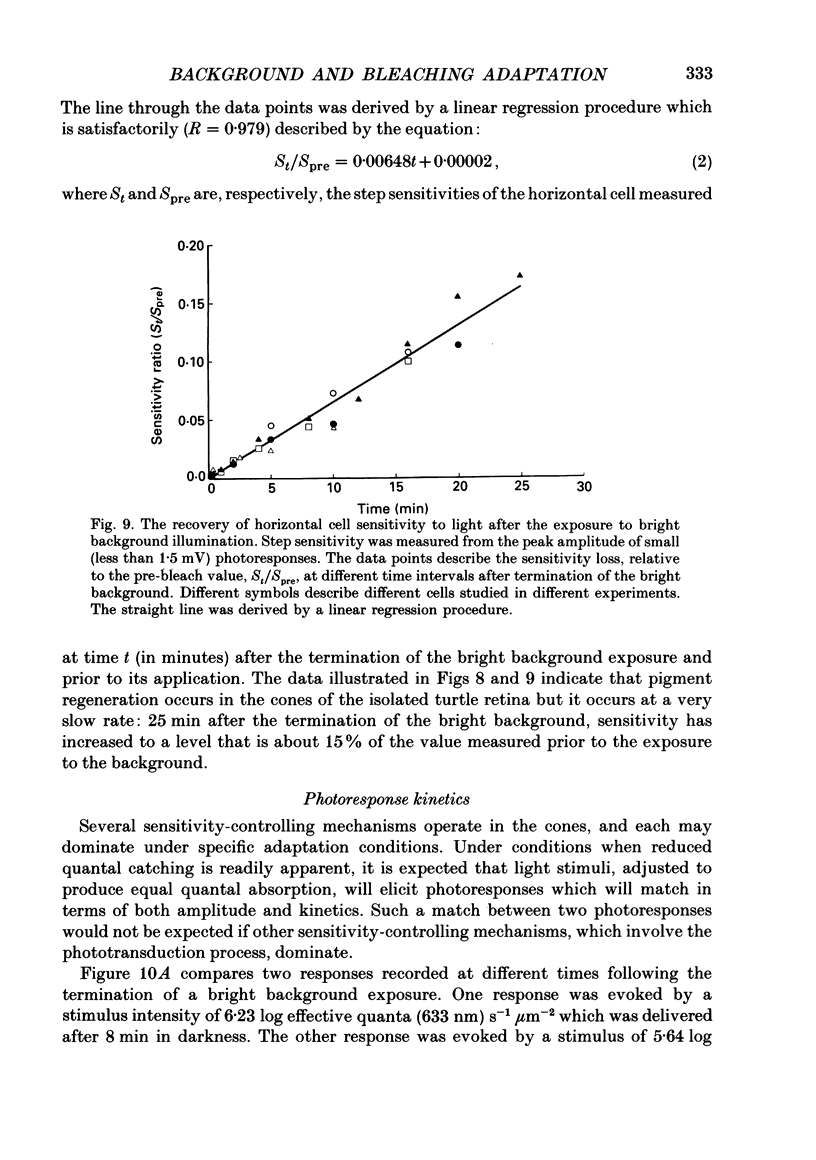
1. The effects of background illumination and bleached photopigment on luminosity type horizontal cells were studied in the isolated turtle retina. 2. Background illumination, which produced less than 60% bleaching, hyperpolarized and desensitized the horizontal cells to a degree which depended upon the background intensity. The desensitization of horizontal cells by these backgrounds is described by a Weber-Fechner type relationship. This desensitization primarily reflects the activation of a 'gain reduction' mechanism and cannot be accounted for by 'response compression'. 3. Following the termination of these backgrounds, horizontal cell sensitivity partially recovered but did not return to the pre-background, dark-adapted level. This desensitization was attributed to the presence of bleached photoproducts which were produced by the background exposure. 4. Application of very bright backgrounds caused the horizontal cells to initially hyperpolarize, and then to gradually depolarize towards the dark-adapted level along an exponential time course which appeared to reflect the decreased quantal catching associated with very high levels of photopigment bleaching. 5. From the time constant of the exponential decay of horizontal cell potential during the bright background illumination, the photosensitivity to bleaching of the cone photopigment was determined to be 4.5 x 10(7) effective quanta (633 nm) microns-2. 6. After termination of bright backgrounds which bleached more than 99% of the cone photopigment, the horizontal cell sensitivity increased linearly with time and after 25 min reached a level which was about 15% of the pre-background sensitivity. 7. Bleached photopigment reduces light sensitivity via at least two different mechanisms. For moderate degrees of bleaching (less than 95%), the presence of bleached photoproducts plays the major role in sensitivity control, producing a desensitization which is logarithmically related to the fraction of bleached pigment. During extensive bleaching (greater than 99%), the contribution of reduced quantal catching to sensitivity control becomes apparent and produces an additional loss in sensitivity which is linearly related to the fraction of unbleached pigment present.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern M. Rhodopsin kinetics in the human eye. J Physiol. 1971 Sep;217(2):447–471. doi: 10.1113/jphysiol.1971.sp009580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton R. M., Whitten D. N. Visual adaptation in monkey cones: recordings of late receptor potentials. Science. 1970 Dec 25;170(3965):1423–1426. doi: 10.1126/science.170.3965.1423. [DOI] [PubMed] [Google Scholar]
- Clack J. W., Pepperberg D. R. Desensitization of skate photoreceptors by bleaching and background light. J Gen Physiol. 1982 Dec;80(6):863–883. doi: 10.1085/jgp.80.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWLING J. E. NEURAL AND PHOTOCHEMICAL MECHANISMS OF VISUAL ADAPTATION IN THE RAT. J Gen Physiol. 1963 Jul;46:1287–1301. doi: 10.1085/jgp.46.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawis S. M. A model for light adaptation: producing Weber's law with bleaching-type kinetics. Biol Cybern. 1978 Sep 28;30(4):187–193. doi: 10.1007/BF00361040. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. Visual adaptation in the retina of the skate. J Gen Physiol. 1970 Oct;56(4):491–520. doi: 10.1085/jgp.56.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. G., Dowling J. E., Siegel I. M., Ripps H. Retinal mechanisms of visual adaptation in the skate. J Gen Physiol. 1975 Apr;65(4):483–502. doi: 10.1085/jgp.65.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. G. The search for the site of visual adaptation. Vision Res. 1986;26(9):1417–1429. doi: 10.1016/0042-6989(86)90165-3. [DOI] [PubMed] [Google Scholar]
- Hemilä S. The stimulus-response functions of visual systems. Vision Res. 1987;27(8):1253–1261. doi: 10.1016/0042-6989(87)90201-x. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., Obryan P. M. Internal recording of the early receptor potential in turtle cones. J Physiol. 1977 Jun;267(3):737–766. doi: 10.1113/jphysiol.1977.sp011836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood D. C., Hock P. A. Recovery of cone receptor activity in the frog's isolated retina. Vision Res. 1973 Oct;13(10):1943–1951. doi: 10.1016/0042-6989(73)90065-5. [DOI] [PubMed] [Google Scholar]
- Itzhaki A., Perlman I. Light adaptation of red cones and L1-horizontal cells in the turtle retina: effect of the background spatial pattern. Vision Res. 1987;27(5):685–696. doi: 10.1016/0042-6989(87)90065-4. [DOI] [PubMed] [Google Scholar]
- Leibovic K. N., Dowling J. E., Kim Y. Y. Background and bleaching equivalence in steady-state adaptation of vertebrate rods. J Neurosci. 1987 Apr;7(4):1056–1063. doi: 10.1523/JNEUROSCI.07-04-01056.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from luminosity units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):587–599. doi: 10.1113/jphysiol.1966.sp008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann R. A., Anderton P. J. The incremental sensitivity curve of turtle cone photoreceptors. Vision Res. 1983;23(12):1731–1733. doi: 10.1016/0042-6989(83)90190-6. [DOI] [PubMed] [Google Scholar]
- Normann R. A., Perlman I. Evaluating sensitivity changing mechanisms in light-adapted photoreceptors. Vision Res. 1979;19(4):391–394. doi: 10.1016/0042-6989(79)90101-9. [DOI] [PubMed] [Google Scholar]
- Normann R. A., Perlman I. Signal transmission from red cones to horizontal cells in the turtle retina. J Physiol. 1979 Jan;286:509–524. doi: 10.1113/jphysiol.1979.sp012634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann R. A., Perlman I. The effects of background illumination on the photoresponses of red and green cones. J Physiol. 1979 Jan;286:491–507. doi: 10.1113/jphysiol.1979.sp012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann R. A., Werblin F. S. Control of retinal sensitivity. I. Light and dark adaptation of vertebrate rods and cones. J Gen Physiol. 1974 Jan;63(1):37–61. doi: 10.1085/jgp.63.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperberg D. R., Brown P. K., Lurie M., Dowling J. E. Visual pigment and photoreceptor sensitivity in the isolated skate retina. J Gen Physiol. 1978 Apr;71(4):369–396. doi: 10.1085/jgp.71.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperberg D. R. Rhodopsin and visual adaptation: analysis of photoreceptor thresholds in the isolated skate retina. Vision Res. 1984;24(4):357–366. doi: 10.1016/0042-6989(84)90061-0. [DOI] [PubMed] [Google Scholar]
- Perlman I., Auerbach E. The relationship between visual sensitivity and rhodopsin density in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1981 Jun;20(6):758–765. [PubMed] [Google Scholar]
- RUSHTON W. A. Rhodopsin measurement and dark-adaptation in a subject deficient in cone vision. J Physiol. 1961 Apr;156:193–205. doi: 10.1113/jphysiol.1961.sp006668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. VISUAL ADAPTATION. Proc R Soc Lond B Biol Sci. 1965 Mar 16;162:20–46. doi: 10.1098/rspb.1965.0024. [DOI] [PubMed] [Google Scholar]
- Ripps H., Brin K. P., Weale R. A. Rhodopsin and visual threshold in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1978 Aug;17(8):735–745. [PubMed] [Google Scholar]
- Ripps H., Mehaffey L., 3rd, Siegel I. M. Rhodopsin kinetics in the cat retina. J Gen Physiol. 1981 Mar;77(3):317–334. doi: 10.1085/jgp.77.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeton J. M. Photoreceptor light adaptation models: an evaluation. Vision Res. 1983;23(12):1549–1554. doi: 10.1016/0042-6989(83)90168-2. [DOI] [PubMed] [Google Scholar]
- Valeton J. M., van Norren D. Light adaptation of primate cones: an analysis based on extracellular data. Vision Res. 1983;23(12):1539–1547. doi: 10.1016/0042-6989(83)90167-0. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Gallin E., Hollyfield J. G., Ripps H., Bridges C. D. Photoreceptor thresholds and visual pigment levels in normal and vitamin A-deprived Xenopus tadpoles. J Neurophysiol. 1976 Nov;39(6):1272–1287. doi: 10.1152/jn.1976.39.6.1272. [DOI] [PubMed] [Google Scholar]