Abstract
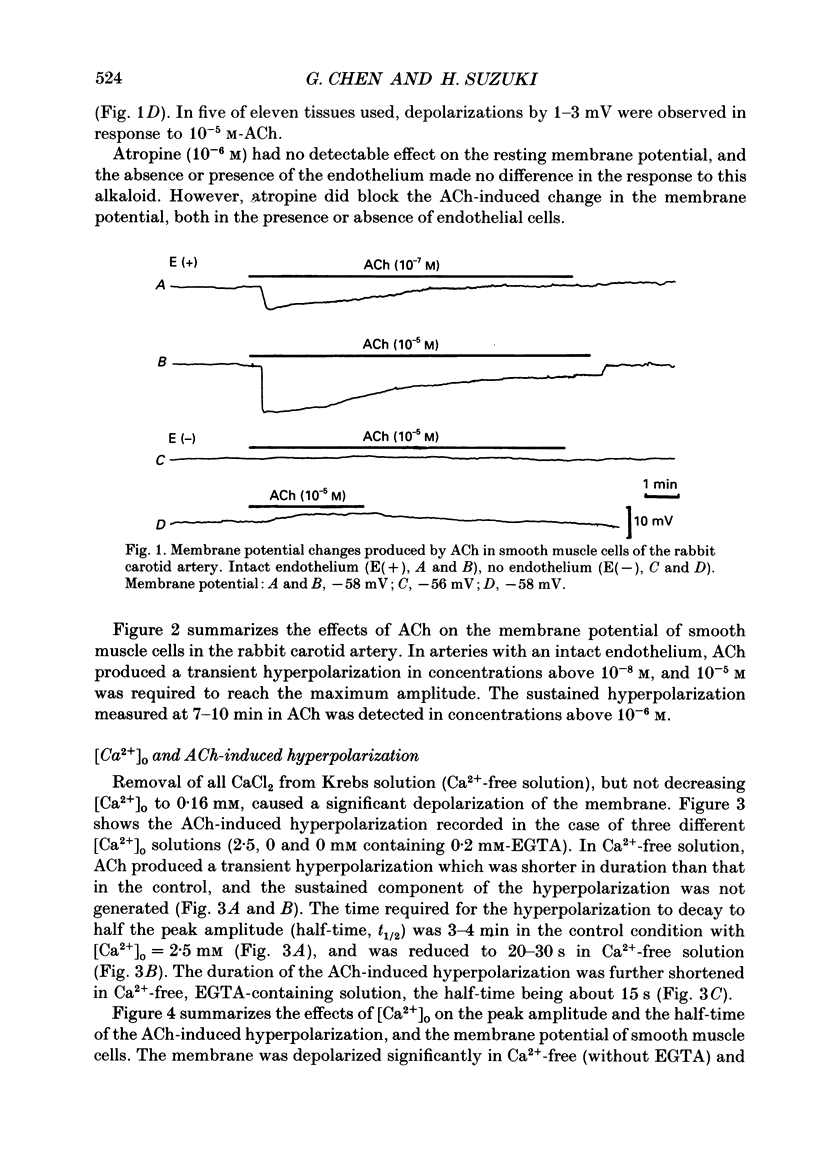
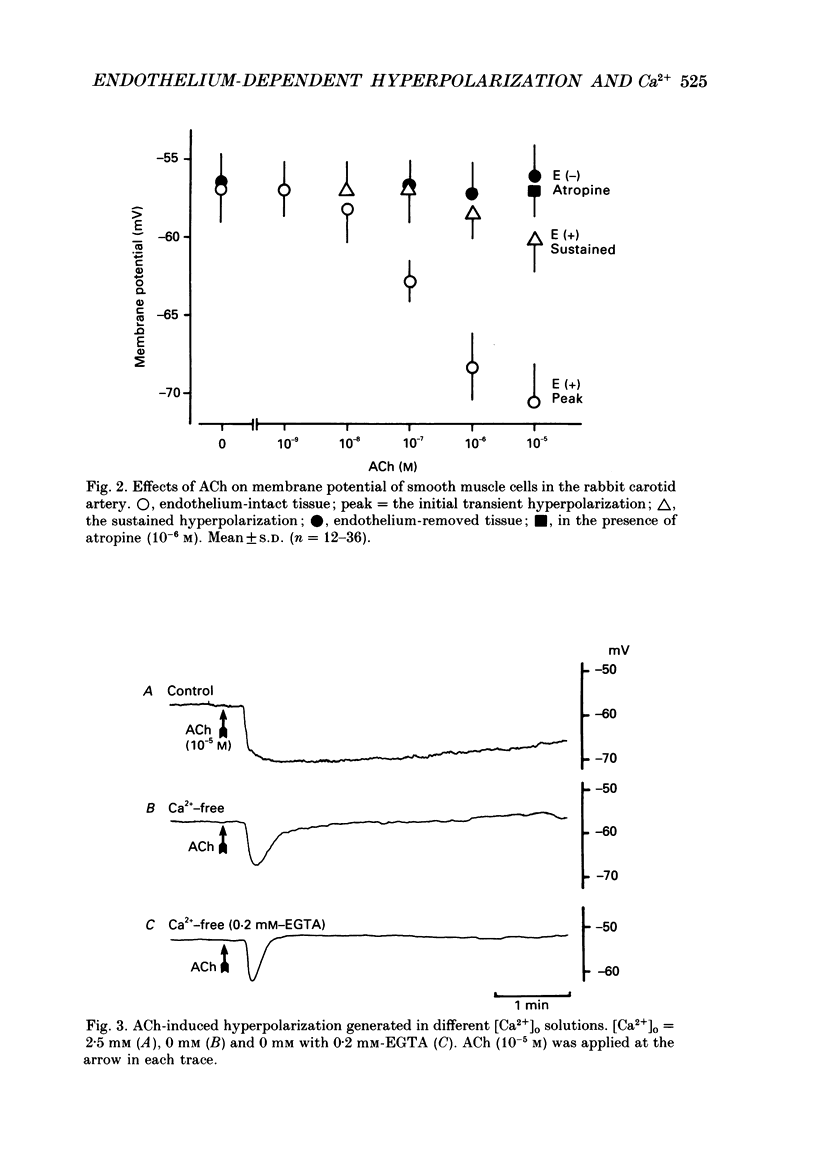
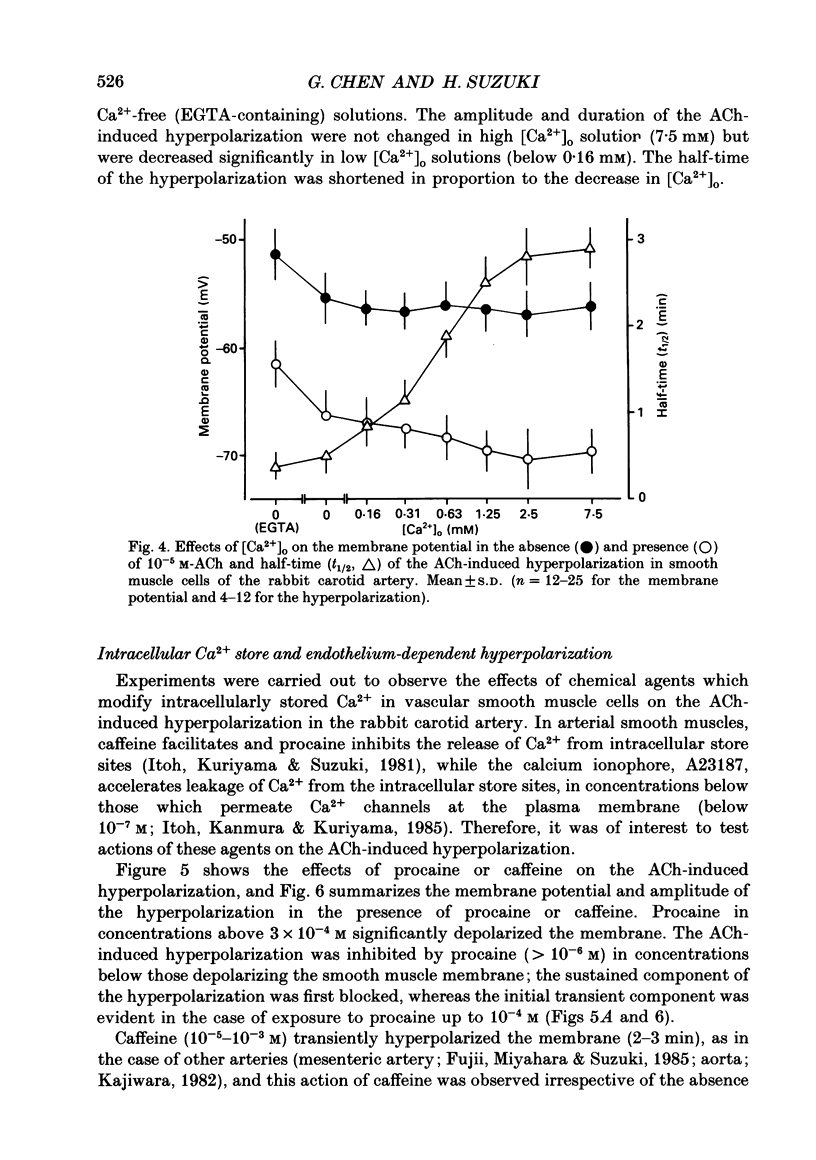
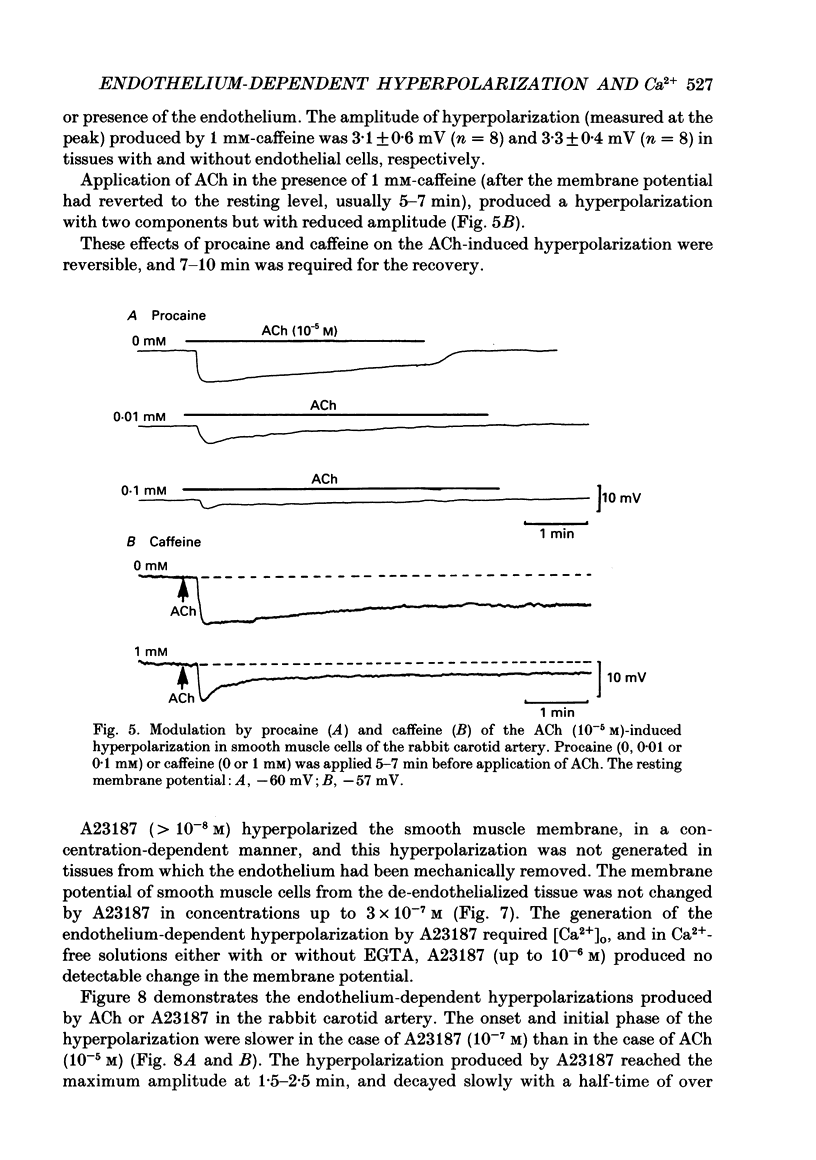
1. In smooth muscle cells of the rabbit carotid artery, ACh (greater than 10(-8) M) generated a hyperpolarization with two components (transient followed by sustained), only in the tissues with an intact endothelium. There were no detectable changes in the membrane potential, as elicited by ACh (up to 10(-5) M) in tissues with no endothelium or in the presence of atropine (10(-6) M). 2. Reduction of [Ca2+]o inhibited the sustained component which was not apparent in [Ca2+]o below 0.16 mM. In Ca2+-free (EGTA-containing) solution, the generation of the transient component of the hyperpolarization remained sustained but with a substantially reduced amplitude. 3. Procaine (greater than 10(-6) M) inhibited the ACh-induced hyperpolarization in a concentration-dependent manner, and at a concentration of procaine (10(-3) M) which caused substantial depolarization of the membrane, no detectable change was elicited by ACh. 4. Caffeine (10(-6)-10(-3) M) produced a transient hyperpolarization, independent of the presence or absence of the endothelium, and inhibited the sustained component of the ACh-induced hyperpolarization more so than the initial component. 5. A23187 (greater than 10(-8) M) hyperpolarized the smooth muscle membrane in a concentration-dependent manner, and this hyperpolarization was not generated in Ca2+-free solution or in the absence of endothelial cells. 6. In intact tissues, pre-treatment with A23187 resulted in a reduction of the subsequently generated ACh-induced hyperpolarization, in an irreversible manner. 7. It would thus appear that in the rabbit carotid artery, the endothelium-dependent hyperpolarization induced by ACh has Ca2+-dependent and Ca2+-independent components, and each may be related to the increase in endothelial [Ca2+]i by release from the intracellular store and by influx from the extracellular medium, respectively. The increased [Ca2+]i would trigger a release of an endothelium-derived hyperpolarizing factor (EDHF) from the endothelial cells.
Full text
PDF


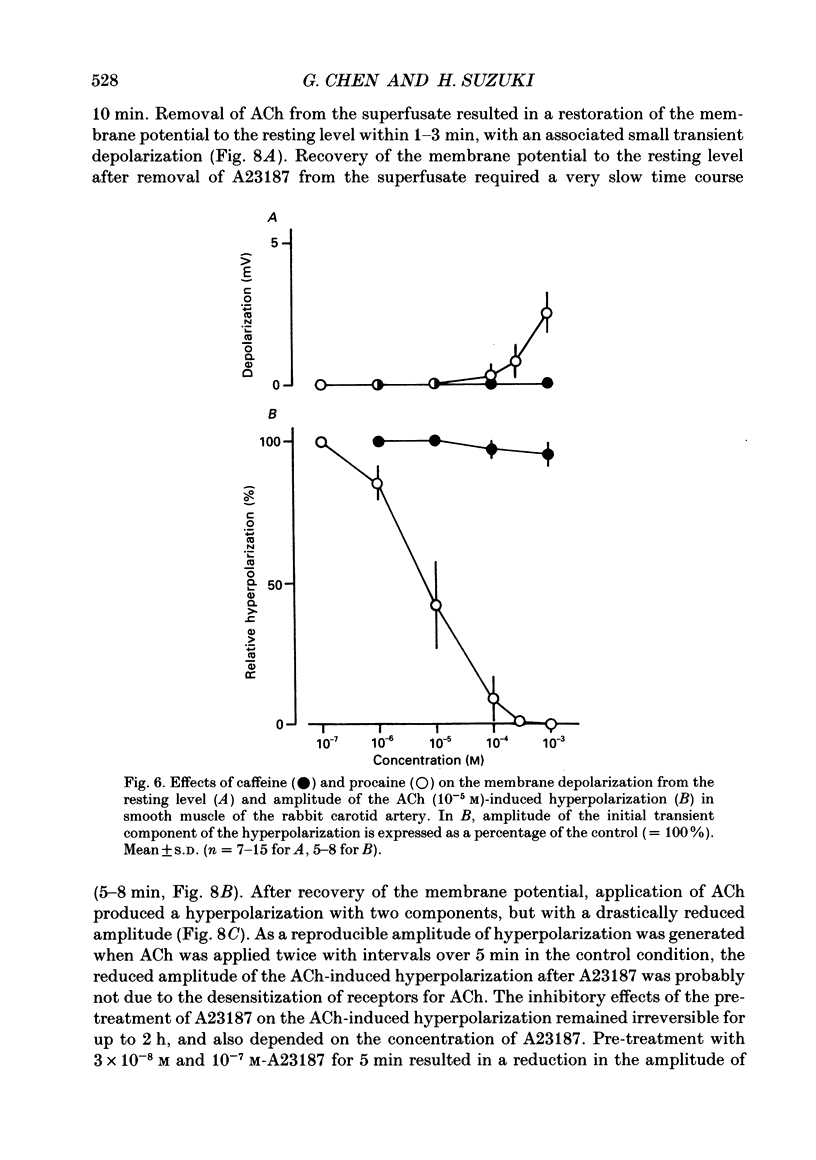
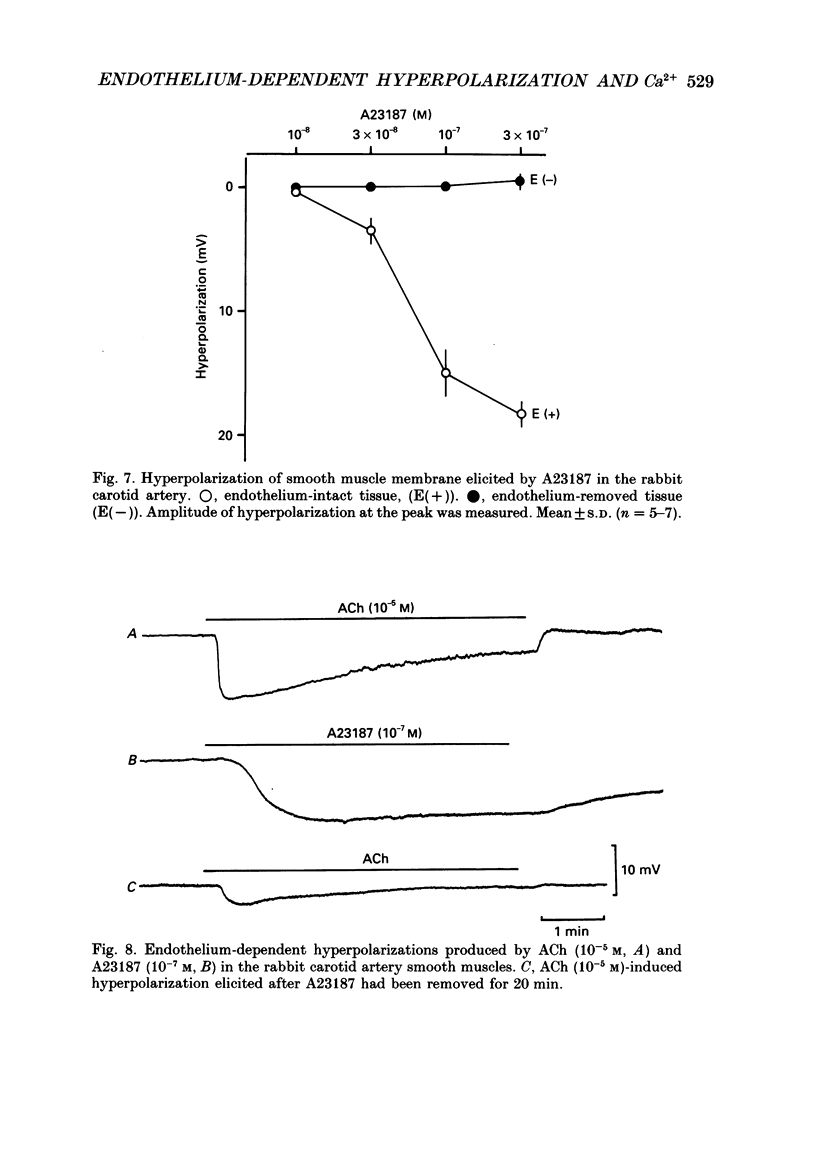





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton T. B., Lang R. J., Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984 Jun;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Fichtner H., Lückhoff A., Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H965–H969. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- Bény J. L., Brunet P. C. Electrophysiological and mechanical effects of substance P and acetylcholine on rabbit aorta. J Physiol. 1988 Apr;398:277–289. doi: 10.1113/jphysiol.1988.sp017042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Suzuki H. Some electrical properties of the endothelium-dependent hyperpolarization recorded from rat arterial smooth muscle cells. J Physiol. 1989 Mar;410:91–106. doi: 10.1113/jphysiol.1989.sp017522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Suzuki H., Weston A. H. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol. 1988 Dec;95(4):1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colden-Stanfield M., Schilling W. P., Ritchie A. K., Eskin S. G., Navarro L. T., Kunze D. L. Bradykinin-induced increases in cytosolic calcium and ionic currents in cultured bovine aortic endothelial cells. Circ Res. 1987 Nov;61(5):632–640. doi: 10.1161/01.res.61.5.632. [DOI] [PubMed] [Google Scholar]
- Danthuluri N. R., Cybulsky M. I., Brock T. A. ACh-induced calcium transients in primary cultures of rabbit aortic endothelial cells. Am J Physiol. 1988 Dec;255(6 Pt 2):H1549–H1553. doi: 10.1152/ajpheart.1988.255.6.H1549. [DOI] [PubMed] [Google Scholar]
- Derian C. K., Moskowitz M. A. Polyphosphoinositide hydrolysis in endothelial cells and carotid artery segments. Bradykinin-2 receptor stimulation is calcium-independent. J Biol Chem. 1986 Mar 15;261(8):3831–3837. [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K., Miyahara H., Suzuki H. Comparison of the effects of caffeine and procaine on noradrenergic transmission in the guinea-pig mesenteric artery. Br J Pharmacol. 1985 Mar;84(3):675–684. doi: 10.1111/j.1476-5381.1985.tb16149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Newby A. C., Lewis M. J., Henderson A. H. Production of endothelium derived relaxant factor is dependent on oxidative phosphorylation and extracellular calcium. Cardiovasc Res. 1986 Jan;20(1):7–12. doi: 10.1093/cvr/20.1.7. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D. Exogenous ATP raises cytoplasmic free calcium in fura-2 loaded piglet aortic endothelial cells. FEBS Lett. 1986 Oct 20;207(1):95–99. doi: 10.1016/0014-5793(86)80019-9. [DOI] [PubMed] [Google Scholar]
- Hong S. L., Deykin D. Activation of phospholipases A2 and C in pig aortic endothelial cells synthesizing prostacyclin. J Biol Chem. 1982 Jun 25;257(12):7151–7154. [PubMed] [Google Scholar]
- Ignarro L. J., Kadowitz P. J. The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Annu Rev Pharmacol Toxicol. 1985;25:171–191. doi: 10.1146/annurev.pa.25.040185.001131. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H. A23187 increases calcium permeability of store sites more than of surface membranes in the rabbit mesenteric artery. J Physiol. 1985 Feb;359:467–484. doi: 10.1113/jphysiol.1985.sp015597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Excitation--contraction coupling in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1981 Dec;321:513–535. doi: 10.1113/jphysiol.1981.sp014000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns A., Lategan T. W., Lodge N. J., Ryan U. S., Van Breemen C., Adams D. J. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell. 1987;19(6):733–745. doi: 10.1016/0040-8166(87)90015-2. [DOI] [PubMed] [Google Scholar]
- Kajiwara M. General features of electrical and mechanical properties of smooth muscle cells in the guinea-pig abdominal aorta. Pflugers Arch. 1982 Mar;393(1):109–117. doi: 10.1007/BF00582402. [DOI] [PubMed] [Google Scholar]
- Komori K., Lorenz R. R., Vanhoutte P. M. Nitric oxide, ACh, and electrical and mechanical properties of canine arterial smooth muscle. Am J Physiol. 1988 Jul;255(1 Pt 2):H207–H212. doi: 10.1152/ajpheart.1988.255.1.H207. [DOI] [PubMed] [Google Scholar]
- Komori K., Suzuki H. Electrical responses of smooth muscle cells during cholinergic vasodilation in the rabbit saphenous artery. Circ Res. 1987 Oct;61(4):586–593. doi: 10.1161/01.res.61.4.586. [DOI] [PubMed] [Google Scholar]
- Komori K., Suzuki H. Heterogeneous distribution of muscarinic receptors in the rabbit saphenous artery. Br J Pharmacol. 1987 Nov;92(3):657–664. doi: 10.1111/j.1476-5381.1987.tb11369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Long C. J., Stone T. W. The release of endothelium-derived relaxant factor is calcium dependent. Blood Vessels. 1985;22(4):205–208. doi: 10.1159/000158602. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Increased free calcium in endothelial cells under stimulation with adenine nucleotides. J Cell Physiol. 1986 Mar;126(3):414–420. doi: 10.1002/jcp.1041260312. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Pohl U., Mülsch A., Busse R. Differential role of extra- and intracellular calcium in the release of EDRF and prostacyclin from cultured endothelial cells. Br J Pharmacol. 1988 Sep;95(1):189–196. doi: 10.1111/j.1476-5381.1988.tb16564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. W., Wysolmerski R. B. Ca2+-dependent and Ca2+-independent pathways for release of arachidonic acid from phosphatidylinositol in endothelial cells. J Biol Chem. 1987 Sep 25;262(27):13086–13092. [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Nagao T., Suzuki H. Non-neural electrical responses of smooth muscle cells of the rabbit basilar artery to electrical field stimulation. Jpn J Physiol. 1987;37(3):497–513. doi: 10.2170/jjphysiol.37.497. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Davies P. F., Clapham D. E. Muscarinic-activated K+ current in bovine aortic endothelial cells. Circ Res. 1988 Jun;62(6):1059–1064. doi: 10.1161/01.res.62.6.1059. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Peach M. J., Singer H. A., Izzo N. J., Jr, Loeb A. L. Role of calcium in endothelium-dependent relaxation of arterial smooth muscle. Am J Cardiol. 1987 Jan 23;59(2):35A–43A. doi: 10.1016/0002-9149(87)90174-3. [DOI] [PubMed] [Google Scholar]
- Rhodin J. A. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967 Apr;18(1):181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- Schwarz W., Passow H. Ca2+-activated K+ channels in erythrocytes and excitable cells. Annu Rev Physiol. 1983;45:359–374. doi: 10.1146/annurev.ph.45.030183.002043. [DOI] [PubMed] [Google Scholar]
- Suzuki H. The electrogenic Na-K pump does not contribute to endothelium-dependent hyperpolarization in the rabbit ear artery. Eur J Pharmacol. 1988 Nov 1;156(2):295–297. doi: 10.1016/0014-2999(88)90337-8. [DOI] [PubMed] [Google Scholar]
- Takeda K., Schini V., Stoeckel H. Voltage-activated potassium, but not calcium currents in cultured bovine aortic endothelial cells. Pflugers Arch. 1987 Nov;410(4-5):385–393. doi: 10.1007/BF00586515. [DOI] [PubMed] [Google Scholar]
- Taugner R., Kirchheim H., Forssmann W. G. Myoendothelial contacts in glomerular arterioles and in renal interlobular arteries of rat, mouse and Tupaia belangeri. Cell Tissue Res. 1984;235(2):319–325. doi: 10.1007/BF00217856. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Southerton J. S., Weston A. H., Baker J. R. Endothelium-dependent effects of acetylcholine in rat aorta: a comparison with sodium nitroprusside and cromakalim. Br J Pharmacol. 1988 Jul;94(3):853–863. doi: 10.1111/j.1476-5381.1988.tb11597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


