Abstract
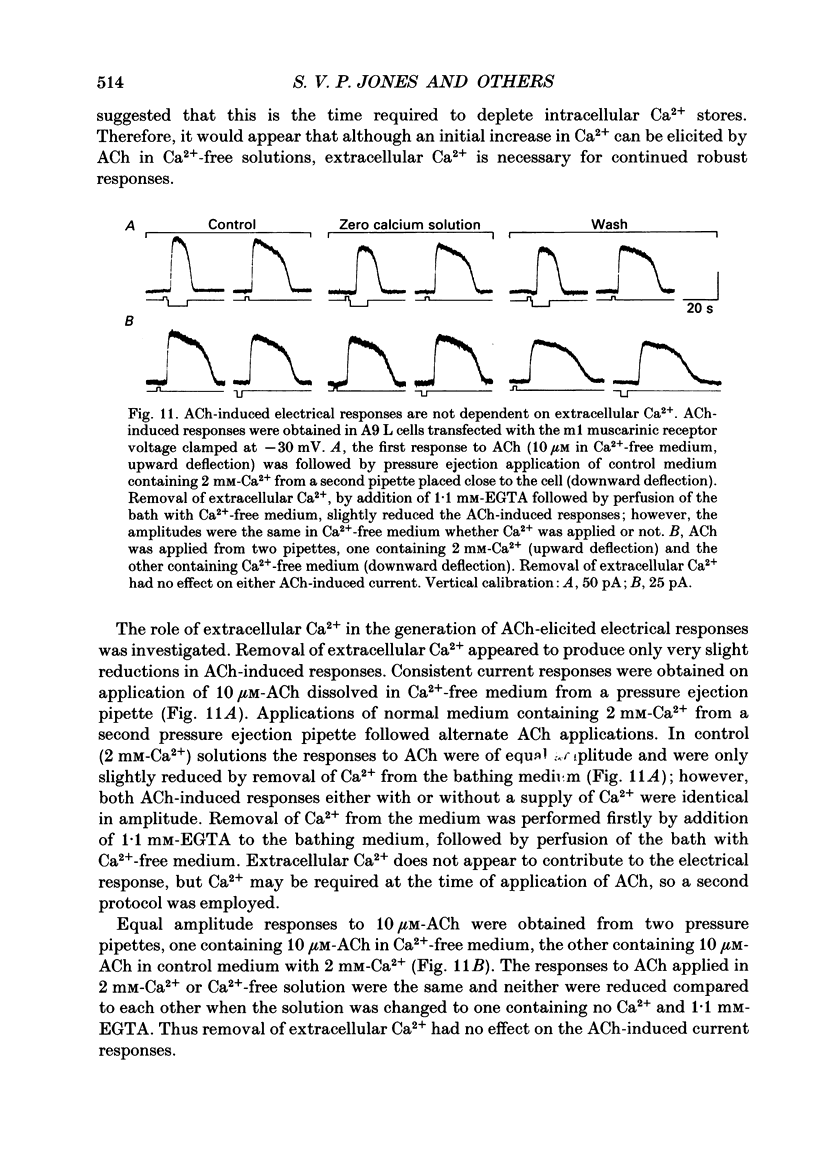
1. The mechanism by which cloned m1 and m3 muscarinic receptor subtypes activate Ca2+-dependent channels was investigated with whole-cell and cell-attached patch-clamp recording techniques and with Fura-2 Ca2+ indicator dye measurements in cultured A9 L cells transfected with rat m1 and m3 cDNAs. 2. The Ca2+-dependent K+ and Cl- currents induced by muscarinic receptor stimulation were dependent on GTP. Responses were reduced when GTP was excluded from the intracellular recording solution or when GDP-beta-S was added. Intracellular GTP-gamma-S activated spontaneous fluctuations and permitted only one acetylcholine-(ACh) induced current response. These results implicate GTP-binding proteins (G protein) in the signal transduction pathway. This G protein is probably not pertussis toxin-sensitive as the ACh-induced electrical response was not abolished by pertussis toxin treatment. 3. Cell-attached single-channel recordings revealed activation of ion channels within the patch during application of ACh outside the patch, implying that second messengers might be involved in the ACh-induced response. Two types of K+ channel were activated, a discrete channel of 36 pS and channel activity calculated to be about 5 pS. 4. Application of 8-bromo cyclic AMP or 1-oleoyl-1,2-acetylglycerol (OAG) produced no electrical response and did not affect the ACh-induced responses. Phorbol myristic acetate (PMA) evoked no electrical response, but reduced the ACh-induced responses. 5. Inclusion of inositol 1,4,5-trisphosphate (IP3) in the intracellular pipette solution activated outward currents at -50 mV associated with an increase in conductance. The IP3-induced current response reversed polarity at -65 mV and showed a dependence on K+. Increasing the intracellular free Ca2+ concentration ([Ca2+]i) from 20 nM to 1 microM also induced an outward current response associated with an increase in conductance. Inclusion of inositol 1,3,4,5-tetrakisphosphate (IP4) in the intracellular solution had no effect on the A9 L cells. 6. Fura-2 measurements revealed ACh-induced increases in Cai2+. The Ca2+ responses were abolished by atropine showing that they were muscarinic in nature. Removal of extracellular Ca2+ did not affect the initial ACh-induced increase in Cai2+ but subsequent Cai2+ responses to ACh were depressed, suggesting depletion of Ca2+ intracellular stores. Residual though small responses continued to be elicited by ACh. Barium (5 mM) had little effect and cobalt slightly reduced the ACh-induced Ca2+ response. 7. The ACh-induced currents recorded at -50 mV were unaffected by removal of extracellular Ca2+.(ABSTRACT TRUNCATED AT 400 WORDS)
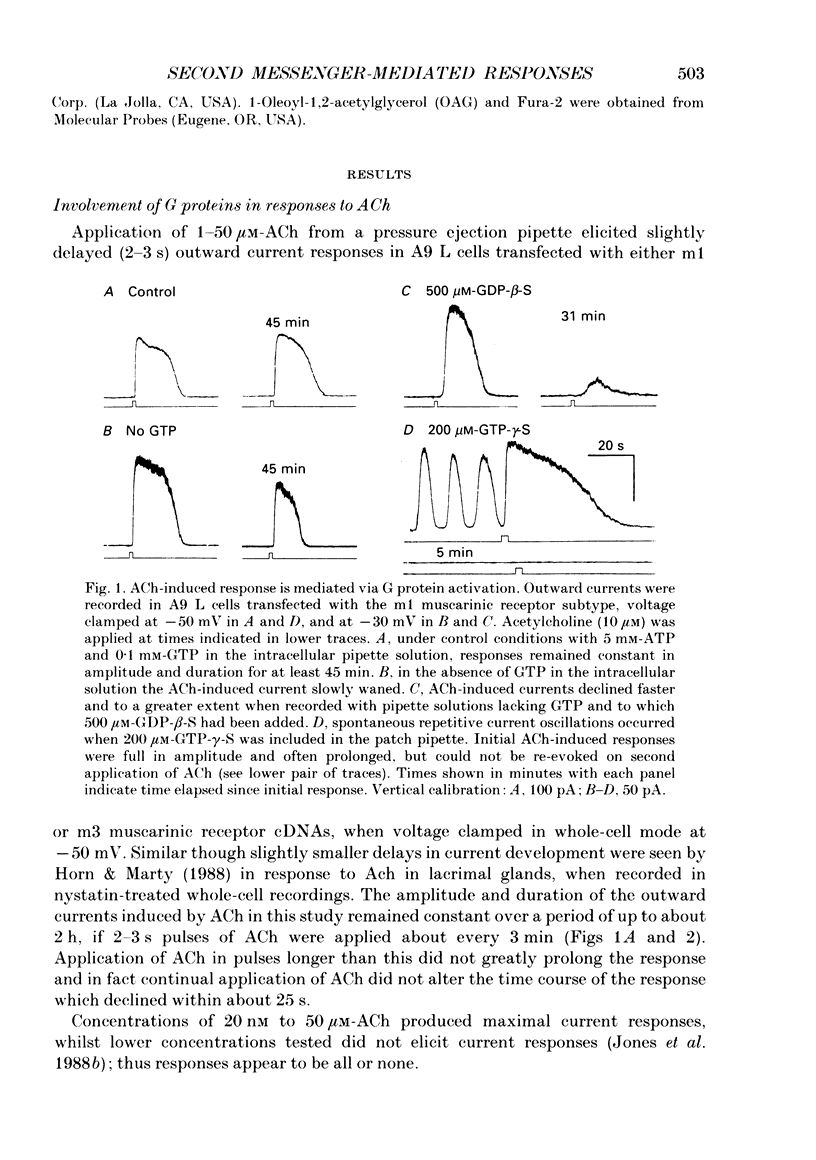
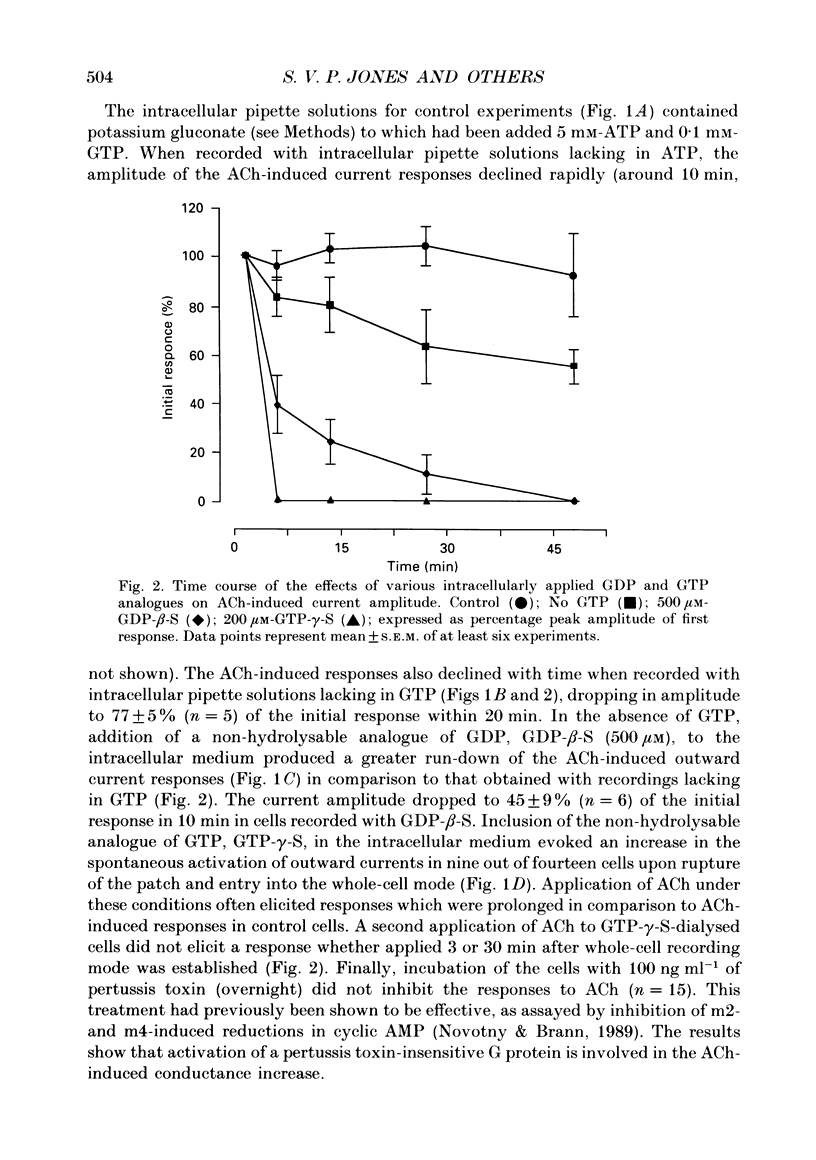
Full text
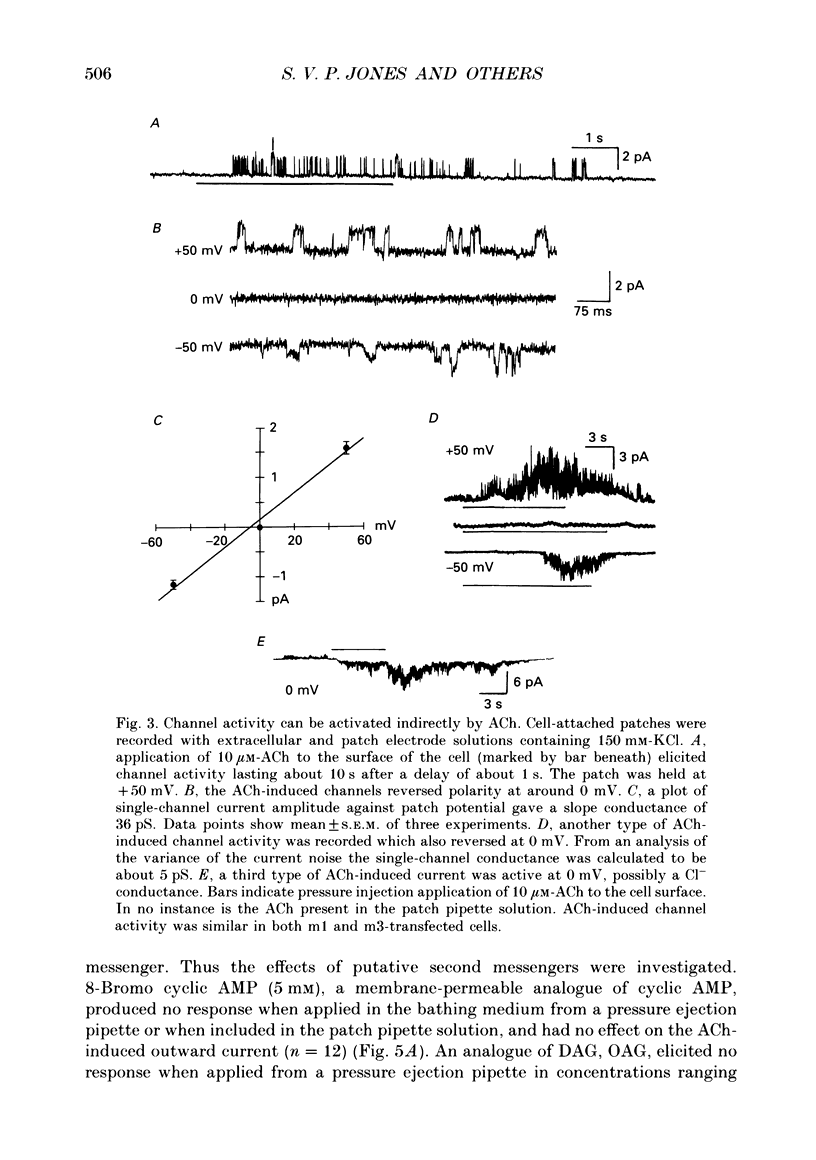
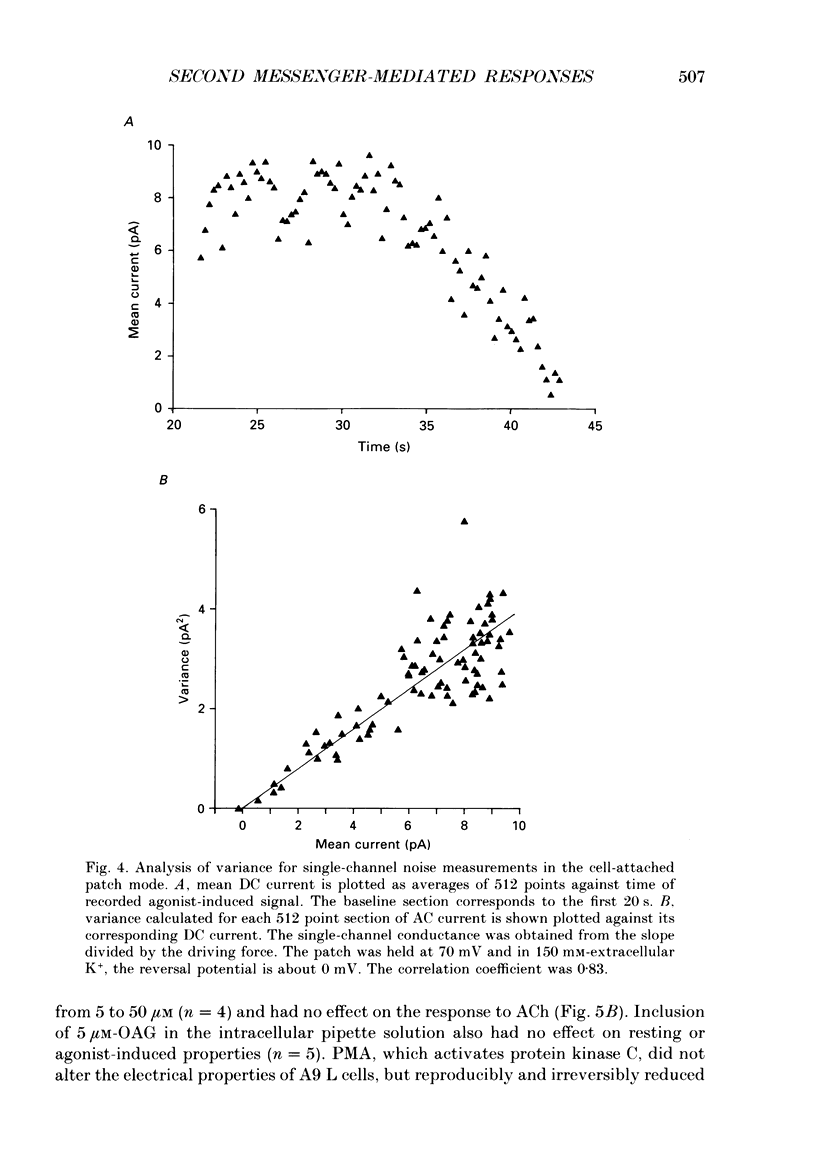
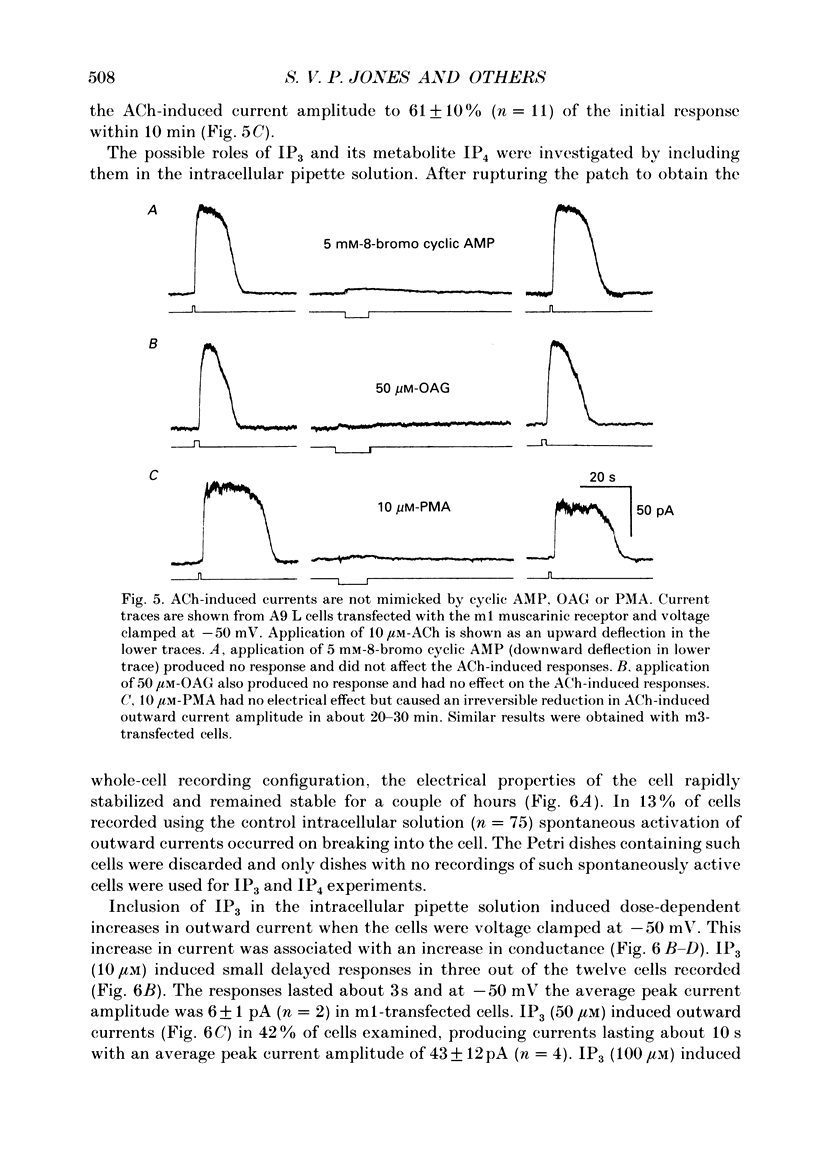
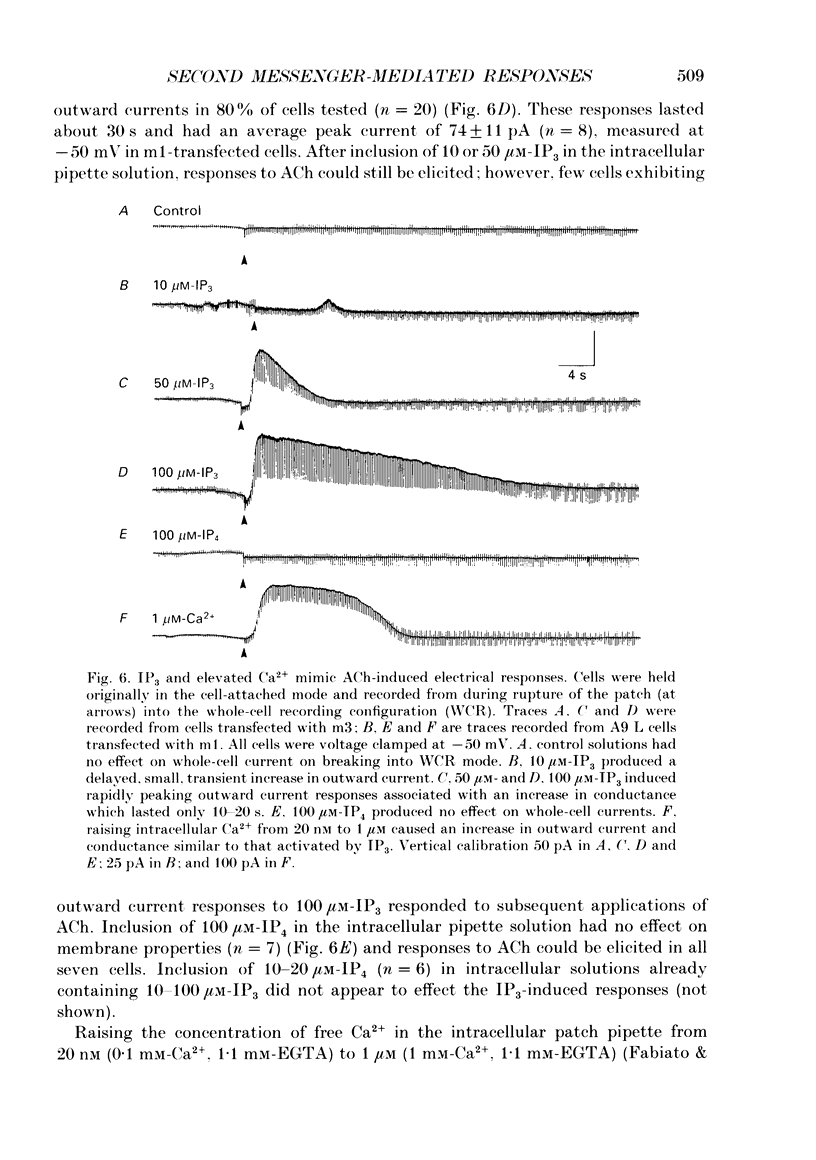
PDF









Images in this article
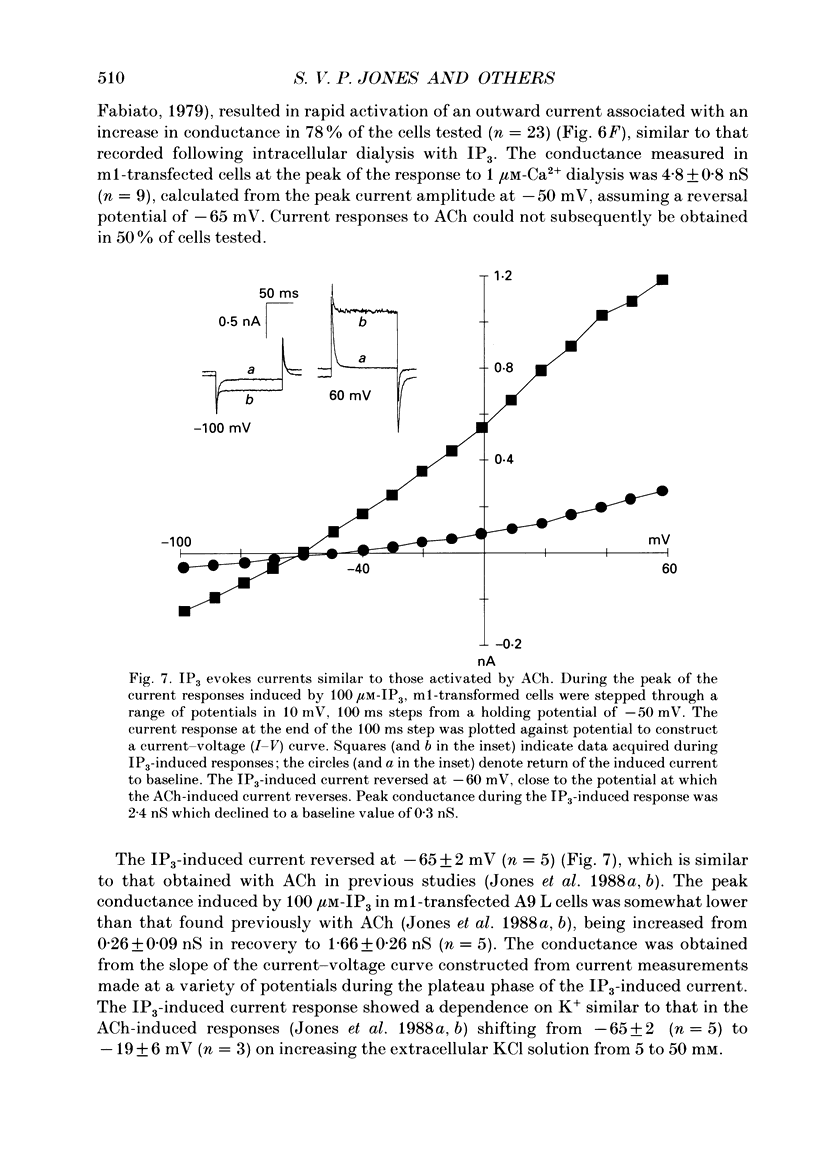
Selected References
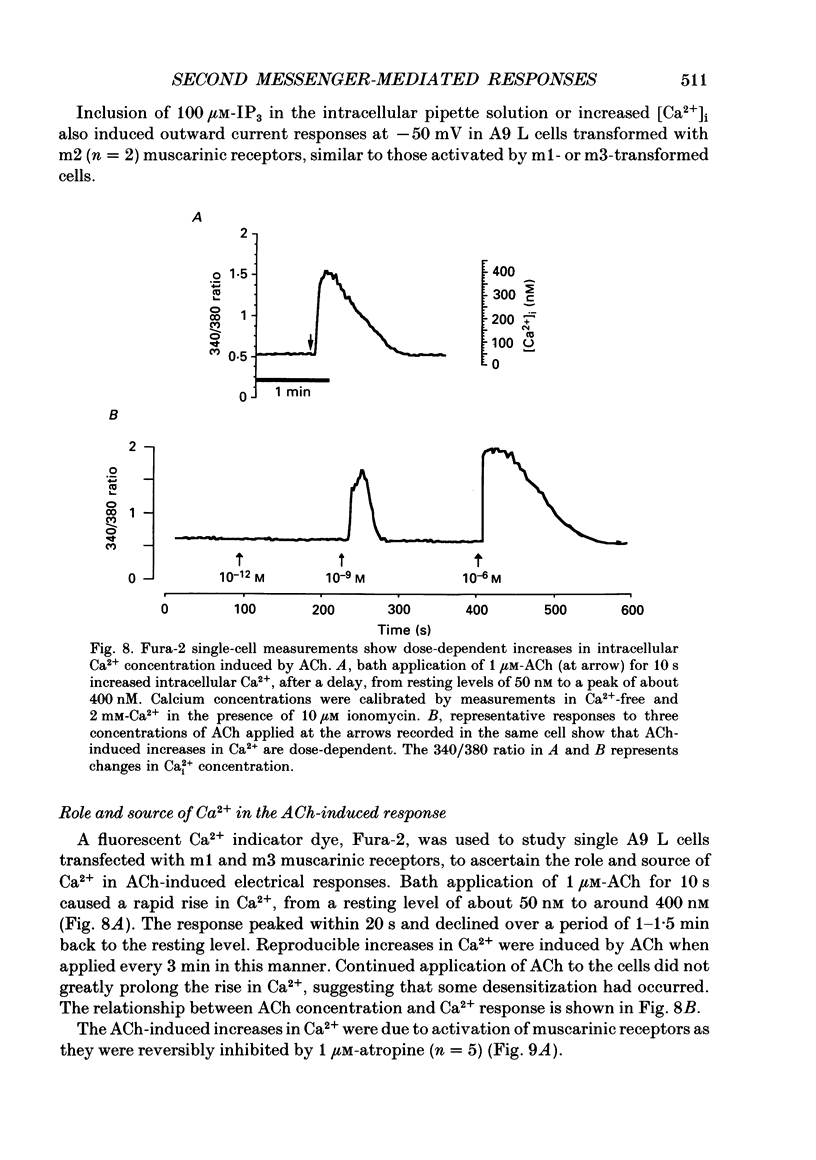
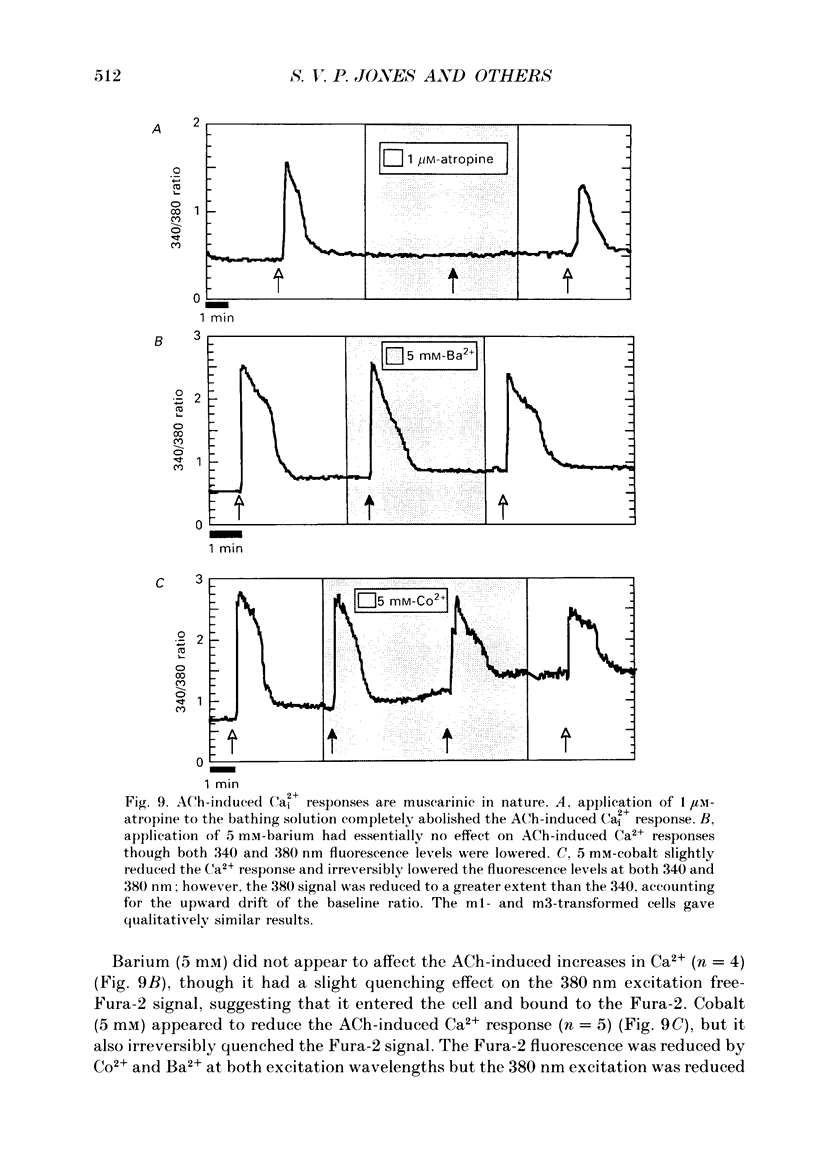
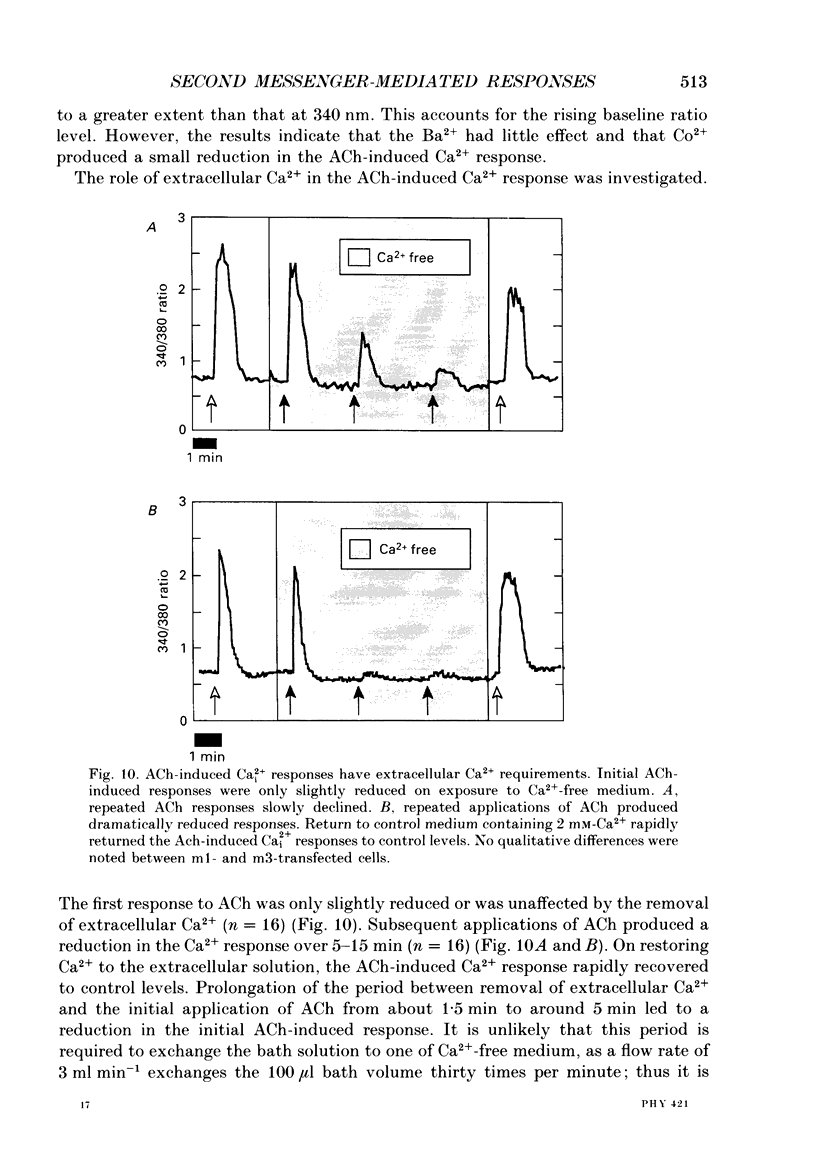
These references are in PubMed. This may not be the complete list of references from this article.
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Young A. C., Brann M. R., Buckley N. J. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988 Jul;1(5):403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Inositol 1,4,5-trisphosphate and diacylglycerol mimic bradykinin effects on mouse neuroblastoma x rat glioma hybrid cells. J Physiol. 1988 Mar;397:185–207. doi: 10.1113/jphysiol.1988.sp016995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. Neuropharmacology. Acetylcholine and brain cells. 1986 Jan 30-Feb 5Nature. 319(6052):358–359. doi: 10.1038/319358a0. [DOI] [PubMed] [Google Scholar]
- Capiod T., Ogden D. C. The properties of calcium-activated potassium ion channels in guinea-pig isolated hepatocytes. J Physiol. 1989 Feb;409:285–295. doi: 10.1113/jphysiol.1989.sp017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie M. J., North R. A. Control of ion conductances by muscarinic receptors. Trends Pharmacol Sci. 1988 Feb;Suppl:30–34. [PubMed] [Google Scholar]
- Conklin B. R., Brann M. R., Buckley N. J., Ma A. L., Bonner T. I., Axelrod J. Stimulation of arachidonic acid release and inhibition of mitogenesis by cloned genes for muscarinic receptor subtypes stably expressed in A9 L cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8698–8702. doi: 10.1073/pnas.85.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Steinberg T. H., Swanson J. A., Silverstein S. C. Fura-2 secretion and sequestration in macrophages. A blocker of organic anion transport reveals that these processes occur via a membrane transport system for organic anions. J Immunol. 1988 Feb 1;140(3):915–920. [PubMed] [Google Scholar]
- Evans M. G., Marty A. Potentiation of muscarinic and alpha-adrenergic responses by an analogue of guanosine 5'-triphosphate. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4099–4103. doi: 10.1073/pnas.83.11.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Forsythe I. D., Coates R. T. A chamber for electrophysiological recording from cultured neurones allowing perfusion and temperature control. J Neurosci Methods. 1988 Aug;25(1):19–27. doi: 10.1016/0165-0270(88)90116-1. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Higashida H., Kubo T., Maeda A., Akiba I., Bujo H., Mishina M., Numa S. Selective coupling with K+ currents of muscarinic acetylcholine receptor subtypes in NG108-15 cells. Nature. 1988 Sep 22;335(6188):355–358. doi: 10.1038/335355a0. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Kubo T., Akiba I., Maeda A., Mishina M., Numa S. Molecular distinction between muscarinic acetylcholine receptor subtypes. Nature. 1987 Jun 18;327(6123):623–625. doi: 10.1038/327623a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Higashida H., Brown D. A. Ca2+-dependent K+ channels in neuroblastoma hybrid cells activated by intracellular inositol trisphosphate and extracellular bradykinin. FEBS Lett. 1988 Oct 10;238(2):395–400. doi: 10.1016/0014-5793(88)80519-2. [DOI] [PubMed] [Google Scholar]
- Higashida H., Brown D. A. Two polyphosphatidylinositide metabolites control two K+ currents in a neuronal cell. 1986 Sep 25-Oct 1Nature. 323(6086):333–335. doi: 10.1038/323333a0. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. V., Barker J. L., Bonner T. I., Buckley N. J., Brann M. R. Electrophysiological characterization of cloned m1 muscarinic receptors expressed in A9 L cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4056–4060. doi: 10.1073/pnas.85.11.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. V., Barker J. L., Buckley N. J., Bonner T. I., Collins R. M., Brann M. R. Cloned muscarinic receptor subtypes expressed in A9 L cells differ in their coupling to electrical responses. Mol Pharmacol. 1988 Oct;34(4):421–426. [PubMed] [Google Scholar]
- Kubo T., Fukuda K., Mikami A., Maeda A., Takahashi H., Mishina M., Haga T., Haga K., Ichiyama A., Kangawa K. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986 Oct 2;323(6087):411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- Kubo T., Maeda A., Sugimoto K., Akiba I., Mikami A., Takahashi H., Haga T., Haga K., Ichiyama A., Kangawa K. Primary structure of porcine cardiac muscarinic acetylcholine receptor deduced from the cDNA sequence. FEBS Lett. 1986 Dec 15;209(2):367–372. doi: 10.1016/0014-5793(86)81144-9. [DOI] [PubMed] [Google Scholar]
- Kudo Y., Ogura A., Iijima T. Stimulation of muscarinic receptor in hippocampal neuron induces characteristic increase in cytosolic free Ca2+ concentration. Neurosci Lett. 1988 Mar 10;85(3):345–350. doi: 10.1016/0304-3940(88)90590-3. [DOI] [PubMed] [Google Scholar]
- Marty A., Evans M. G., Tan Y. P., Trautmann A. Muscarinic response in rat lacrimal glands. J Exp Biol. 1986 Sep;124:15–32. doi: 10.1242/jeb.124.1.15. [DOI] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Nathanson N. M. Molecular properties of the muscarinic acetylcholine receptor. Annu Rev Neurosci. 1987;10:195–236. doi: 10.1146/annurev.ne.10.030187.001211. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A., Fukuda K., Kubo T., Numa S. Intracellular calcium release mediated by two muscarinic receptor subtypes. FEBS Lett. 1988 Nov 21;240(1-2):88–94. doi: 10.1016/0014-5793(88)80345-4. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988 Jul 29;241(4865):545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- Nomura Y., Kaneko S., Kato K., Yamagishi S., Sugiyama H. Inositol phosphate formation and chloride current responses induced by acetylcholine and serotonin through GTP-binding proteins in Xenopus oocyte after injection of rat brain messenger RNA. Brain Res. 1987 Jul;388(2):113–123. doi: 10.1016/s0006-8993(87)80004-5. [DOI] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Ramachandran J., Capon D. J. Differential regulation of PI hydrolysis and adenylyl cyclase by muscarinic receptor subtypes. Nature. 1988 Aug 4;334(6181):434–437. doi: 10.1038/334434a0. [DOI] [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Smith D. H., Ramachandran J., Capon D. J. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987 Dec 20;6(13):3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H. Calcium-activated potassium channels and fluid secretion by exocrine glands. Am J Physiol. 1986 Jul;251(1 Pt 1):G1–13. doi: 10.1152/ajpgi.1986.251.1.G1. [DOI] [PubMed] [Google Scholar]