Abstract
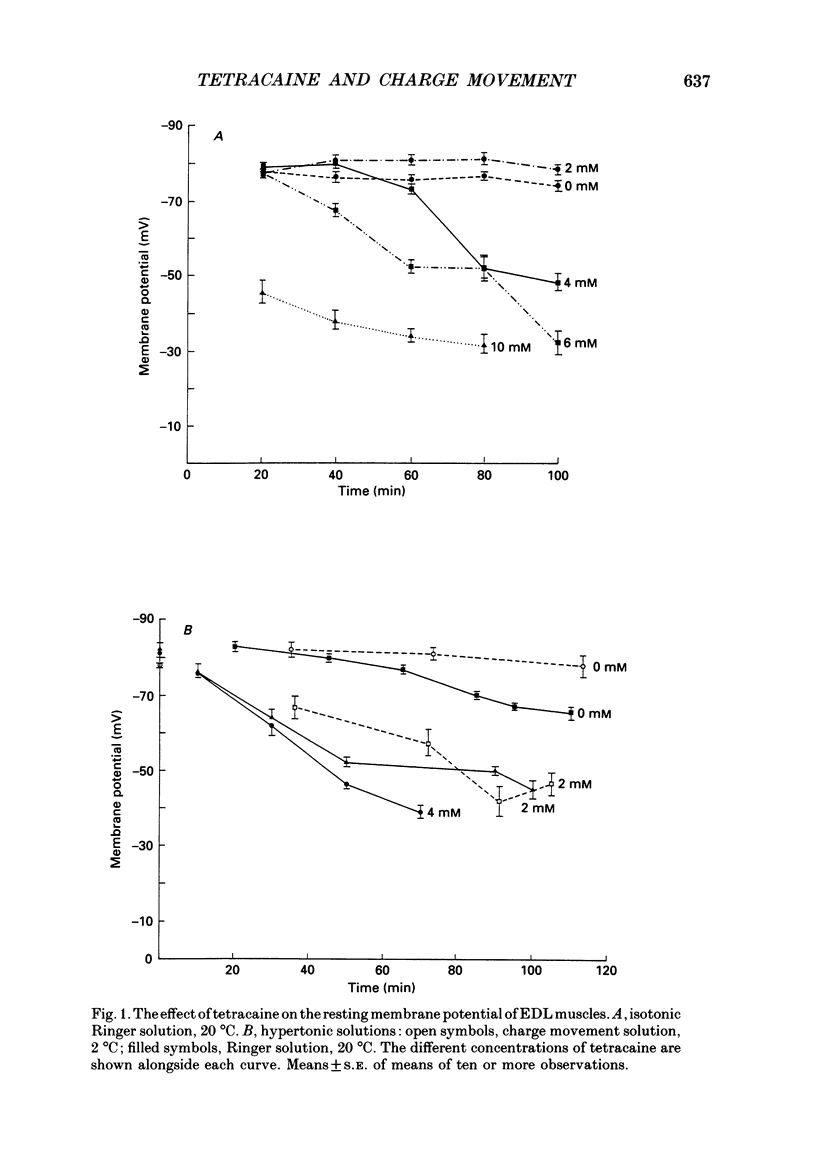
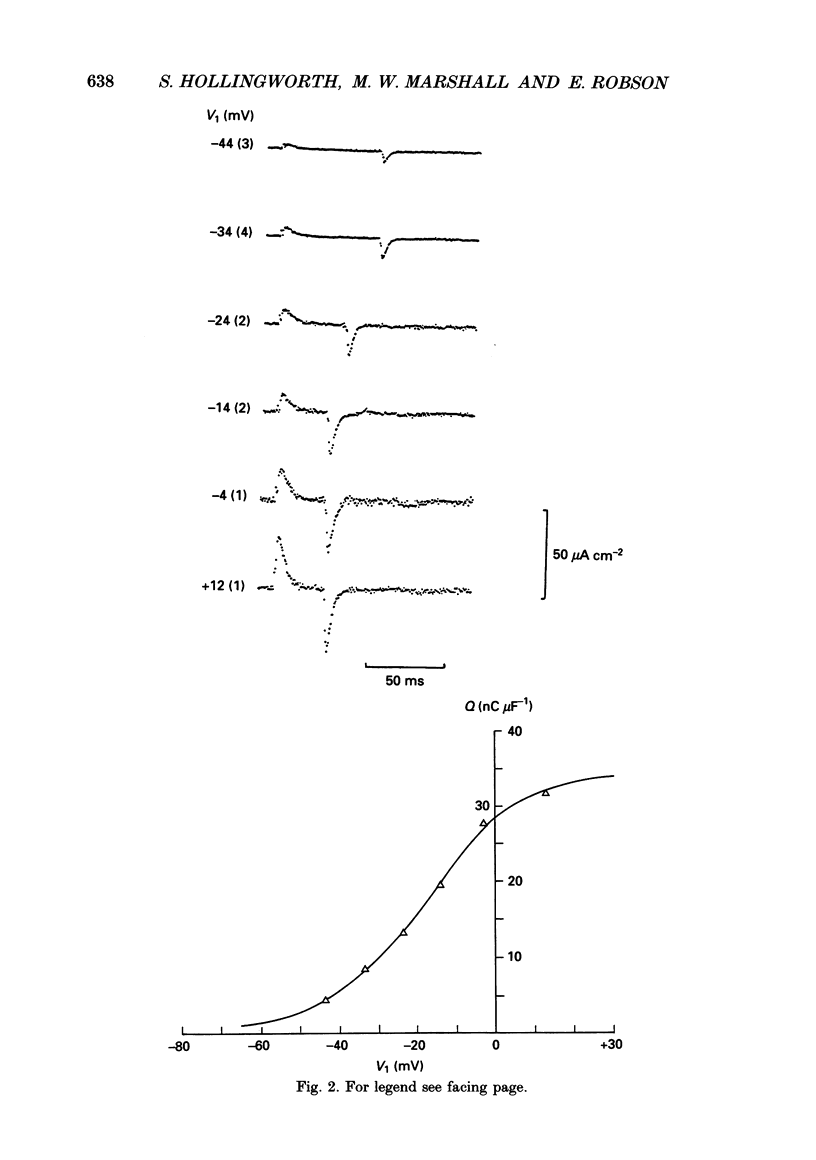
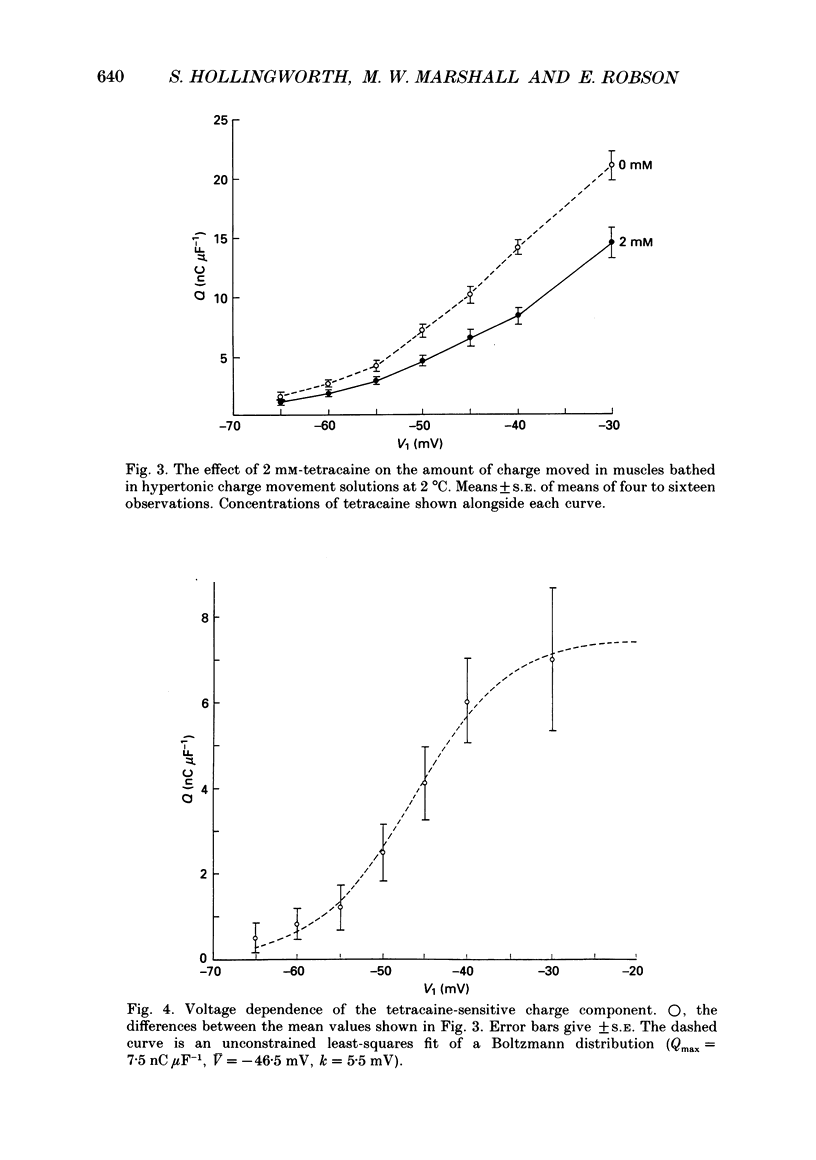
1. The effects of tetracaine, a local anaesthetic that inhibits muscle contraction, on membrane potential and intramembrane charge movements were investigated in fast twitch rat muscle fibres (extensor digitorum longus). 2. The resting membrane potentials of surface fibres from muscles bathed in isotonic Ringer solution containing 2 mM-tetracaine were well maintained, but higher concentrations of tetracaine caused a time-dependent fall of potential. Muscle fibres bathed in hypertonic solutions containing 2 mM-tetracaine were rapidly depolarized. In both isotonic and hypertonic solutions, the depolarizing effect of tetracaine could not be reversed. 3. Charge movement measurements were made using the middle-of-the-fibre voltage clamp technique. The voltage dependence of charge movements measured in cold isotonic solutions was well fitted by a Boltzmann distribution (Q(V) = Qmax/(1 + exp(-(V-V)/k] where Qmax = 37.3 +/- 2.8 nC muF-1, V = -17.9 +/- 1.2 mV and k = 12.6 +/- 0.8 mV (n = 6, 2 degrees C; means +/- S.E. of means). Similar values were obtained when 2 mM-tetracaine was added to the isotonic bathing fluid (Qmax = 40.6 +/- 2.3 nC microF-1, V = -14.1 +/- 1.3 mV, k = 15.3 +/- 0.8 mV; n = 8, 2 degrees C). 4. Charge movements measured around mechanical threshold in muscle fibres bathed in hypertonic solutions were reduced when 2 mM-tetracaine was added to the bathing fluid. The tetracaine-sensitive component of charge was well fitted with an unconstrained Boltzmann distribution which gave: Qmax = 7.5 nC microF-1, V = -46.5 mV, k = 5.5 mV. The e-fold rise of the foot of the curve was 9.3 mV.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Almers W. The voltage dependence of membrane capacity. J Physiol. 1976 Jan;254(2):317–338. doi: 10.1113/jphysiol.1976.sp011234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Marshall M. W. Sodium currents in mammalian muscle. J Physiol. 1977 Jun;268(1):223–250. doi: 10.1113/jphysiol.1977.sp011855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Peres A. Charge movement and membrane capacity in frog muscle. J Physiol. 1979 Apr;289:83–97. doi: 10.1113/jphysiol.1979.sp012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Best P. M. Effects of tetracaine on displacement currents and contraction of frog skeletal muscle. J Physiol. 1976 Nov;262(3):583–611. doi: 10.1113/jphysiol.1976.sp011611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel A., Brown B. H. Some abnormal effects of stimulating nerve trunks through the skin. J Physiol. 1968 Feb;194(2):57–9P. [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J Physiol. 1983 Nov;344:625–666. doi: 10.1113/jphysiol.1983.sp014959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S., Marshall M. W. Effects of intracellular ruthenium red on excitation-contraction coupling in intact frog skeletal muscle fibres. J Physiol. 1989 Jan;408:617–635. doi: 10.1113/jphysiol.1989.sp017480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernoch L., Huang C. L., Szucs G., Kovacs L. Differential effects of tetracaine on charge movements and Ca2+ signals in frog skeletal muscle. J Gen Physiol. 1988 Nov;92(5):601–612. doi: 10.1085/jgp.92.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty A. F., Gage P. W. Asymmetrical charge movement in slow- and fast-twitch mammalian muscle fibres in normal and paraplegic rats. J Physiol. 1983 Aug;341:213–231. doi: 10.1113/jphysiol.1983.sp014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth S., Marshall M. W. A comparative study of charge movement in rat and frog skeletal muscle fibres. J Physiol. 1981 Dec;321:583–602. doi: 10.1113/jphysiol.1981.sp014004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth S., Marshall M. W., Robson E. Ionic currents and charge movements in organ-cultured rat skeletal muscle. J Physiol. 1984 Dec;357:369–386. doi: 10.1113/jphysiol.1984.sp015505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Dielectric components of charge movements in skeletal muscle. J Physiol. 1981;313:187–205. doi: 10.1113/jphysiol.1981.sp013658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Effects of local anaesthetics on the relationship between charge movements and contractile thresholds in frog skeletal muscle. J Physiol. 1981 Nov;320:381–391. doi: 10.1113/jphysiol.1981.sp013956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Intramembrane charge movements in skeletal muscle. Physiol Rev. 1988 Oct;68(4):1197–1147. doi: 10.1152/physrev.1988.68.4.1197. [DOI] [PubMed] [Google Scholar]
- Huang C. L. Pharmacological separation of charge movement components in frog skeletal muscle. J Physiol. 1982 Mar;324:375–387. doi: 10.1113/jphysiol.1982.sp014118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. The differential effects of twitch potentiators on charge movements in frog skeletal muscle. J Physiol. 1986 Nov;380:17–33. doi: 10.1113/jphysiol.1986.sp016269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S. Pharmacological studies of charge movement in frog skeletal muscle. J Physiol. 1983 Apr;337:509–529. doi: 10.1113/jphysiol.1983.sp014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D. Components of charge movement in rabbit skeletal muscle: the effect of tetracaine and nifedipine. J Physiol. 1986 Jul;376:85–100. doi: 10.1113/jphysiol.1986.sp016143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D. Components of charge movement in rabbit skeletal muscle: the effect of tetracaine and nifedipine. J Physiol. 1986 Jul;376:85–100. doi: 10.1113/jphysiol.1986.sp016143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Chandler W. K. Calcium signals recorded from cut frog twitch fibers containing antipyrylazo III. J Gen Physiol. 1987 Jan;89(1):83–143. doi: 10.1085/jgp.89.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Nakajima S., Parker I., Takahashi T. Effects of membrane polarization on sarcoplasmic calcium release in skeletal muscle. Proc R Soc Lond B Biol Sci. 1981 Sep 17;213(1190):1–13. doi: 10.1098/rspb.1981.0049. [DOI] [PubMed] [Google Scholar]
- Nakazato Y., Onoda Y. Barium and strontium can substitute for calcium in noradrenaline output induced by excess potassium in the guinea-pig. J Physiol. 1980 Aug;305:59–71. doi: 10.1113/jphysiol.1980.sp013349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]



