Abstract
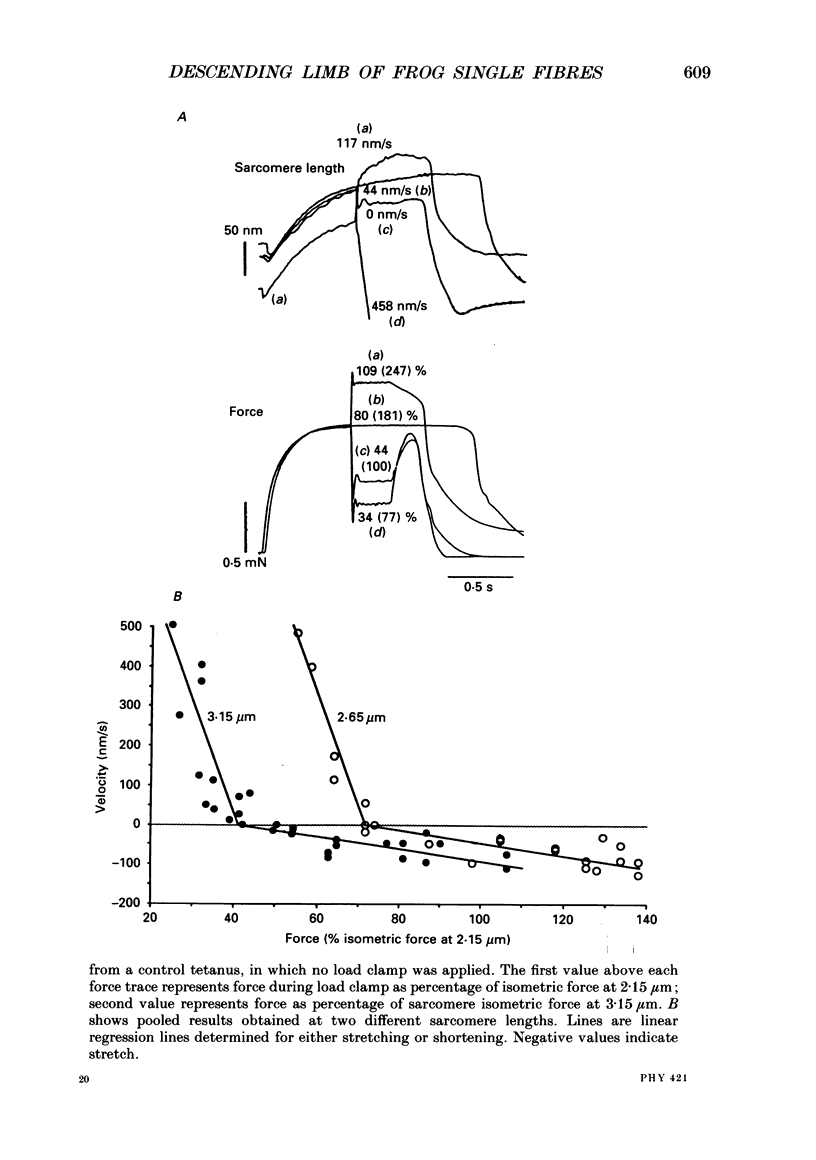
1. We studied the descending limb of the force-sarcomere length relation in single frog muscle fibres using sarcomere isometric contractions. 2. Sarcomere length was measured simultaneously with two independent methods: a laser diffraction method and a segment length method that detects the distance between two markers attached to the surface of the fibre, about 800 microns apart. Both methods were used to keep sarcomeres at constant length during contraction. 3. Fibres were selected for low resting tension since it was known from previous experiments that for such fibres the force developed by fixed-end tetani is much higher than that predicted by the degree of filament overlap. 4. With fixed-end tetani, force decline with increase of sarcomere length was small between 2.0 and 3.0 microns. At a sarcomere length of 3.0 microns, force was about 90% of maximal. 5. With sarcomere isometric tetani, force was considerably lower than with fixed-end tetani. Force was maximal at about 2.1 microns and decreased to zero at about 3.6 microns. At intermediate lengths the descending limb was within 80 nm of the values predicted from filament overlap. 6. We investigated why force of fixed-end contractions was much higher than that generated by sarcomere isometric contractions. 7. During the force plateau of fixed-end tetani at sarcomere lengths longer than about 2.0-2.2 microns, sarcomeres in the fibres mid-region were not isometric, but instead stretched slowly. By measuring the force-velocity relation it was shown that this slow stretch elevates active force well beyond sarcomere isometric force. 8. Stretch of the central region was also observed during the tetanic force rise. This was shown to result in an increase of passive force that grew larger at longer sarcomere lengths. At about 3.6 microns the increase of passive force was similar to the total force generated by fixed-end contractions at this length. 9. Laser diffraction and segment length methods gave the same results, diminishing the chance that any systematic artifact underlies our findings. 10. While earlier experiments from this laboratory carried out on fibres held at constant length during contraction did not reveal a linear descending limb, the present results support the linear descending limb as a characteristic feature of isometrically contracting sarcomeres.
Full text
PDF



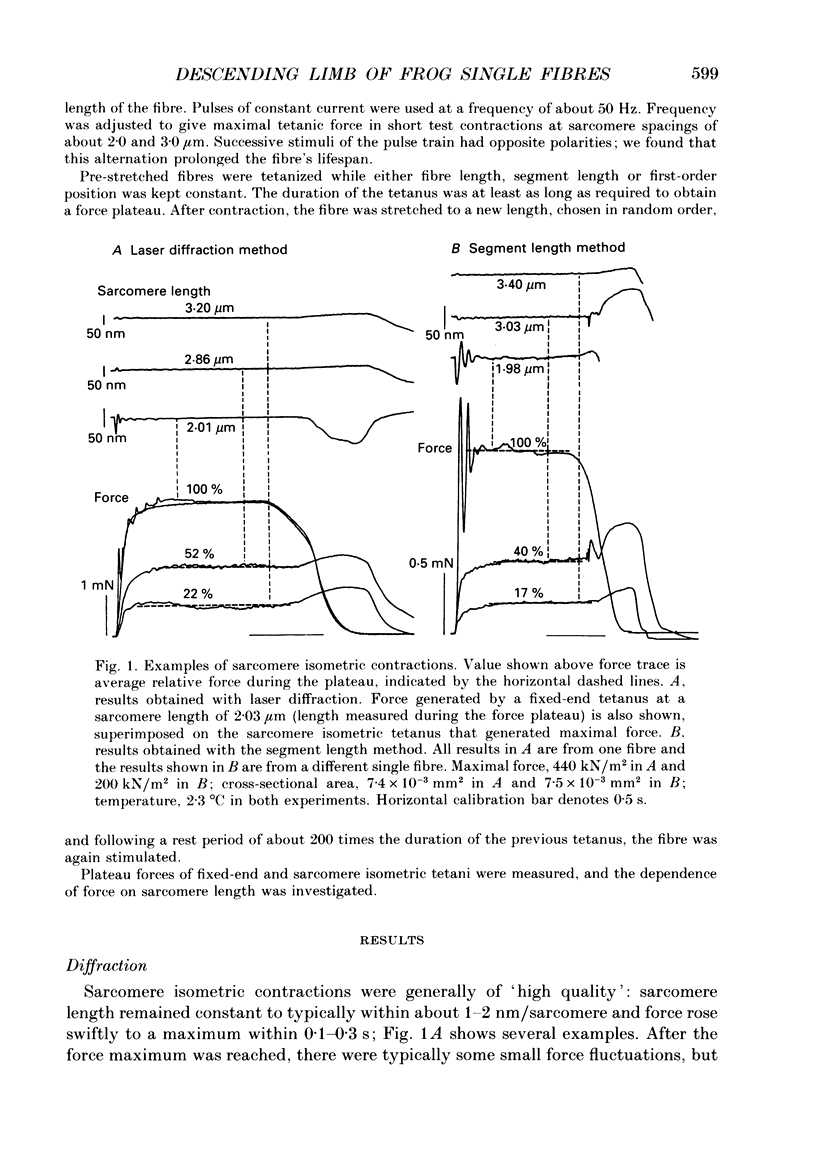
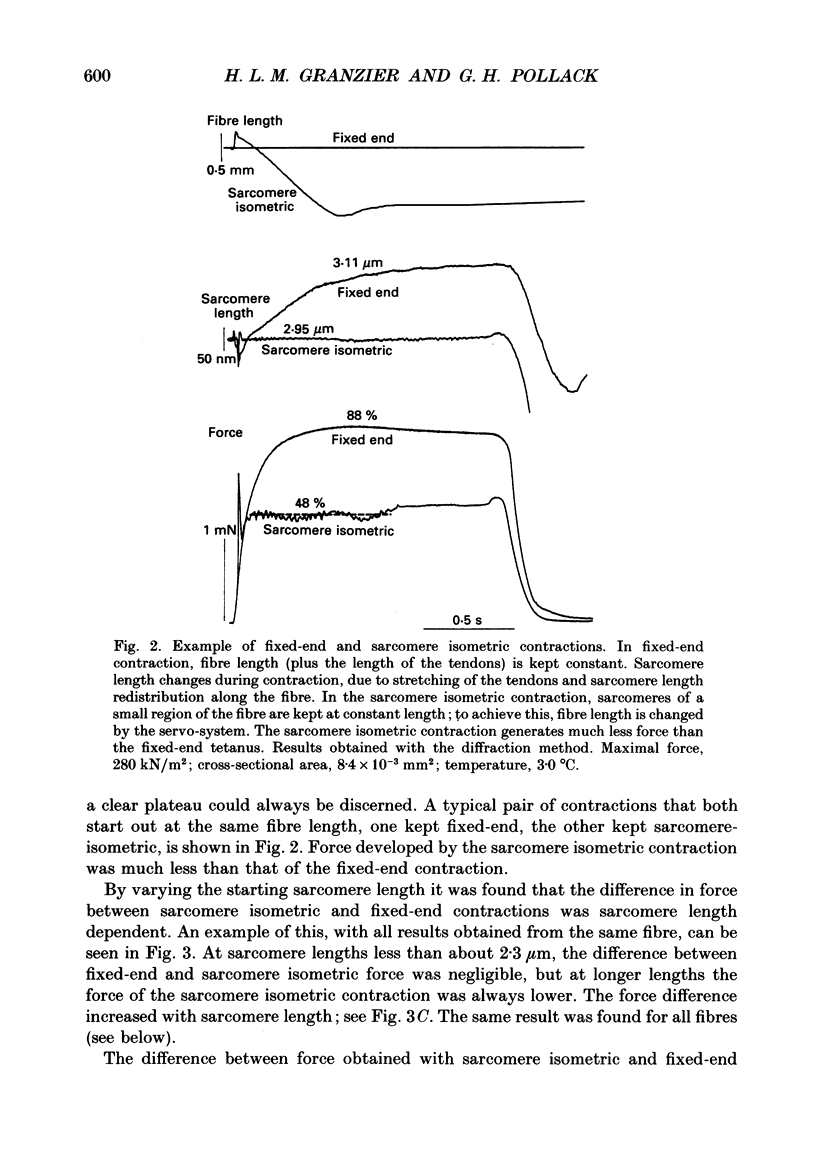
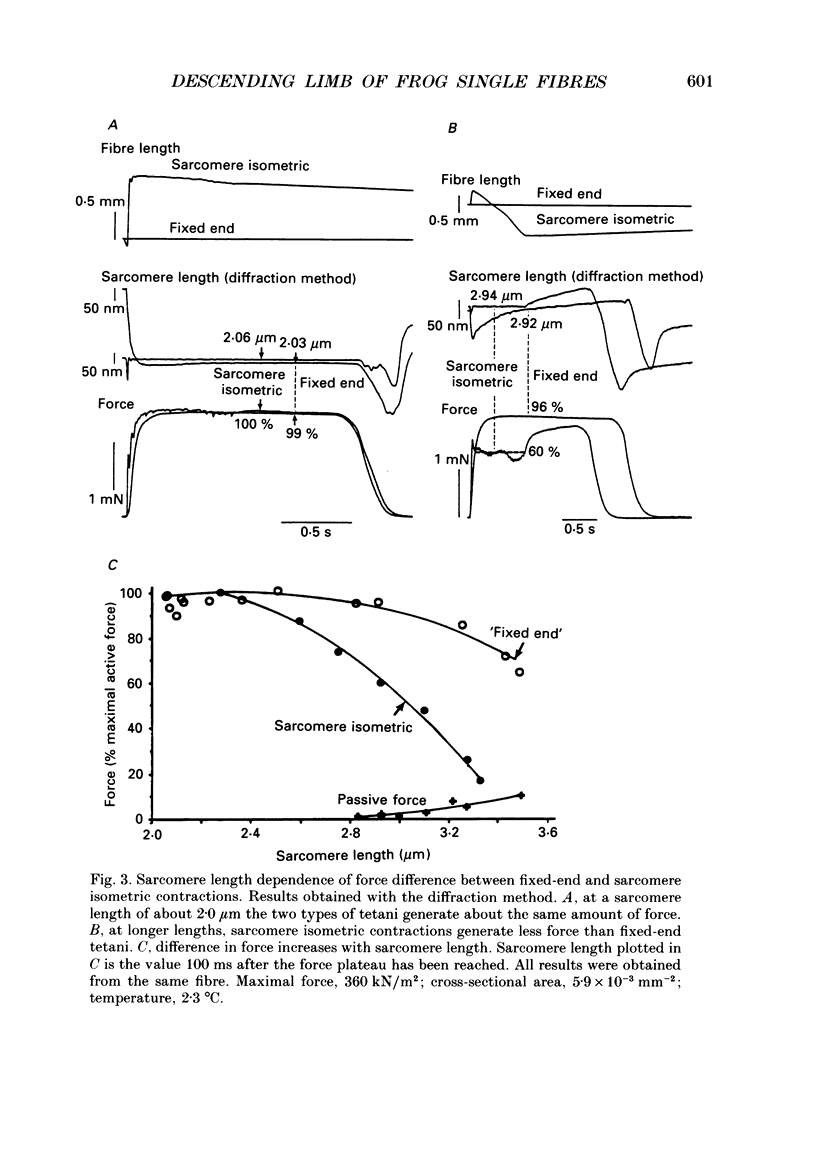
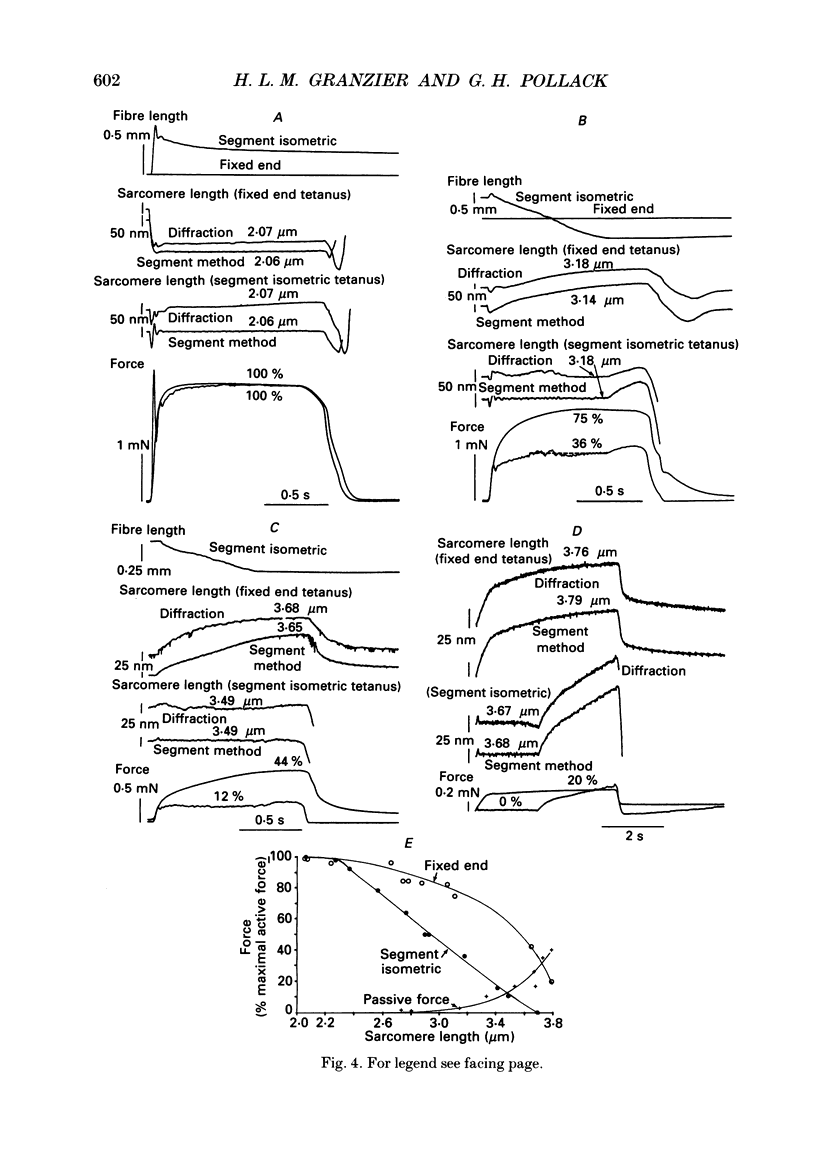


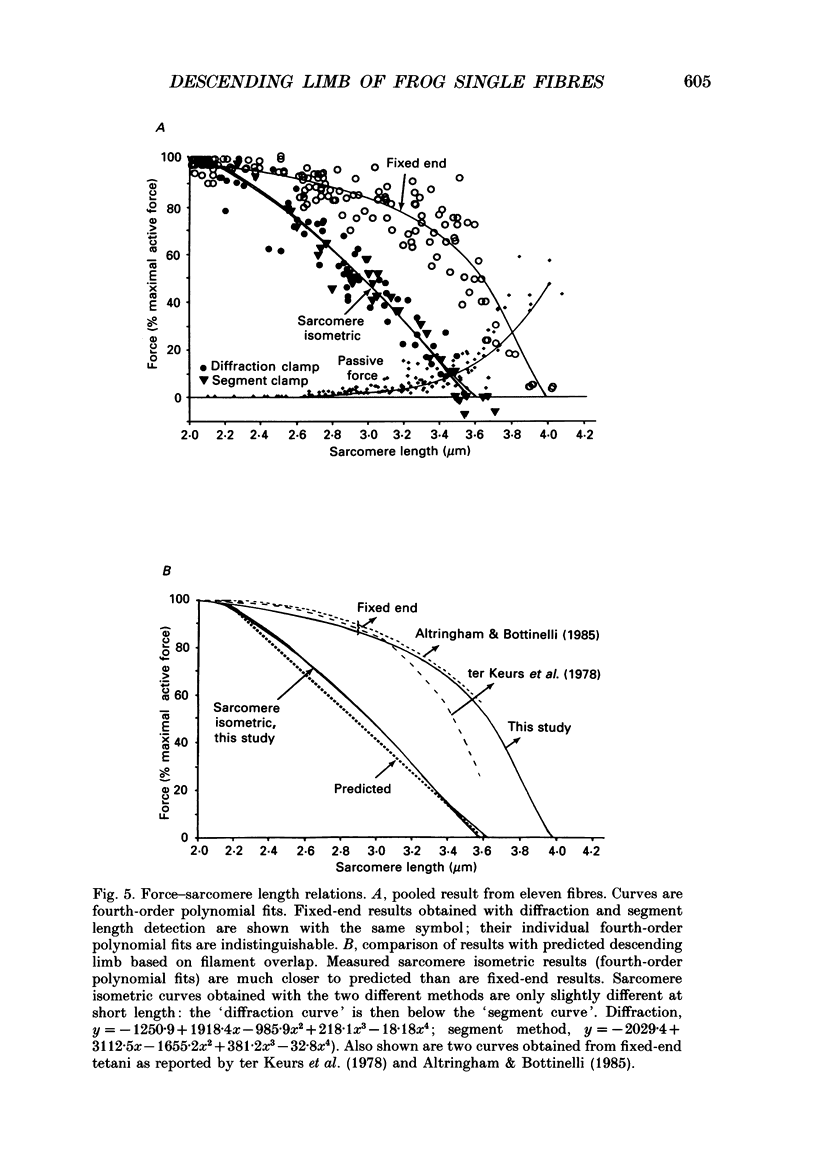






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altringham J. D., Bottinelli R. The descending limb of the sarcomere length-force relation in single muscle fibres of the frog. J Muscle Res Cell Motil. 1985 Oct;6(5):585–600. doi: 10.1007/BF00711916. [DOI] [PubMed] [Google Scholar]
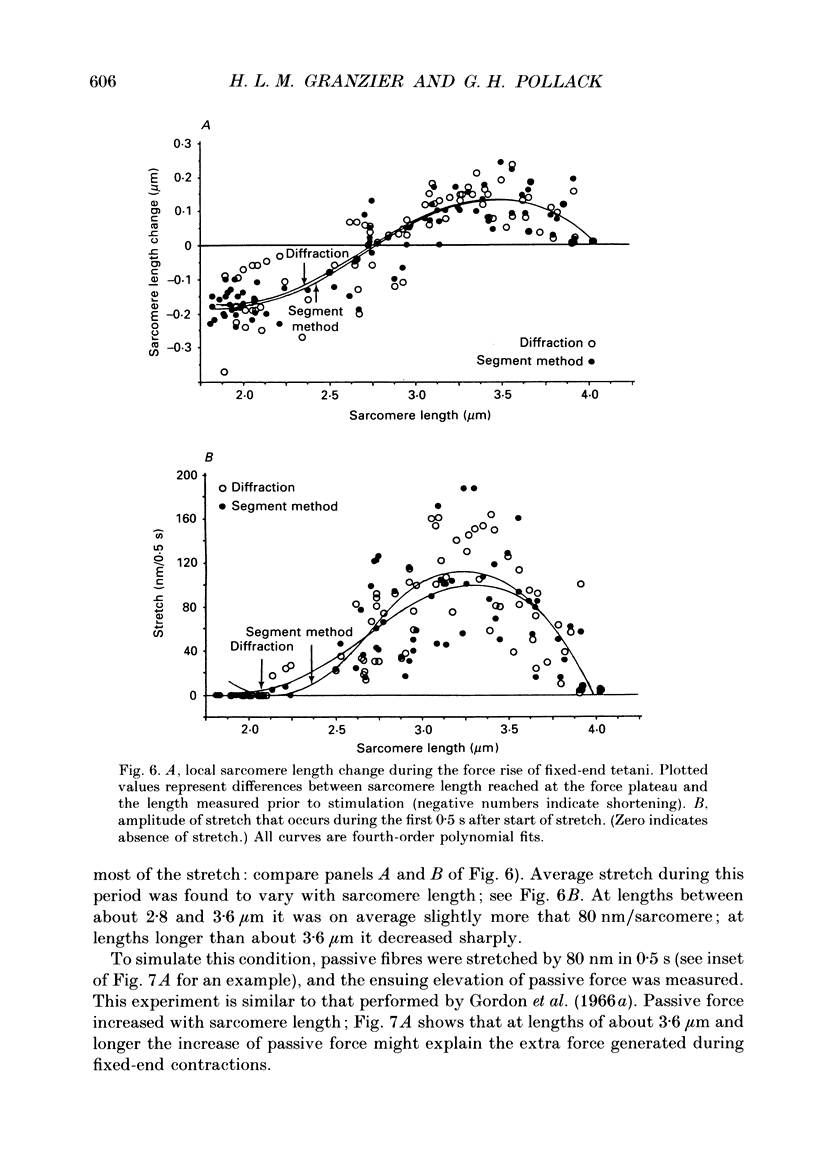
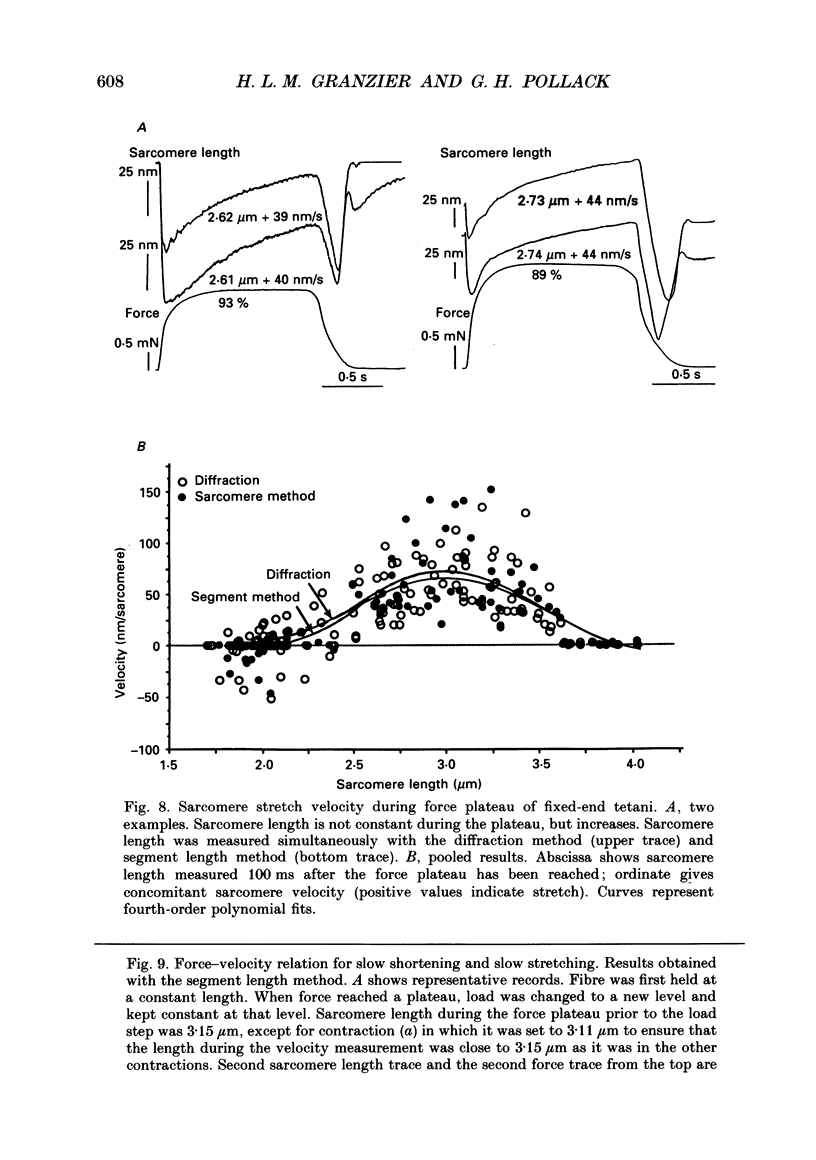
- Altringham J. D., Pollack G. H. Sarcomere length changes in single frog muscle fibres during tetani at long sarcomere lengths. Adv Exp Med Biol. 1984;170:473–493. doi: 10.1007/978-1-4684-4703-3_42. [DOI] [PubMed] [Google Scholar]
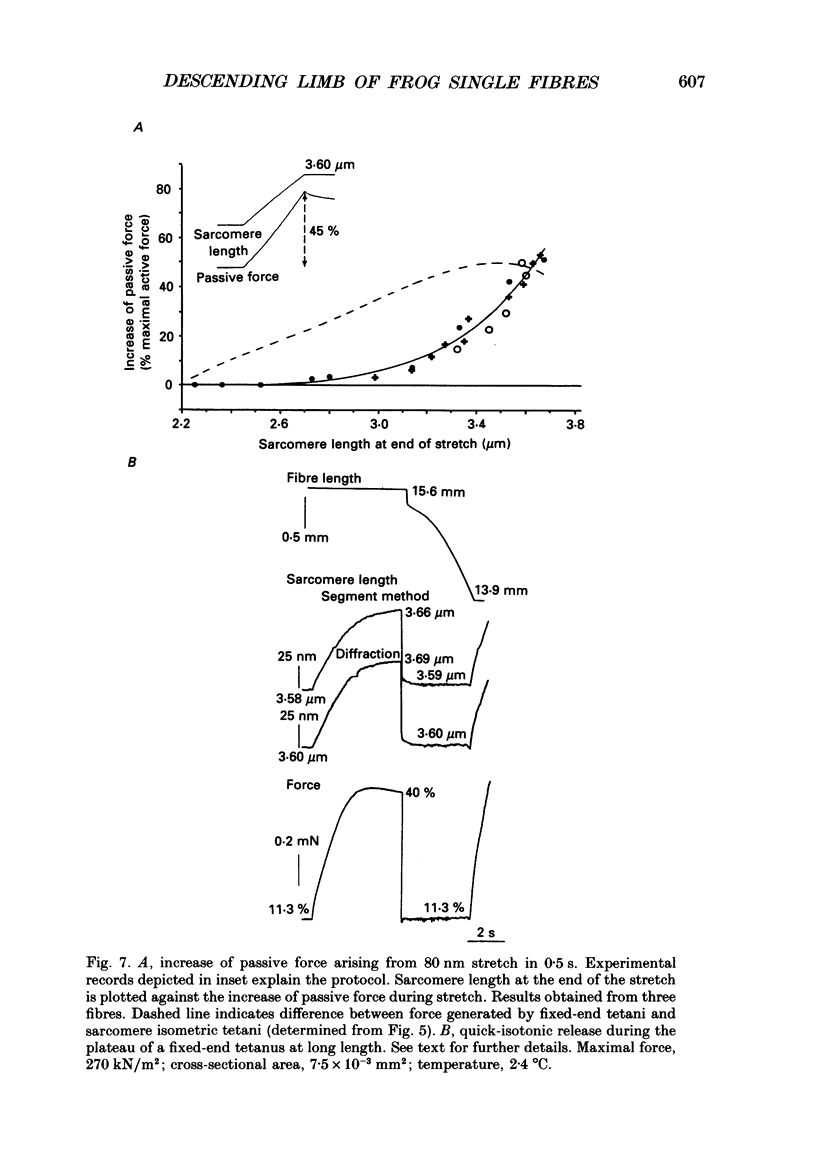
- April E. W. The myofilament lattice: studies on isolated fibers. IV. Lattice equilibria in striated muscle. J Mechanochem Cell Motil. 1975;3(2):111–121. [PubMed] [Google Scholar]
- Bagni M. A., Cecchi G., Colomo F., Tesi C. Plateau and descending limb of the sarcomere length-tension relation in short length-clamped segments of frog muscle fibres. J Physiol. 1988 Jul;401:581–595. doi: 10.1113/jphysiol.1988.sp017181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. M., González-Serratos H., Huxley A. F. Sarcomere and filament lengths in passive muscle fibres with wavy myofibrils. J Muscle Res Cell Motil. 1984 Jun;5(3):293–314. doi: 10.1007/BF00713109. [DOI] [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V. The relation between sarcomere length and isometric tetanic tension of isolated frog muscle fibres at 20 degrees C. Boll Soc Ital Biol Sper. 1976 May 30;52(10):716–718. [PubMed] [Google Scholar]
- Chaplain R. A., Frommelt B. On the contractile mechanism of insect fibrillar flight muscle. I. The dynamics and energetics of the linearized system. Kybernetik. 1968 Jul;5(1):1–17. doi: 10.1007/BF00288894. [DOI] [PubMed] [Google Scholar]
- Colomo F., Lombardi V., Piazzesi G. The mechanisms of force enhancement during constant velocity lengthening in tetanized single fibres of frog muscle. Adv Exp Med Biol. 1988;226:489–502. [PubMed] [Google Scholar]
- Edman K. A., Reggiani C. Redistribution of sarcomere length during isometric contraction of frog muscle fibres and its relation to tension creep. J Physiol. 1984 Jun;351:169–198. doi: 10.1113/jphysiol.1984.sp015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Reggiani C. The sarcomere length-tension relation determined in short segments of intact muscle fibres of the frog. J Physiol. 1987 Apr;385:709–732. doi: 10.1113/jphysiol.1987.sp016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The relation between sarcomere length and active tension in isolated semitendinosus fibres of the frog. J Physiol. 1966 Mar;183(2):407–417. doi: 10.1113/jphysiol.1966.sp007873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Myofilament-generated tension oscillations during partial calcium activation and activation dependence of the sarcomere length-tension relation of skinned cardiac cells. J Gen Physiol. 1978 Nov;72(5):667–699. doi: 10.1085/jgp.72.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. Tension development in highly stretched vertebrate muscle fibres. J Physiol. 1966 May;184(1):143–169. doi: 10.1113/jphysiol.1966.sp007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H. L., Myers J. A., Pollack G. H. Stepwise shortening of muscle fibre segments. J Muscle Res Cell Motil. 1987 Jun;8(3):242–251. doi: 10.1007/BF01574592. [DOI] [PubMed] [Google Scholar]
- Granzier H. L., Pollack G. H. Effect of active pre-shortening on isometric and isotonic performance of single frog muscle fibres. J Physiol. 1989 Aug;415:299–327. doi: 10.1113/jphysiol.1989.sp017723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., PEACHEY L. D. The maximum length for contraction in vertebrate straiated muscle. J Physiol. 1961 Apr;156:150–165. doi: 10.1113/jphysiol.1961.sp006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington W. F. A mechanochemical mechanism for muscle contraction. Proc Natl Acad Sci U S A. 1971 Mar;68(3):685–689. doi: 10.1073/pnas.68.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. Muscular contraction. Annu Rev Physiol. 1988;50:1–16. doi: 10.1146/annurev.ph.50.030188.000245. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishino A., Yanagida T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature. 1988 Jul 7;334(6177):74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- PAGE S. G., HUXLEY H. E. FILAMENT LENGTHS IN STRIATED MUSCLE. J Cell Biol. 1963 Nov;19:369–390. doi: 10.1083/jcb.19.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S. G. Fine structure of tortoise skeletal muscle. J Physiol. 1968 Aug;197(3):709–715. doi: 10.1113/jphysiol.1968.sp008583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack G. H. A proposed mechanism of contraction in which stepwise shortening is a basic feature. Adv Exp Med Biol. 1984;170:787–792. doi: 10.1007/978-1-4684-4703-3_75. [DOI] [PubMed] [Google Scholar]
- Pollack G. H. The cross-bridge theory. Physiol Rev. 1983 Jul;63(3):1049–1113. doi: 10.1152/physrev.1983.63.3.1049. [DOI] [PubMed] [Google Scholar]
- Robinson T. F., Winegrad S. The measurement and dynamic implications of thin filament lengths in heart muscle. J Physiol. 1979 Jan;286:607–619. doi: 10.1113/jphysiol.1979.sp012640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Pollack G. H. Bridgelike interconnections between thick filaments in stretched skeletal muscle fibers observed by the freeze-fracture method. J Cell Biol. 1986 Mar;102(3):1093–1098. doi: 10.1083/jcb.102.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Wright J. Architecture of the sarcomere matrix of skeletal muscle: immunoelectron microscopic evidence that suggests a set of parallel inextensible nebulin filaments anchored at the Z line. J Cell Biol. 1988 Dec;107(6 Pt 1):2199–2212. doi: 10.1083/jcb.107.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. The elasticity of relaxed insect fibrillar flight muscle. J Physiol. 1983 Oct;343:31–57. doi: 10.1113/jphysiol.1983.sp014880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Keurs H. E., Iwazumi T., Pollack G. H. The sarcomere length-tension relation in skeletal muscle. J Gen Physiol. 1978 Oct;72(4):565–592. doi: 10.1085/jgp.72.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Keurs H. E., Luff A. R., Luff S. E. Force--sarcomere-length relation and filament length in rat extensor digitorum muscle. Adv Exp Med Biol. 1984;170:511–525. doi: 10.1007/978-1-4684-4703-3_44. [DOI] [PubMed] [Google Scholar]


