Abstract
1. Comparisons have been made between rabbit thoracic aorta and main pulmonary artery of the effects of hypoxia upon contractions evoked by noradrenaline (NA) and KCl (K+). 2. Contractions were evoked in cylindrical sections of pulmonary artery and aorta, mounted for isometric recording of tension, by NA and K+ (at ED80) in normoxia (PO2 110 mmHg) and hypoxia (PO2 23 or 7 mmHg). Contractions were also evoked in Ca2(+)-free conditions with EGTA to prevent influx of extracellular Ca2+. All contractions are expressed as a percentage of normoxic response in the presence of Ca2+. 3. Potassium-evoked contractions of aorta and pulmonary artery were reduced to a similar extent by both levels of hypoxia, to 85 and 92% respectively. As expected K(+)-evoked contractions were virtually abolished by Ca2(+)-free conditions. It is proposed that hypoxia has a small inhibitory effect upon contraction mediated by Ca2+ influx via voltage-operated Ca2+ channels. 4. In the aorta in the presence of Ca2+, hypoxia reduced NA-evoked contractions to 84% at PO2 23 mmHg and 34% at PO2 7 mmHg. In the absence of Ca2+, NA-evoked contractions reached 73% in normoxia, but only 43 and 21% at PO2 23 and 7 mmHg respectively. These results suggest that hypoxia reduces the component of contraction that is mediated by release of intracellular Ca2+ and possibly that mediated by agonist-induced Ca2+ influx. 5. In the pulmonary artery also, NA-evoked responses in the absence of Ca2+ were reduced from 60% in normoxia, to 49 and 38% at PO2 23 and 7 mmHg. But, in the presence of Ca2+, hypoxia potentiated NA-evoked contractions to 113 and 111% at PO2 23 and 7 mmHg respectively. It is proposed that in the pulmonary artery, hypoxia reduces the component of contraction mediated by release of intracellular Ca2+, but facilitates that mediated by extracellular Ca2+. Possible mechanisms are discussed.
Full text
PDF



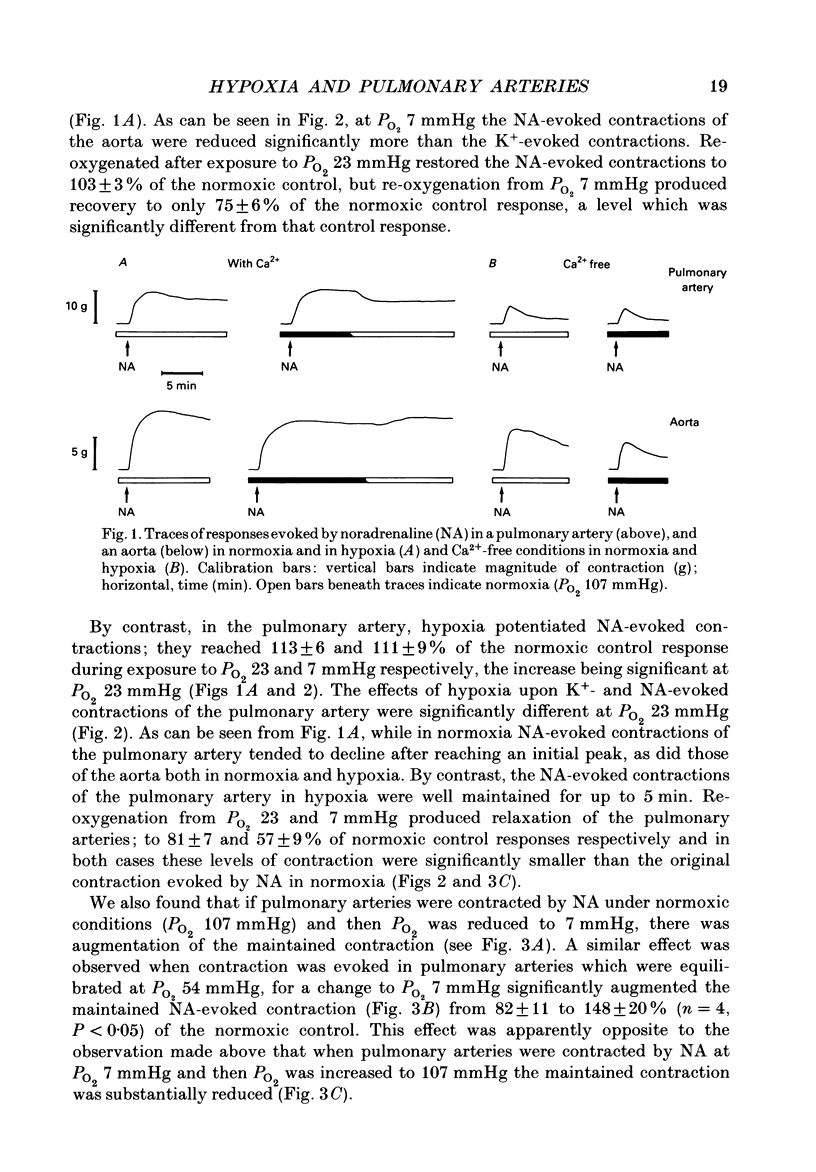
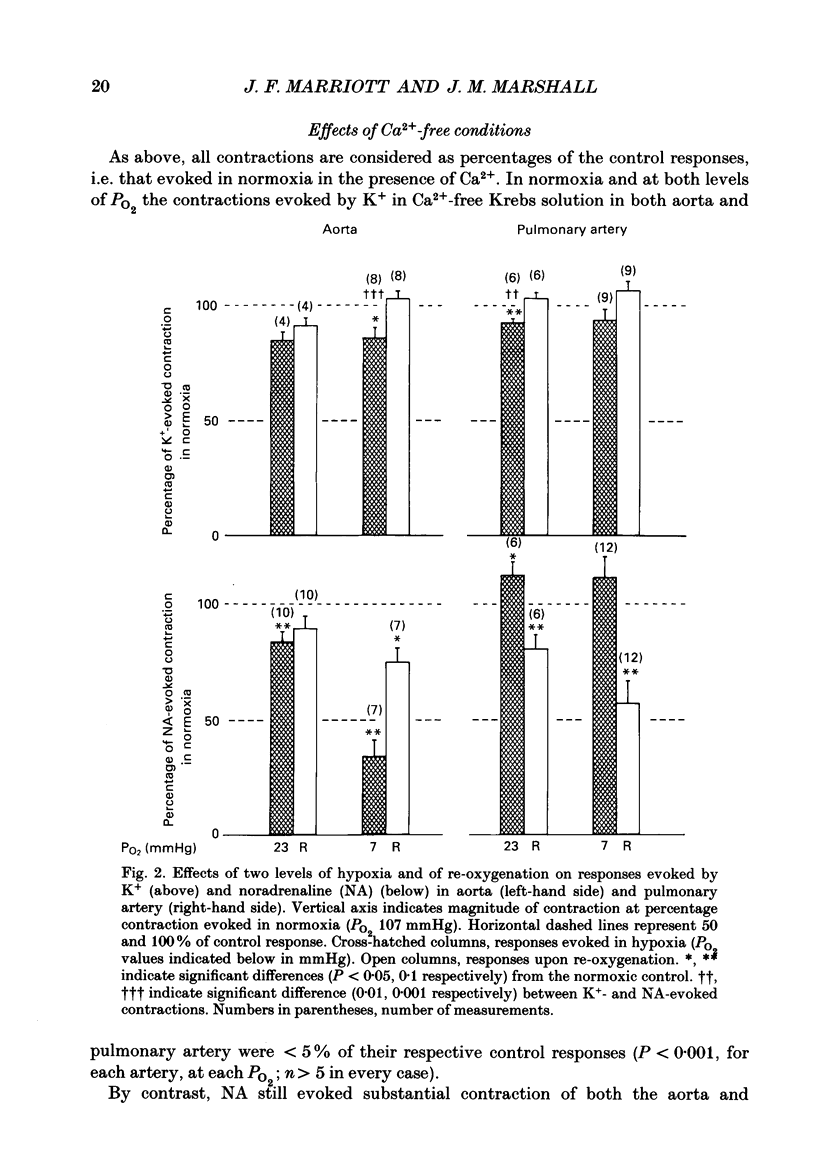
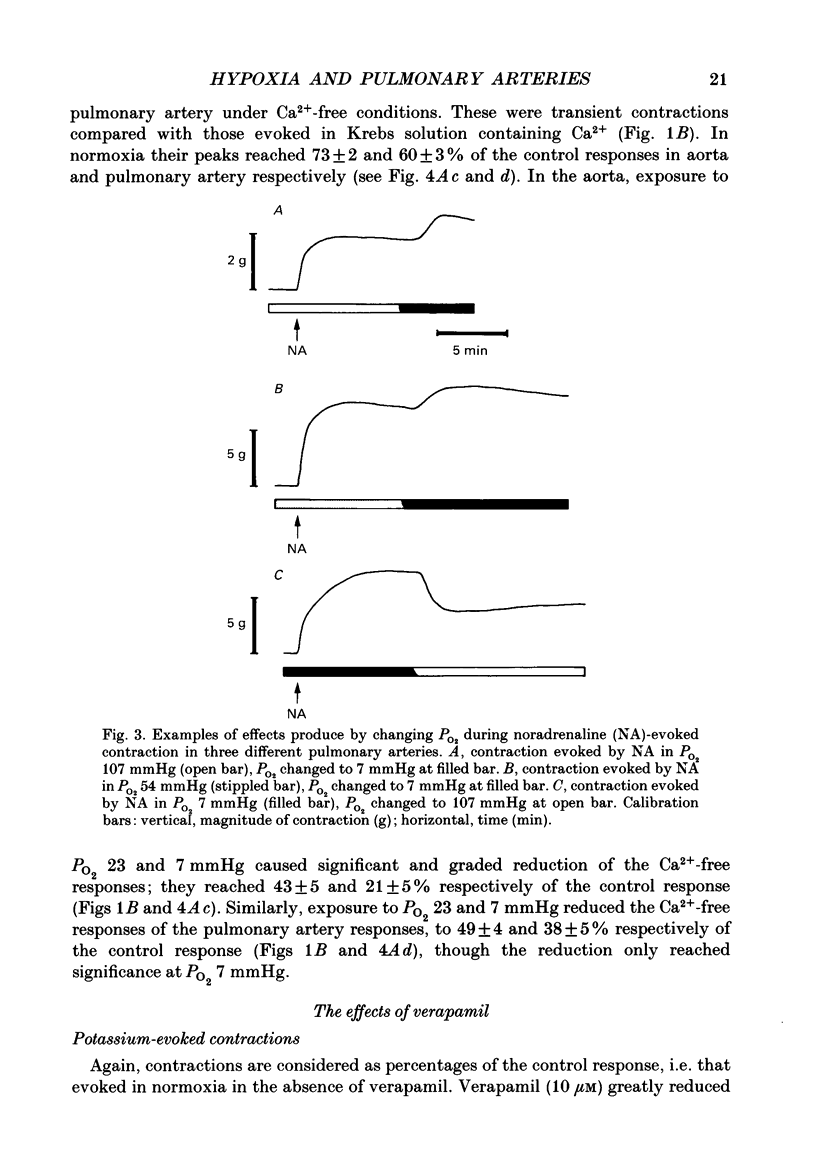
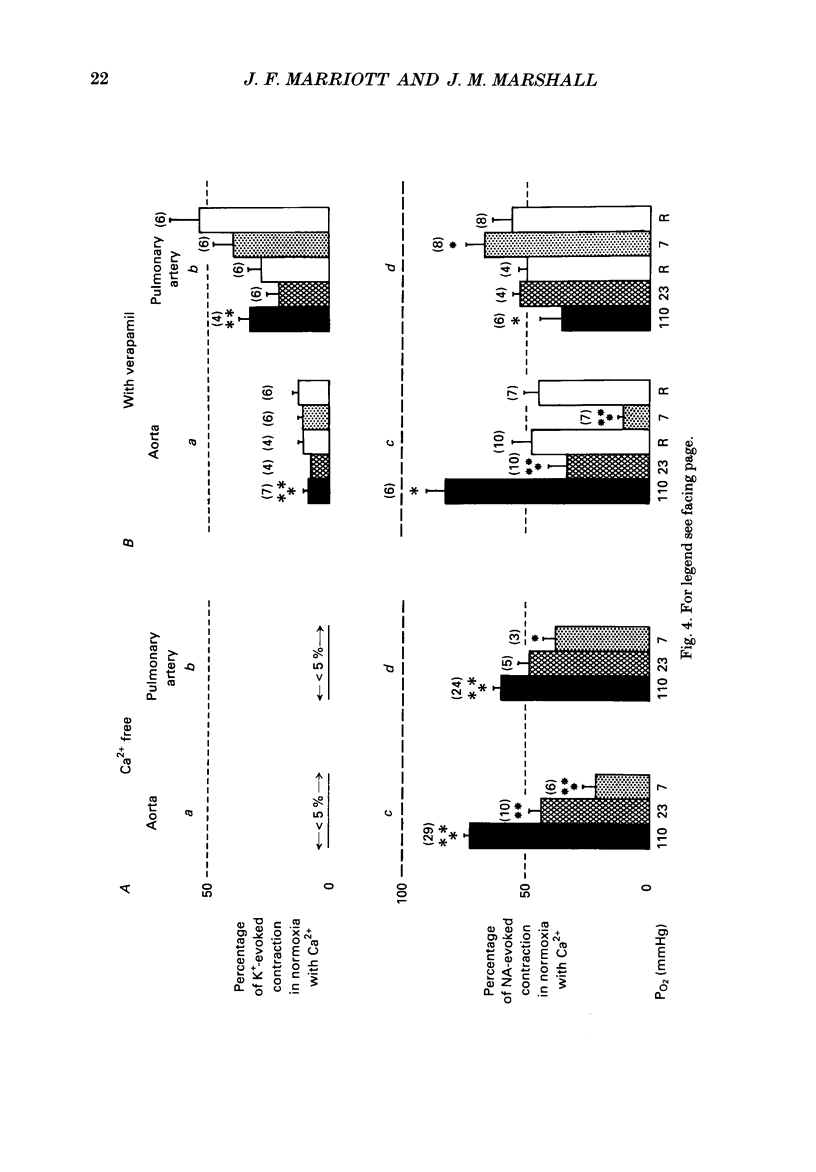






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barer G. R. The physiology of the pulmonary circulation and methods of study. Pharmacol Ther B. 1976;2(2):247–273. doi: 10.1016/s0306-039x(76)80008-6. [DOI] [PubMed] [Google Scholar]
- Cauvin C., Loutzenhiser R., Van Breemen C. Mechanisms of calcium antagonist-induced vasodilation. Annu Rev Pharmacol Toxicol. 1983;23:373–396. doi: 10.1146/annurev.pa.23.040183.002105. [DOI] [PubMed] [Google Scholar]
- Dawson C. A. Role of pulmonary vasomotion in physiology of the lung. Physiol Rev. 1984 Apr;64(2):544–616. doi: 10.1152/physrev.1984.64.2.544. [DOI] [PubMed] [Google Scholar]
- Detar R., Bohr D. F. Contractile responses of isolated vascular smooth muscle during prolonged exposure to anoxia. Am J Physiol. 1972 May;222(5):1269–1277. doi: 10.1152/ajplegacy.1972.222.5.1269. [DOI] [PubMed] [Google Scholar]
- Detar R., Gellai M. Oxygen and isolated vascular smooth muscle from the main pulmonary artery of the rabbit. Am J Physiol. 1971 Dec;221(6):1791–1794. doi: 10.1152/ajplegacy.1971.221.6.1791. [DOI] [PubMed] [Google Scholar]
- Ebeigbe A. B. Influence of hypoxia on contractility and calcium uptake in rabbit aorta. Experientia. 1982 Aug 15;38(8):935–937. doi: 10.1007/BF01953662. [DOI] [PubMed] [Google Scholar]
- Fishman A. P. Hypoxia on the pulmonary circulation. How and where it acts. Circ Res. 1976 Apr;38(4):221–231. doi: 10.1161/01.res.38.4.221. [DOI] [PubMed] [Google Scholar]
- Haeusler G. Contraction, membrane potential, and calcium fluxes in rabbit pulmonary arterial muscle. Fed Proc. 1983 Feb;42(2):263–268. [PubMed] [Google Scholar]
- Lloyd T. C., Jr Hypoxic pulmonary vasoconstriction: role of perivascular tissue. J Appl Physiol. 1968 Nov;25(5):560–565. doi: 10.1152/jappl.1968.25.5.560. [DOI] [PubMed] [Google Scholar]
- Lloyd T. C., Jr Responses to hypoxia of pulmonary arterial strips in nonaqueous baths. J Appl Physiol. 1970 May;28(5):566–569. doi: 10.1152/jappl.1970.28.5.566. [DOI] [PubMed] [Google Scholar]
- Marriott J. F. A comparison of the effects of the calcium entry blockers, verapamil, diltiazem and flunarizine against contractions of the rat isolated aorta and portal vein. Br J Pharmacol. 1988 Sep;95(1):145–154. doi: 10.1111/j.1476-5381.1988.tb16558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott J. F., Marshall J. M. Differential effects of hypoxia upon contractions evoked by potassium and noradrenaline in rabbit arteries in vitro. J Physiol. 1990 Mar;422:1–13. doi: 10.1113/jphysiol.1990.sp017968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell L. C., Herlihy J. T., Riedel G. L. Effects of ascorbic acid and EDTA on vascular concentration-response to catecholamines. Microvasc Res. 1983 Jul;26(1):81–88. doi: 10.1016/0026-2862(83)90057-2. [DOI] [PubMed] [Google Scholar]
- McMurtry I. F., Rounds S., Stanbrook H. S. Studies of the mechanism of hypoxic pulmonary vasoconstriction. Adv Shock Res. 1982;8:21–33. [PubMed] [Google Scholar]
- Szidon J. P., Fishman A. P. Participation of pulmonary circulation in the defense reaction. Am J Physiol. 1971 Feb;220(2):364–370. doi: 10.1152/ajplegacy.1971.220.2.364. [DOI] [PubMed] [Google Scholar]
- Weiss G. B. Calcium kinetics in vascular smooth muscle. Chest. 1985 Oct;88(4 Suppl):220S–223S. doi: 10.1378/chest.88.4_supplement.220s. [DOI] [PubMed] [Google Scholar]


