Abstract
1. Micellar solubilization of lipolytic products is an important step in lipid absorption. However, micelles are not absorbed intact; dissociation of lipolytic products from bile salt micelles must occur. The dissociation of micelles has been postulated to occur in an acidic microclimate. 2. The effect of an acidic microclimate on the uptake of micellar fatty acid was examined in the rat intestine. We reported that the presence of a lower pH microclimate is associated with a higher fatty acid uptake, suggesting that a lower pH enhances fatty acid uptakes from the micelles. 3. Fatty acid uptake from solutions containing a constant amount of bile salt (10 mM) and varying amounts of fatty acid (3.3-26.4 mM) revealed a saturation phenomenon which reflects the fatty acid carrying capacity of a 10 mM-taurocholate solution. 4. There was a linear relationship between fatty acid uptake and fatty acid concentration when the micellar solutions contained a constant ratio of fatty acid and taurocholate (1.32). 5. Our results indicate that the fatty acid carrying capacity of the micelle and the number of micelles in the solution are both important determinants for the amount of fatty acids delivered to the microclimate. The amount of fatty acids derived from the dissociation of micelles within the microclimate determines fatty acid uptake by the intestine.
Full text
PDF


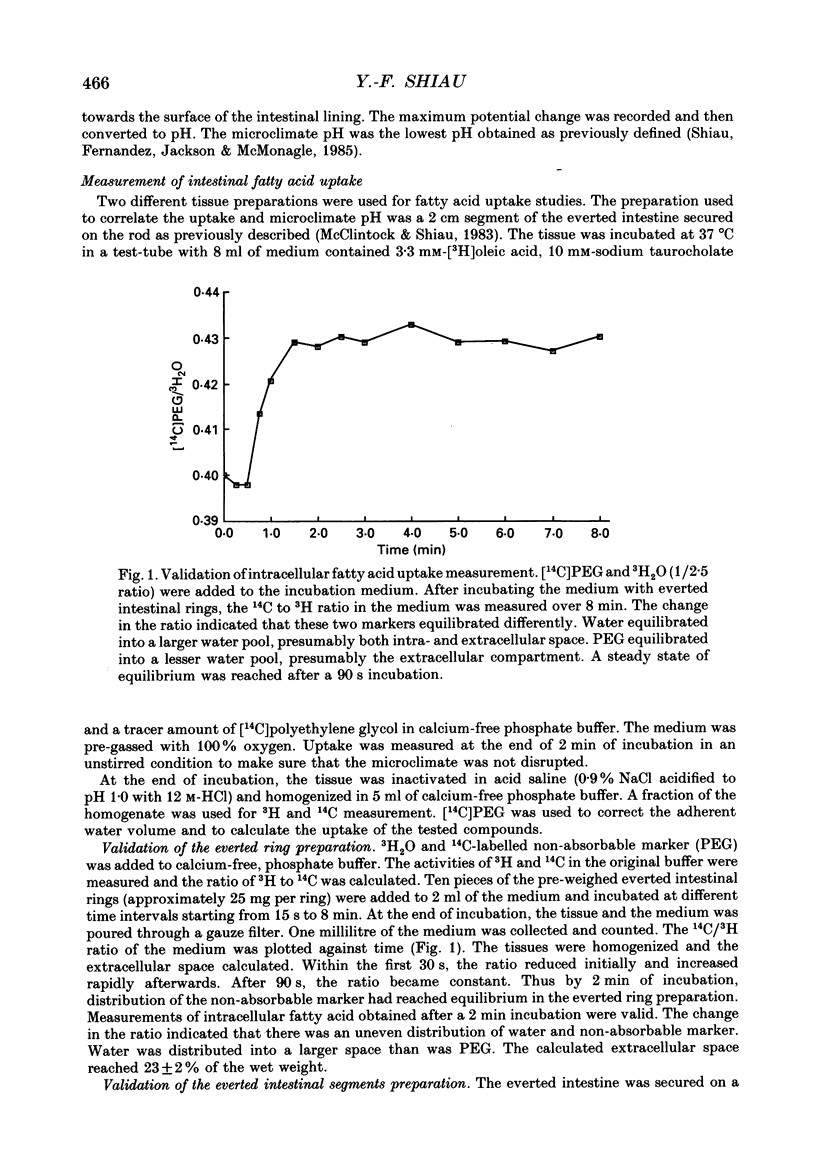

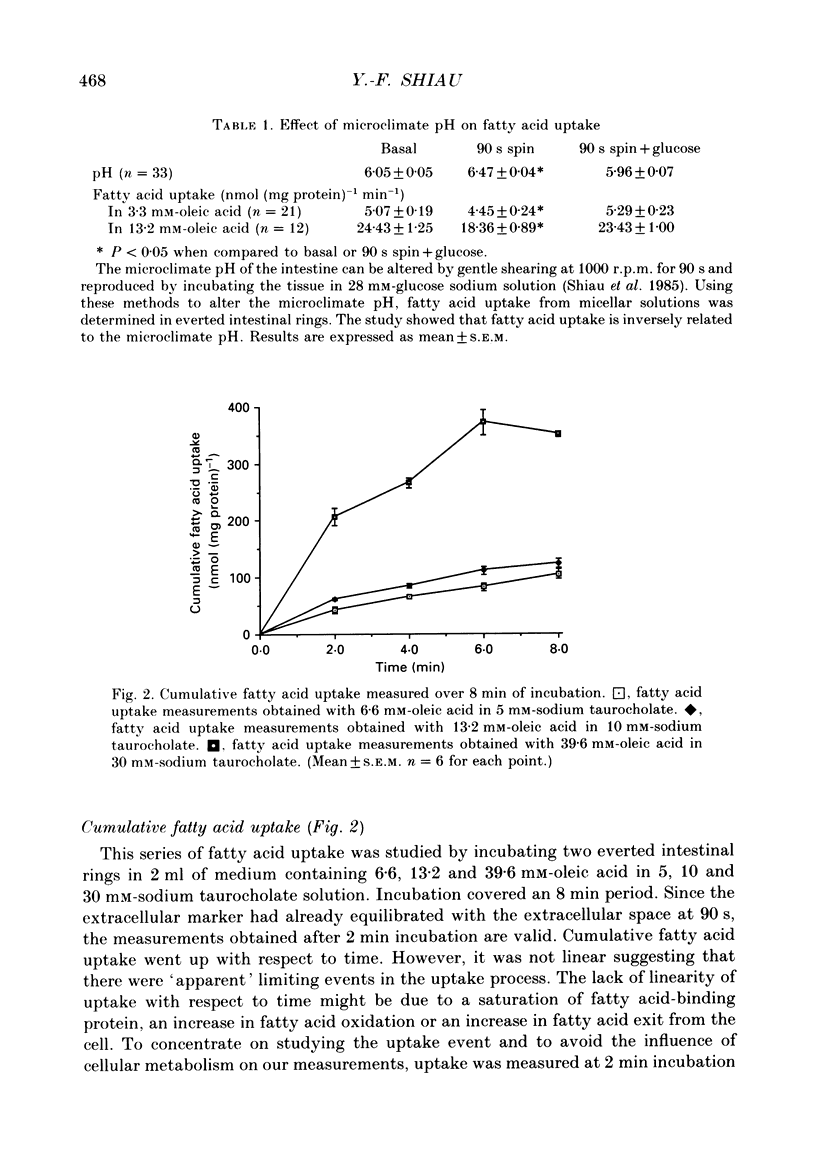
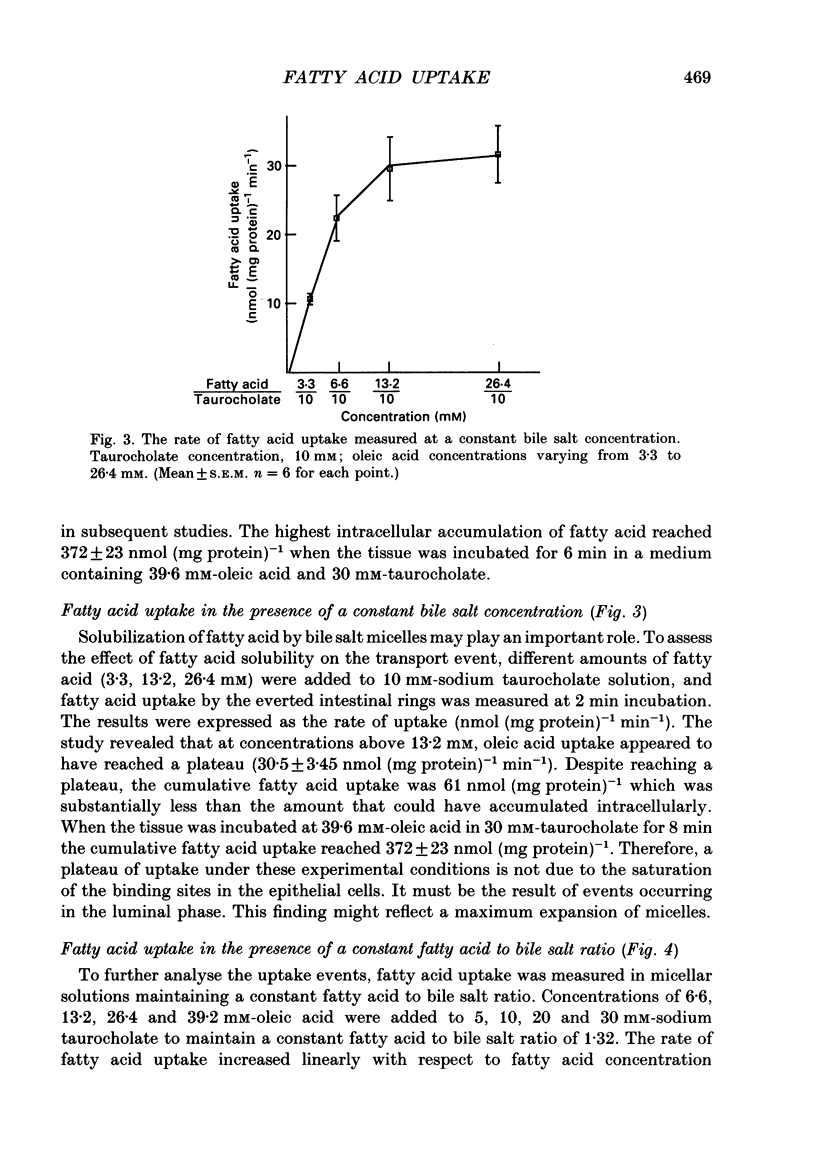
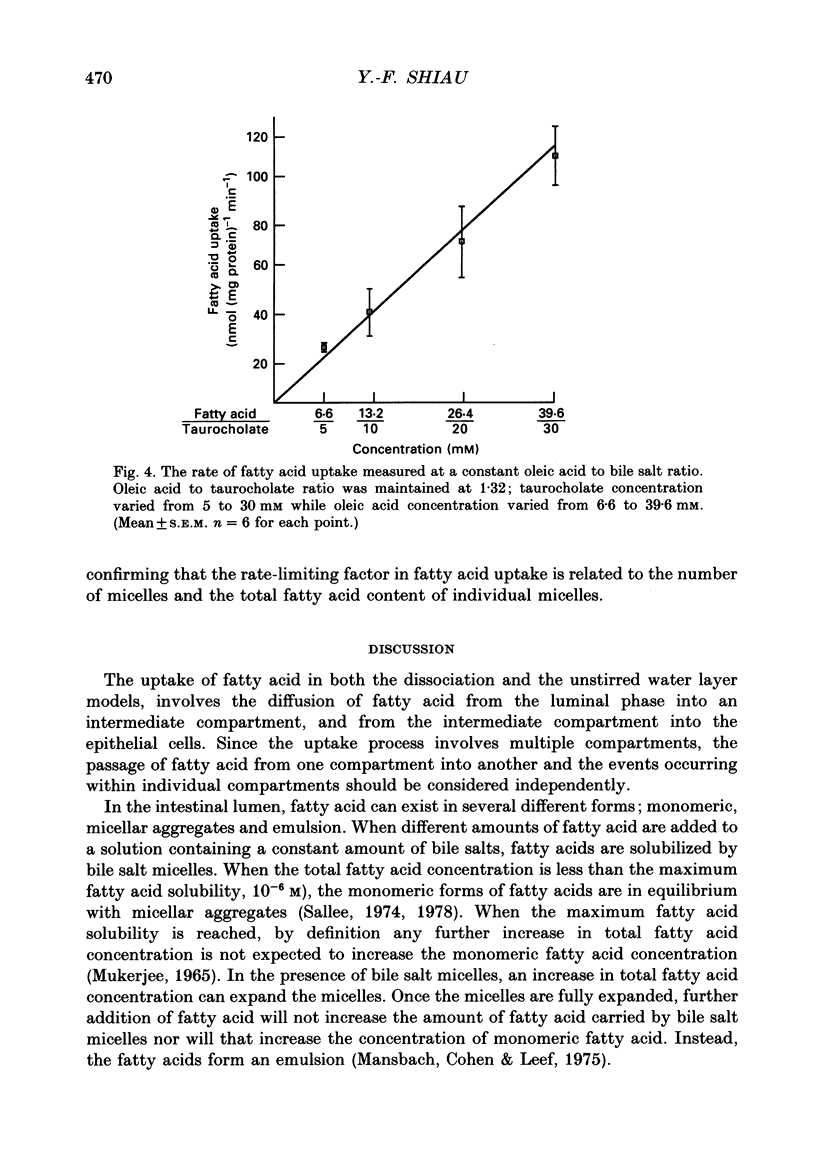




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carey M. C., Small D. M. The characteristics of mixed micellar solutions with particular reference to bile. Am J Med. 1970 Nov;49:590–608. doi: 10.1016/s0002-9343(70)80127-9. [DOI] [PubMed] [Google Scholar]
- Chijiiwa K., Linscheer W. G. Distribution and monomer activity of cholesterol in micellar bile salt: effect of cholesterol level. Am J Physiol. 1987 Mar;252(3 Pt 1):G309–G314. doi: 10.1152/ajpgi.1987.252.3.G309. [DOI] [PubMed] [Google Scholar]
- Chijiiwa K., Linscheer W. G. Mechanism of pH effect on oleic acid and cholesterol absorption in the rat. Am J Physiol. 1987 Apr;252(4 Pt 1):G506–G510. doi: 10.1152/ajpgi.1987.252.4.G506. [DOI] [PubMed] [Google Scholar]
- HOFMANN A. F., BORGSTROM B. Physico-chemical state of lipids in intestinal content during their digestion and absorption. Fed Proc. 1962 Jan-Feb;21:43–50. [PubMed] [Google Scholar]
- HOGBEN C. A., TOCCO D. J., BRODIE B. B., SCHANKER L. S. On the mechanism of intestinal absorption of drugs. J Pharmacol Exp Ther. 1959 Apr;125(4):275–282. [PubMed] [Google Scholar]
- Hoffman N. E., Simmonds W. J. The intestinal uptake and esterification, in vitro, of fatty acid as a diffusion limited process. Biochim Biophys Acta. 1971 Aug 13;241(2):331–333. doi: 10.1016/0005-2736(71)90032-0. [DOI] [PubMed] [Google Scholar]
- Hoffman N. E. The relationship between uptake in vitro of oleic acid and micellar solubilization. Biochim Biophys Acta. 1970;196(2):193–203. doi: 10.1016/0005-2736(70)90006-4. [DOI] [PubMed] [Google Scholar]
- Hofmann A. F., Small D. M. Detergent properties of bile salts: correlation with physiological function. Annu Rev Med. 1967;18:333–376. doi: 10.1146/annurev.me.18.020167.002001. [DOI] [PubMed] [Google Scholar]
- Lucas M. L., Schneider W., Haberich F. J., Blair J. A. Direct measurement by pH-microelectrode of the pH microclimate in rat proximal jejunum. Proc R Soc Lond B Biol Sci. 1975 Dec 31;192(1106):39–48. doi: 10.1098/rspb.1975.0150. [DOI] [PubMed] [Google Scholar]
- Mansbach C. M., 2nd, Cohen R. S., Leff P. B. Isolation and properties of the mixed lipid micelles present in intestinal content during fat digestion in man. J Clin Invest. 1975 Oct;56(4):781–791. doi: 10.1172/JCI108156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock C., Shiau Y. F. Jejunum is more important than terminal ileum for taurocholate absorption in rats. Am J Physiol. 1983 May;244(5):G507–G514. doi: 10.1152/ajpgi.1983.244.5.G507. [DOI] [PubMed] [Google Scholar]
- Sallee V. L. Apparent monomer activity of saturated fatty acids im micellar bile salt solutions measured by a polyethylene partitioning system. J Lipid Res. 1974 Jan;15(1):56–64. [PubMed] [Google Scholar]
- Sallee V. L. Determinants of fatty acid and alcohol monomer activities in mixed micellar solutions. J Lipid Res. 1978 Feb;19(2):207–214. [PubMed] [Google Scholar]
- Sallee V. L. Permeation of long-chain fatty acids and alcohols in rat intestine. Am J Physiol. 1979 Jun;236(6):E721–E727. doi: 10.1152/ajpendo.1979.236.6.E721. [DOI] [PubMed] [Google Scholar]
- Sallee V. L., Wilson F. A., Dietschy J. M. Determination of unidirectional uptake rates for lipids across the intestinal brush border. J Lipid Res. 1972 Mar;13(2):184–192. [PubMed] [Google Scholar]
- Shiau Y. F., Fernandez P., Jackson M. J., McMonagle S. Mechanisms maintaining a low-pH microclimate in the intestine. Am J Physiol. 1985 Jun;248(6 Pt 1):G608–G617. doi: 10.1152/ajpgi.1985.248.6.G608. [DOI] [PubMed] [Google Scholar]
- Shiau Y. F., Levine G. M. pH dependence of micellar diffusion and dissociation. Am J Physiol. 1980 Sep;239(3):G177–G182. doi: 10.1152/ajpgi.1980.239.3.G177. [DOI] [PubMed] [Google Scholar]
- Westergaard H., Dietschy J. M. Delineation of the dimensions and permeability characteristics of the two major diffusion barriers to passive mucosal uptake in the rabbit intestine. J Clin Invest. 1974 Sep;54(3):718–732. doi: 10.1172/JCI107810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard H., Dietschy J. M. The mechanism whereby bile acid micelles increase the rate of fatty acid and cholesterol uptake into the intestinal mucosal cell. J Clin Invest. 1976 Jul;58(1):97–108. doi: 10.1172/JCI108465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F. A., Dietschy J. M. Characterization of bile acid absorption across the unstirred water layer and brush border of the rat jejunum. J Clin Invest. 1972 Dec;51(12):3015–3025. doi: 10.1172/JCI107129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F. A., Sallee V. L., Dietschy J. M. Unstirred water layers in intestine: rate determinant of fatty acid absorption from micellar solutions. Science. 1971 Dec 3;174(4013):1031–1033. doi: 10.1126/science.174.4013.1031. [DOI] [PubMed] [Google Scholar]


