Abstract
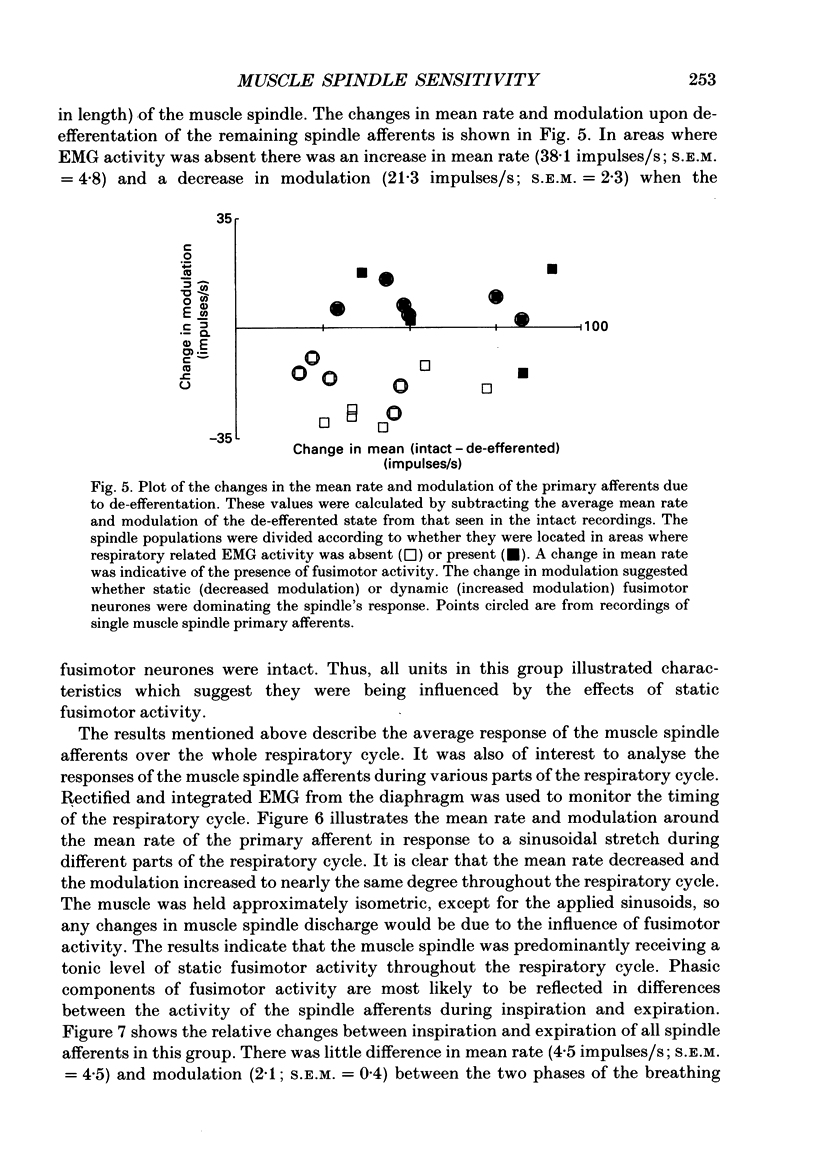
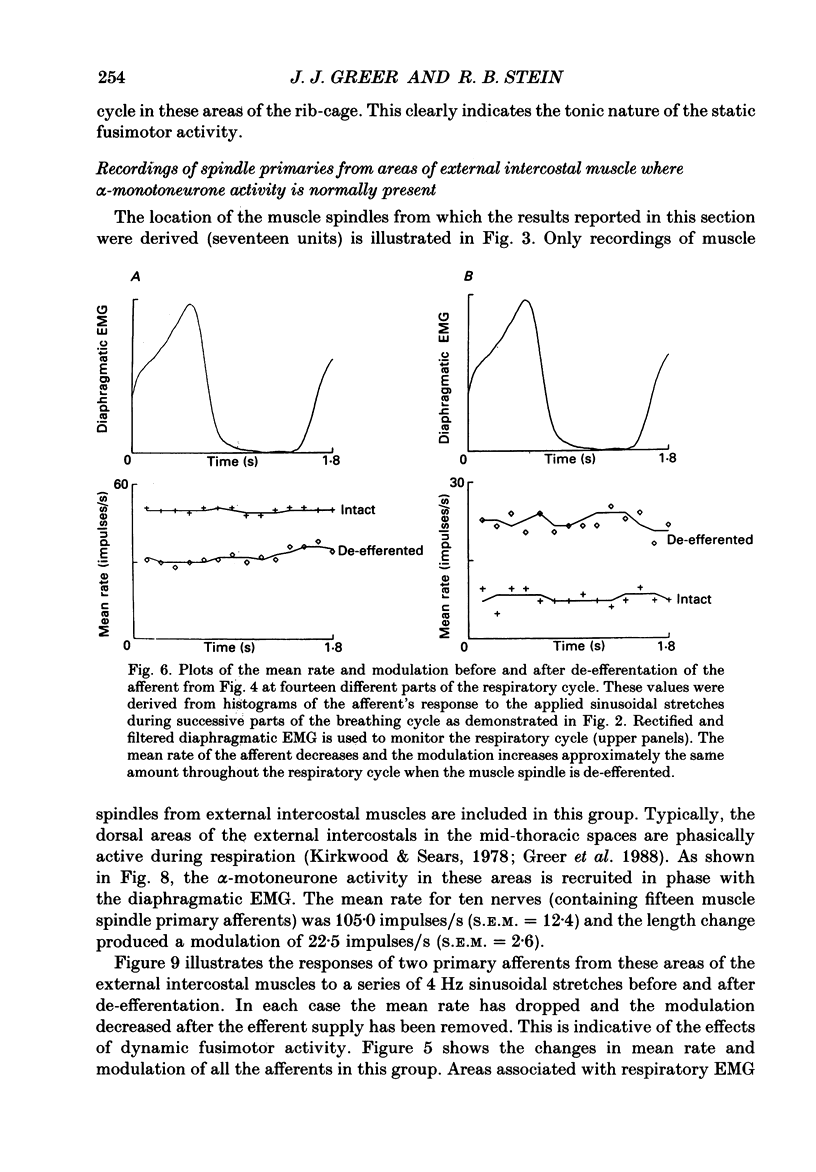
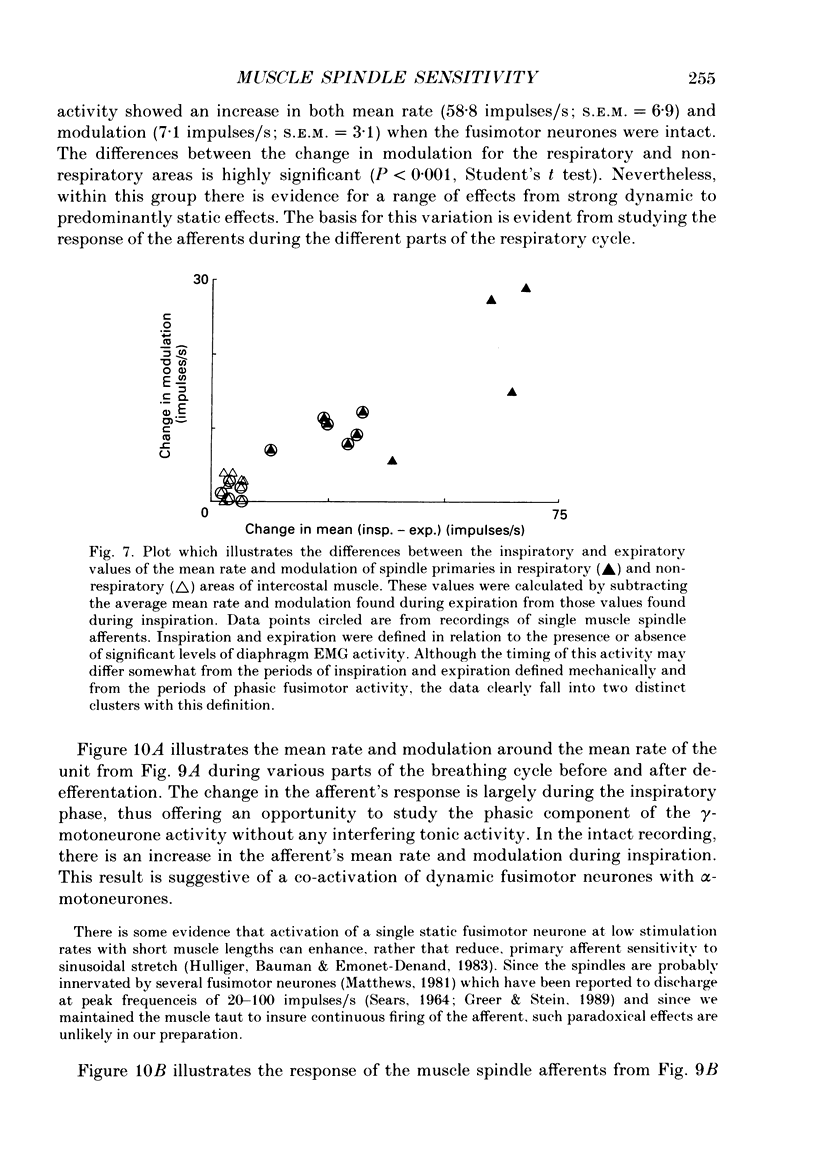
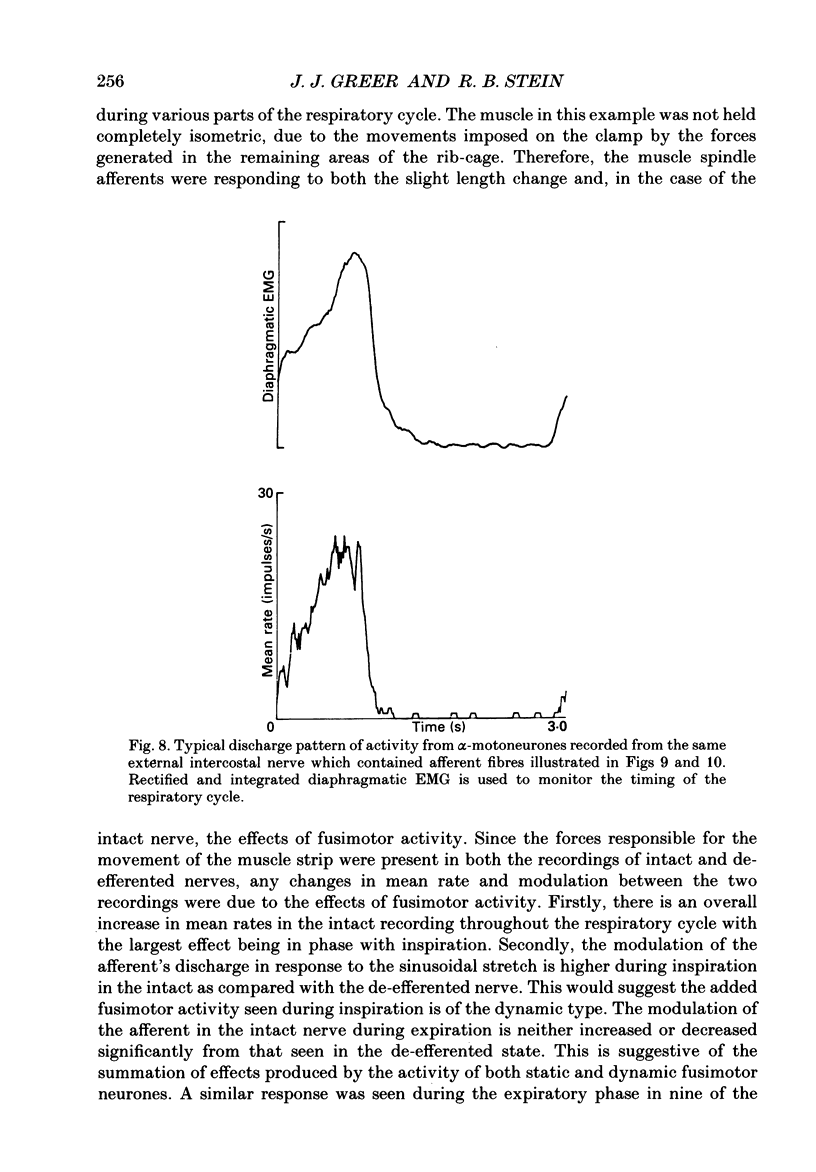
1. The two types of fusimotor neurones, dynamic and static, can be differentiated by their effects on muscle spindle afferents. We have recorded the activity of muscle spindle primary afferents from the intercostal nerves of anaesthetized or decerebrate cats. A 4 Hz sinusoidal stretch was applied to the muscle containing the spindles of interest before and after crushing the nerve proximal to the recording site to eliminate fusimotor effects. The relative activity of the dynamic and static fusimotor neurones was inferred from the change in the spindle afferents' response. 2. Some areas of intercostal muscle normally showed phasic activity linked to respiration, where as other areas of intercostal muscle showed no EMG activity under our experimental conditions. In areas of intercostal muscle lacking EMG activity, the afferents' mean rate was higher and the modulation around the mean was lower at all phases of the breathing cycle when the efferent supply was intact. This result suggests the muscle spindles were receiving a steady level of static fusimotor activity. 3. Spindle primary afferents from regions of intercostal muscle that were typically recruited during respiration had an additional increase in mean rate and modulation around the mean rate in phase with the EMG activity. This is suggestive of phasic activation of dynamic fusimotor neurones in addition to static fusimotor discharge. 4. Thus, the two types of fusimotor neurones can be activated separately by the CNS to control the sensitivity of muscle spindles. The regional differences in the recruitment patterns of fusimotor neurones parallels the functional specializations of different areas of the intercostal muscles. The temporal modifications of fusimotor activity during each respiratory cycle means that the segmental reflex gain will vary in those intercostal muscles that are active during respiration. 5. These findings regarding the CNS recruitment of the two types of fusimotor neurones during respiration are similar to those reported for the hindlimb extensors during locomotion, but differ from those reported for jaw muscles during chewing. This may reflect differing control strategies being used by the CNS to meet the unique demands of the various rhythmical movements.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aminoff M. J., Sears T. A. Spinal integration of segmental, cortical and breathing inputs to thoracic respiratory motoneurones. J Physiol. 1971 Jun;215(2):557–575. doi: 10.1113/jphysiol.1971.sp009485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Sears T. A. Medullary activation of intercostal fusimotor and alpha motoneurones. J Physiol. 1970 Aug;209(3):739–755. doi: 10.1113/jphysiol.1970.sp009189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B. F., Lennerstrand G., Thoden U. Fusimotor effects on position and velocity sensitivity of spindle endings in the external intercostal muscles of the cat. Acta Physiol Scand. 1968 Nov;74(3):285–300. doi: 10.1111/j.1748-1716.1968.tb04237.x. [DOI] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Fusimotor reflexes in triceps surae elicited by natural stimulation of muscle afferents from the cat ipsilateral hind limb. J Physiol. 1982 Aug;329:211–229. doi: 10.1113/jphysiol.1982.sp014299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser D. C., Lindsey B. G., Shannon R. Medullary inspiratory activity: influence of intercostal tendon organs and muscle spindle endings. J Appl Physiol (1985) 1987 Mar;62(3):1046–1056. doi: 10.1152/jappl.1987.62.3.1046. [DOI] [PubMed] [Google Scholar]
- Boyd I. A. Two types of static gamma-axon in cat muscle spindles. Q J Exp Physiol. 1986 Apr;71(2):307–327. doi: 10.1113/expphysiol.1986.sp002987. [DOI] [PubMed] [Google Scholar]
- CRITCHLOW V., VON EULER INTERCOSTAL MUSCLE SPINDLE ACTIVITY AND ITS GAMMA MOTOR CONTROL. J Physiol. 1963 Oct;168:820–847. doi: 10.1113/jphysiol.1963.sp007225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelguen J. M. Static and dynamic fusimotor controls in various hindlimb muscles during locomotor activity in the decorticate cat. Brain Res. 1981 May 25;213(1):83–97. doi: 10.1016/0006-8993(81)91249-x. [DOI] [PubMed] [Google Scholar]
- Coffey G. L., Godwin-Austen R. B., Macgillivray B. B., Sears T. A. The form and distribution of the surface evoked responses in cerebellar cortex from intercostal nerves in the cat. J Physiol. 1971 Jan;212(1):129–145. doi: 10.1113/jphysiol.1971.sp009314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda M., von Euler C., Lennerstrand G. Reflex and cerebellar influences on alpha and on 'rhythmic' and 'tonic' gamma activity in the intercostal muscle. J Physiol. 1966 Jun;184(4):898–923. doi: 10.1113/jphysiol.1966.sp007956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAPER M. H., LADEFOGED P., WHITTERIDGE D. Expiratory pressures and air flow during speech. Br Med J. 1960 Jun 18;1(5189):1837–1843. doi: 10.1136/bmj.1.5189.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. N., Sears T. A. The proprioceptive reflex control of the intercostal muscles during their voluntary activation. J Physiol. 1970 Aug;209(3):711–738. doi: 10.1113/jphysiol.1970.sp009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. N. The response to stretch of human intercostal muscle spindles studied in vitro. J Physiol. 1975 Aug;249(3):561–579. doi: 10.1113/jphysiol.1975.sp011030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A., Kelly S., Macklem P. T., Zin W. A. Mechanics of intercostal space and actions of external and internal intercostal muscles. J Clin Invest. 1985 Mar;75(3):850–857. doi: 10.1172/JCI111782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron B., Caillol M. C. Activité électrique des muscles intercostaux au cours du frisson thermique chez le chat anesthésié. J Physiol (Paris) 1971 Jun-Jul;63(4):523–537. [PubMed] [Google Scholar]
- Duron B., Jung-Caillol M. C., Marlot D. Myelinated nerve fiber supply and muscle spindles in the respiratory muscles of cat: quantitative study. Anat Embryol (Berl) 1978 Feb 20;152(2):171–192. doi: 10.1007/BF00315923. [DOI] [PubMed] [Google Scholar]
- Duron B. Postural and ventilatory functions of intercostal muscles. Acta Neurobiol Exp (Wars) 1973;33(1):355–380. [PubMed] [Google Scholar]
- Gottlieb S., Taylor A. Interpretation of fusimotor activity in cat masseter nerve during reflex jaw movements. J Physiol. 1983 Dec;345:423–438. doi: 10.1113/jphysiol.1983.sp014986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer J. J., Stein R. B. Patterns of gamma-motoneuron activity in the external intercostal muscles of the cat during respiration. Brain Res. 1989 Jan 16;477(1-2):369–372. doi: 10.1016/0006-8993(89)91429-7. [DOI] [PubMed] [Google Scholar]
- Hulliger M., Matthews P. B., Noth J. Effects of combining static and dynamic fusimotor stimulation on the response of the muscle spindle primary ending to sinusoidal stretching. J Physiol. 1977 Jun;267(3):839–856. doi: 10.1113/jphysiol.1977.sp011840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M., Matthews P. B., Noth J. Static and dynamic fusimotor action on the response of Ia fibres to low frequency sinusoidal stretching of widely ranging amplitude. J Physiol. 1977 Jun;267(3):811–838. doi: 10.1113/jphysiol.1977.sp011839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Stagg D., Westgaard R. H. Intercostal muscles and purring in the cat: the influence of afferent inputs. Brain Res. 1987 Mar 3;405(1):187–191. doi: 10.1016/0006-8993(87)91007-9. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978 Feb;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson C. R., Smith A., Luschei E. S. Discharge characteristics and stretch sensitivity of jaw muscle afferents in the monkey during controlled isometric bites. J Neurophysiol. 1981 Jul;46(1):130–142. doi: 10.1152/jn.1981.46.1.130. [DOI] [PubMed] [Google Scholar]
- Loeb G. E. Motoneurone task groups: coping with kinematic heterogeneity. J Exp Biol. 1985 Mar;115:137–146. doi: 10.1242/jeb.115.1.137. [DOI] [PubMed] [Google Scholar]
- Matthews P. B. Evolving views on the internal operation and functional role of the muscle spindle. J Physiol. 1981 Nov;320:1–30. doi: 10.1113/jphysiol.1981.sp013931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner T. E., Stein R. B., Gillespie J., Hanley B. Improved estimates of conduction velocity distributions using single unit action potentials. J Neurol Neurosurg Psychiatry. 1981 Jun;44(6):476–484. doi: 10.1136/jnnp.44.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P. R., Stein R. B., Taylor J. Phasic and tonic modulation of impulse rates in gamma-motoneurons during locomotion in premammillary cats. J Neurophysiol. 1984 Aug;52(2):228–243. doi: 10.1152/jn.1984.52.2.228. [DOI] [PubMed] [Google Scholar]
- NATHAN P. W., SEARS T. A. Effects of posterior root section on the activity of some muscles in man. J Neurol Neurosurg Psychiatry. 1960 Feb;23:10–22. doi: 10.1136/jnnp.23.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret C., Berthoz A. Evidence of static and dynamic fusimotor actions on the spindle response to sinusoidal stretch during locomotor activity in the cat. Exp Brain Res. 1973 Sep 29;18(2):178–188. doi: 10.1007/BF00234722. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Hulliger M., Zangger P., Appenteng K. 'Fusimotor set': new evidence for alpha-independent control of gamma-motoneurones during movement in the awake cat. Brain Res. 1985 Jul 22;339(1):136–140. doi: 10.1016/0006-8993(85)90632-8. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. EFFERENT DISCHARGES IN ALPHA AND FUSIMOTOR FIBRES OF INTERCOSTAL NERVES OF THE CAT. J Physiol. 1964 Nov;174:295–315. doi: 10.1113/jphysiol.1964.sp007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant'Ambrogio G., Widdicombe J. G. Respiratory reflexes acting on the diaphragm and inspiratory intercostal muscle of the rabbit. J Physiol. 1965 Oct;180(4):766–779. doi: 10.1113/jphysiol.1965.sp007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. B., Nichols T. R., Jhamandas J., Davis L., Charles D. Stable long-term recordings from cat peripheral nerves. Brain Res. 1977 Jun 3;128(1):21–38. doi: 10.1016/0006-8993(77)90233-5. [DOI] [PubMed] [Google Scholar]
- Taylor J., Stein R. B., Murphy P. R. Impulse rates and sensitivity to stretch of soleus muscle spindle afferent fibers during locomotion in premammillary cats. J Neurophysiol. 1985 Feb;53(2):341–360. doi: 10.1152/jn.1985.53.2.341. [DOI] [PubMed] [Google Scholar]


