Abstract
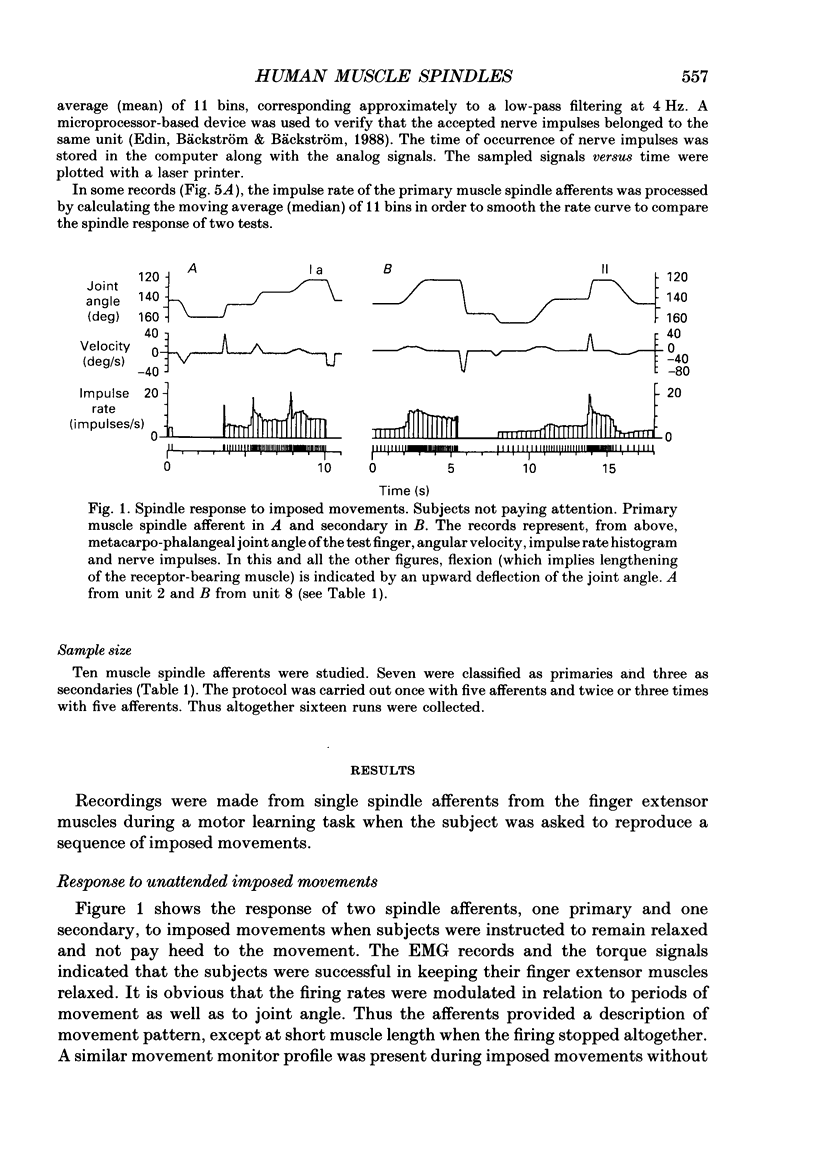
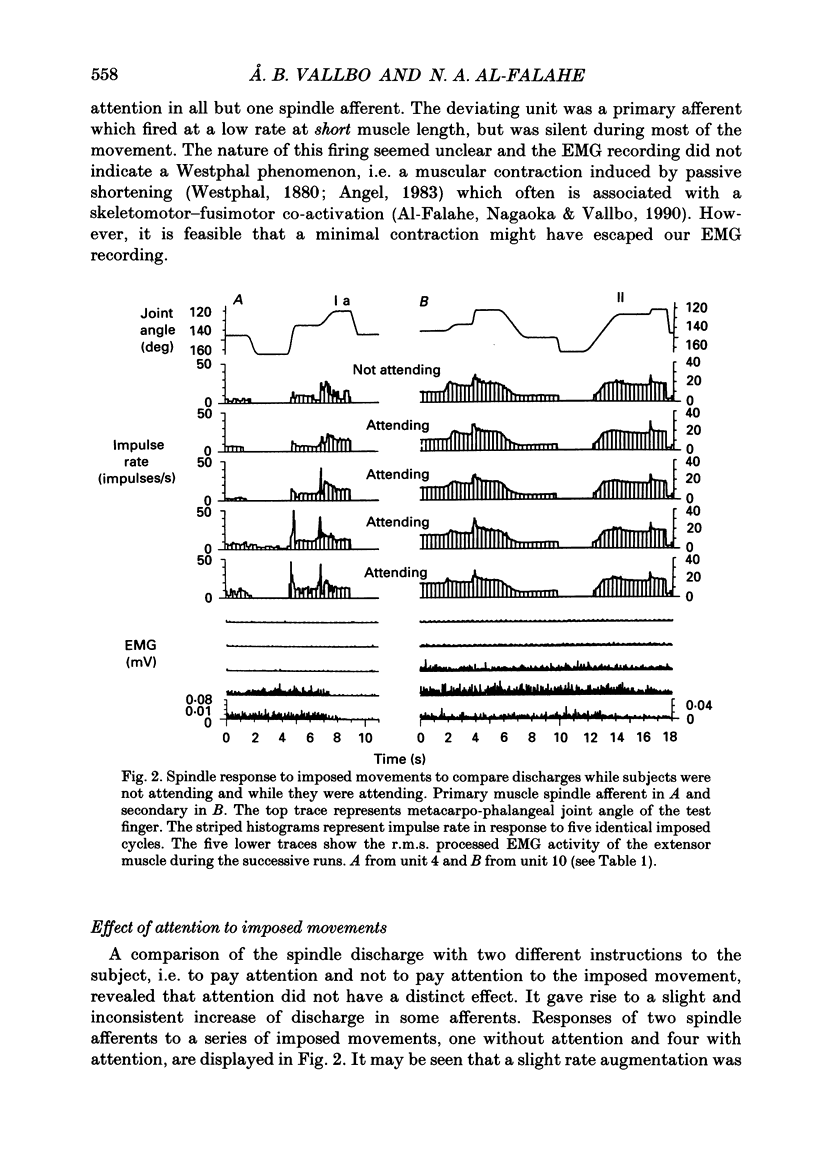
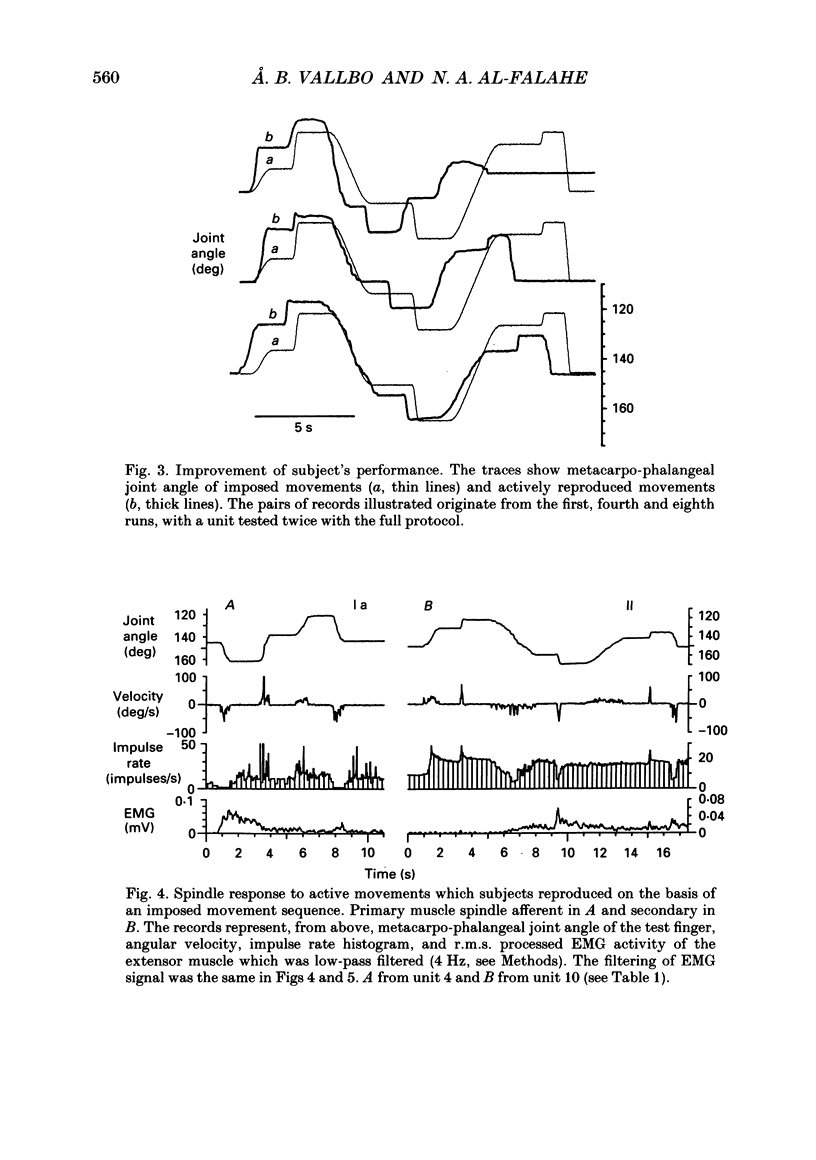
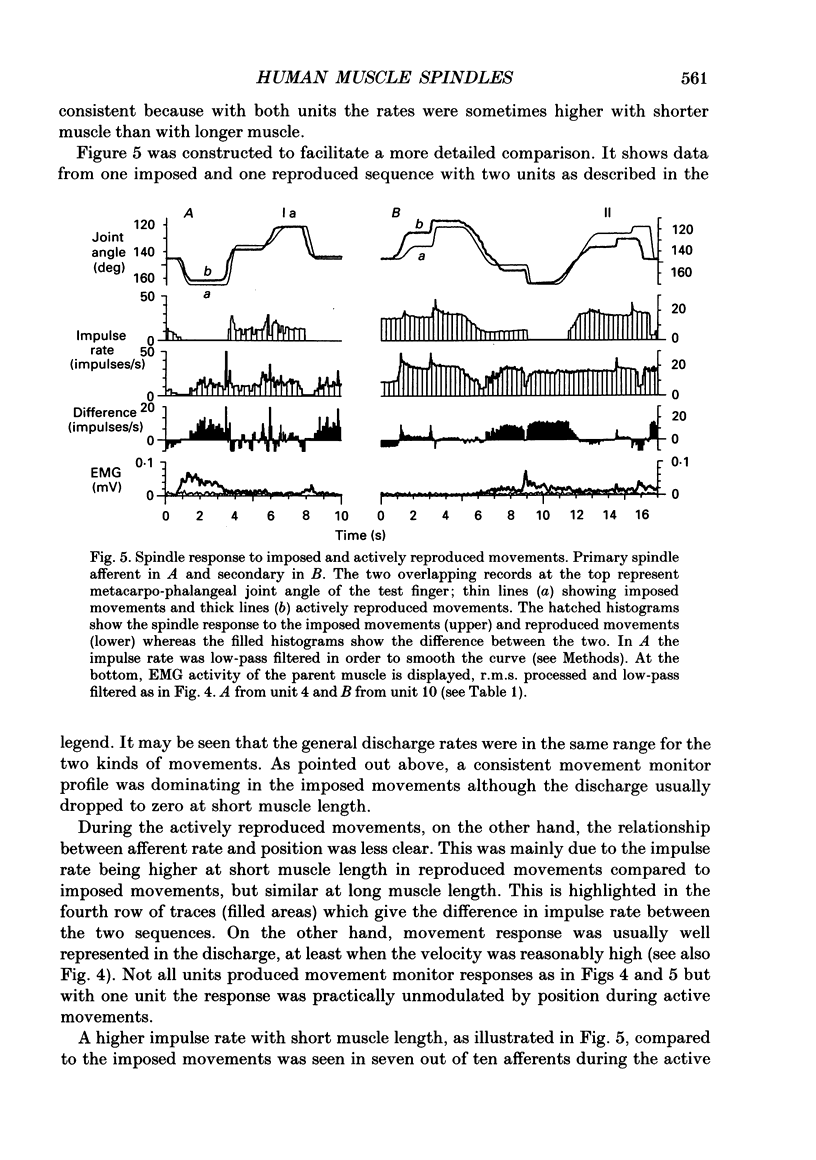
1. Impulse discharge of single muscle spindle afferents from the finger extensor muscles was recorded in the radial nerve of conscious human subjects, during a motor learning task engaging the metacarpo-phalangeal joint of a single finger, using the microneurography technique. 2. Subjects were requested first to pay attention to a complex sequence of imposed single joint movements, and immediately afterwards to reproduce actively the same sequence. No external load was added to the finger and visual control was denied altogether so that subjects relied on mechanoreceptor input exclusively for the sampling and reproduction of movement. In addition, sequences of imposed movements were delivered while subjects were not attending in order to allow analysis of the attention effect. 3. The response of the individual unit was uniform in repeated tests. There were clear differences between spindle firing rate in imposed and actively reproduced movements with most units. However, the difference was complex during the individual sequence, in that firing rate was usually higher during periods of reproduced movements when the muscle was relatively short whereas it was identical when the muscle was relatively long. 4. The hypothesis that reproduction and verification of an imposed movement may be based on simple matching between identical spindle firing in imposed and active movements, was difficult to reject altogether because identical spindle input was present during considerable sections of the movement sequence. It may be speculated that agonists and antagonists cover different ranges of joint excursion, with identical spindle firing rates in imposed and reproduced movements. 5. Attention to imposed movements was associated with a minute and inconsistent increase of spindle firing rate in some afferents and then usually with a slight increase of EMG activity of the parent muscle as well. 6. It was concluded that focusing attention on the kinaesthetic input during imposed movement was not associated with a consistent increase of fusimotor drive.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. A. A closed-loop theory of motor learning. J Mot Behav. 1971 Jun;3(2):111–149. doi: 10.1080/00222895.1971.10734898. [DOI] [PubMed] [Google Scholar]
- Al-Falahe N. A., Vallbo A. B. Role of the human fusimotor system in a motor adaptation task. J Physiol. 1988 Jul;401:77–95. doi: 10.1113/jphysiol.1988.sp017152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel R. W. Muscular contractions elicited by passive shortening. Adv Neurol. 1983;39:555–563. [PubMed] [Google Scholar]
- Bizzi E., Abend W. Posture control and trajectory formation in single- and multi-joint arm movements. Adv Neurol. 1983;39:31–45. [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Löfstedt L. Muscle spindle activity in man during shortening and lengthening contractions. J Physiol. 1978 Apr;277:131–142. doi: 10.1113/jphysiol.1978.sp012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Löfstedt L., Wallin B. G. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. 1976 Oct;261(3):695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., McKeon B., Skuse N. F., Westerman R. A. Anticipation and fusimotor activity in preparation for a voluntary contraction. J Physiol. 1980 Sep;306:337–348. doi: 10.1113/jphysiol.1980.sp013400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin B. B., Bäckström P. A., Bäckström L. O. Single unit retrieval in microneurography: a microprocessor-based device controlled by an operator. J Neurosci Methods. 1988 Jun;24(2):137–144. doi: 10.1016/0165-0270(88)90057-x. [DOI] [PubMed] [Google Scholar]
- Edin B. B., Vallbo A. B. Stretch sensitization of human muscle spindles. J Physiol. 1988 Jun;400:101–111. doi: 10.1113/jphysiol.1988.sp017113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin B. B., Vallbo A. B. Twitch contraction for identification of human muscle afferents. Acta Physiol Scand. 1987 Sep;131(1):129–138. doi: 10.1111/j.1748-1716.1987.tb08214.x. [DOI] [PubMed] [Google Scholar]
- Ferrell W. R., Gandevia S. C., McCloskey D. I. The role of joint receptors in human kinaesthesia when intramuscular receptors cannot contribute. J Physiol. 1987 May;386:63–71. doi: 10.1113/jphysiol.1987.sp016522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia S. C., Burke D. Effect of training on voluntary activation of human fusimotor neurons. J Neurophysiol. 1985 Dec;54(6):1422–1429. doi: 10.1152/jn.1985.54.6.1422. [DOI] [PubMed] [Google Scholar]
- Gandevia S. C., Miller S., Aniss A. M., Burke D. Reflex influences on muscle spindle activity in relaxed human leg muscles. J Neurophysiol. 1986 Jul;56(1):159–170. doi: 10.1152/jn.1986.56.1.159. [DOI] [PubMed] [Google Scholar]
- Goodwin G. M., McCloskey D. I., Matthews P. B. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain. 1972;95(4):705–748. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Hulliger M., Nordh E., Vallbo A. B. Discharge in muscle spindle afferents related to direction of slow precision movements in man. J Physiol. 1985 May;362:437–453. doi: 10.1113/jphysiol.1985.sp015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M., Nordh E., Vallbo A. B. The absence of position response in spindle afferent units from human finger muscles during accurate position holding. J Physiol. 1982 Jan;322:167–179. doi: 10.1113/jphysiol.1982.sp014030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M., Prochazka A. A new simulation method to deduce fusimotor activity from afferent discharge recorded in freely moving cats. J Neurosci Methods. 1983 Jun;8(2):197–204. doi: 10.1016/0165-0270(83)90121-8. [DOI] [PubMed] [Google Scholar]
- Jeannerod M., Michel F., Prablanc C. The control of hand movements in a case of hemianaesthesia following a parietal lesion. Brain. 1984 Sep;107(Pt 3):899–920. doi: 10.1093/brain/107.3.899. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., HUNT C. C., QUILLIAM J. P. Function of medullated small-nerve fibers in mammalian ventral roots; efferent muscle spindle innervation. J Neurophysiol. 1951 Jan;14(1):29–54. doi: 10.1152/jn.1951.14.1.29. [DOI] [PubMed] [Google Scholar]
- Matthews P. B., Stein R. B. The sensitivity of muscle spindle afferents to small sinusoidal changes of length. J Physiol. 1969 Feb;200(3):723–743. doi: 10.1113/jphysiol.1969.sp008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey D. I. Kinesthetic sensibility. Physiol Rev. 1978 Oct;58(4):763–820. doi: 10.1152/physrev.1978.58.4.763. [DOI] [PubMed] [Google Scholar]
- Polit A., Bizzi E. Characteristics of motor programs underlying arm movements in monkeys. J Neurophysiol. 1979 Jan;42(1 Pt 1):183–194. doi: 10.1152/jn.1979.42.1.183. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Hulliger M., Zangger P., Appenteng K. 'Fusimotor set': new evidence for alpha-independent control of gamma-motoneurones during movement in the awake cat. Brain Res. 1985 Jul 22;339(1):136–140. doi: 10.1016/0006-8993(85)90632-8. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Muscle spindle function during normal movement. Int Rev Physiol. 1981;25:47–90. [PubMed] [Google Scholar]
- Prochazka A., Stephens J. A., Wand P. Muscle spindle discharge in normal and obstructed movements. J Physiol. 1979 Feb;287:57–66. doi: 10.1113/jphysiol.1979.sp012645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell J. C., Traub M. M., Day B. L., Obeso J. A., Thomas P. K., Marsden C. D. Manual motor performance in a deafferented man. Brain. 1982 Sep;105(Pt 3):515–542. doi: 10.1093/brain/105.3.515. [DOI] [PubMed] [Google Scholar]
- Sanes J. N., Mauritz K. H., Dalakas M. C., Evarts E. V. Motor control in humans with large-fiber sensory neuropathy. Hum Neurobiol. 1985;4(2):101–114. [PubMed] [Google Scholar]
- Vallbo A. B., Hagbarth K. E. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp Neurol. 1968 Jul;21(3):270–289. doi: 10.1016/0014-4886(68)90041-1. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B., Hagbarth K. E., Torebjörk H. E., Wallin B. G. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979 Oct;59(4):919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]


