Abstract
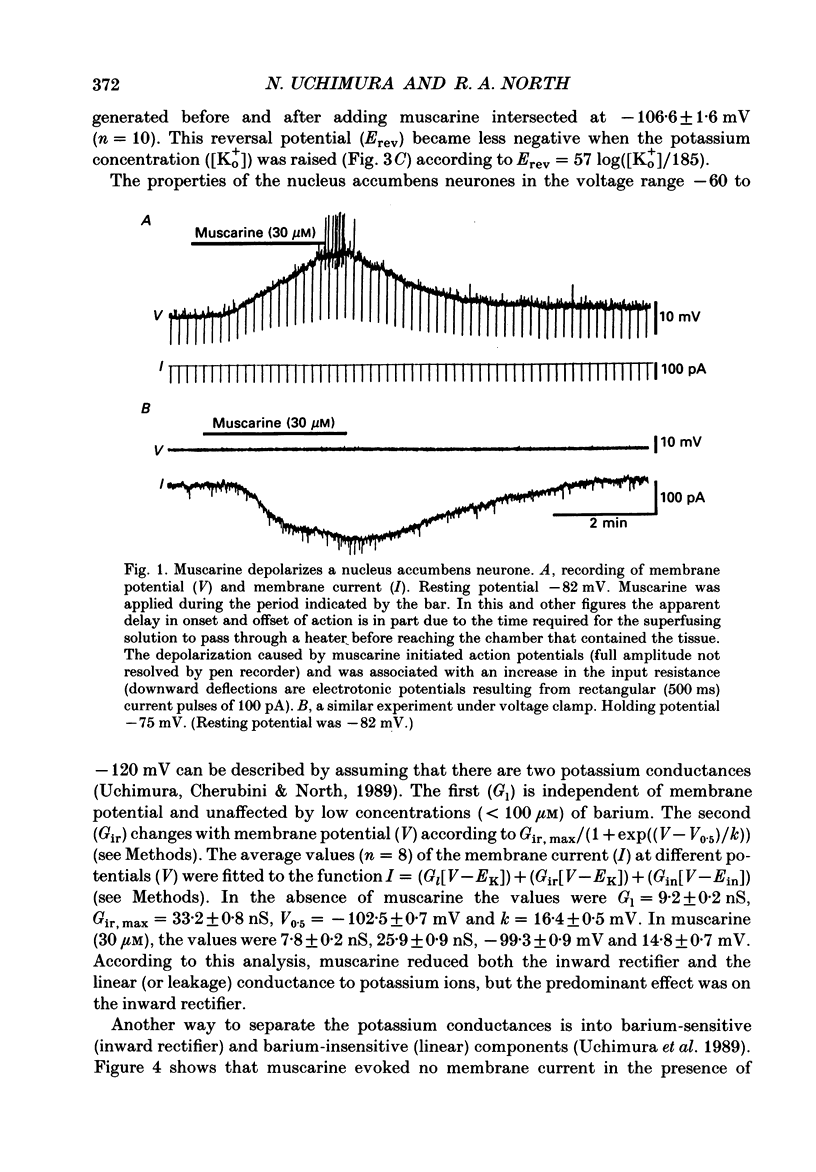
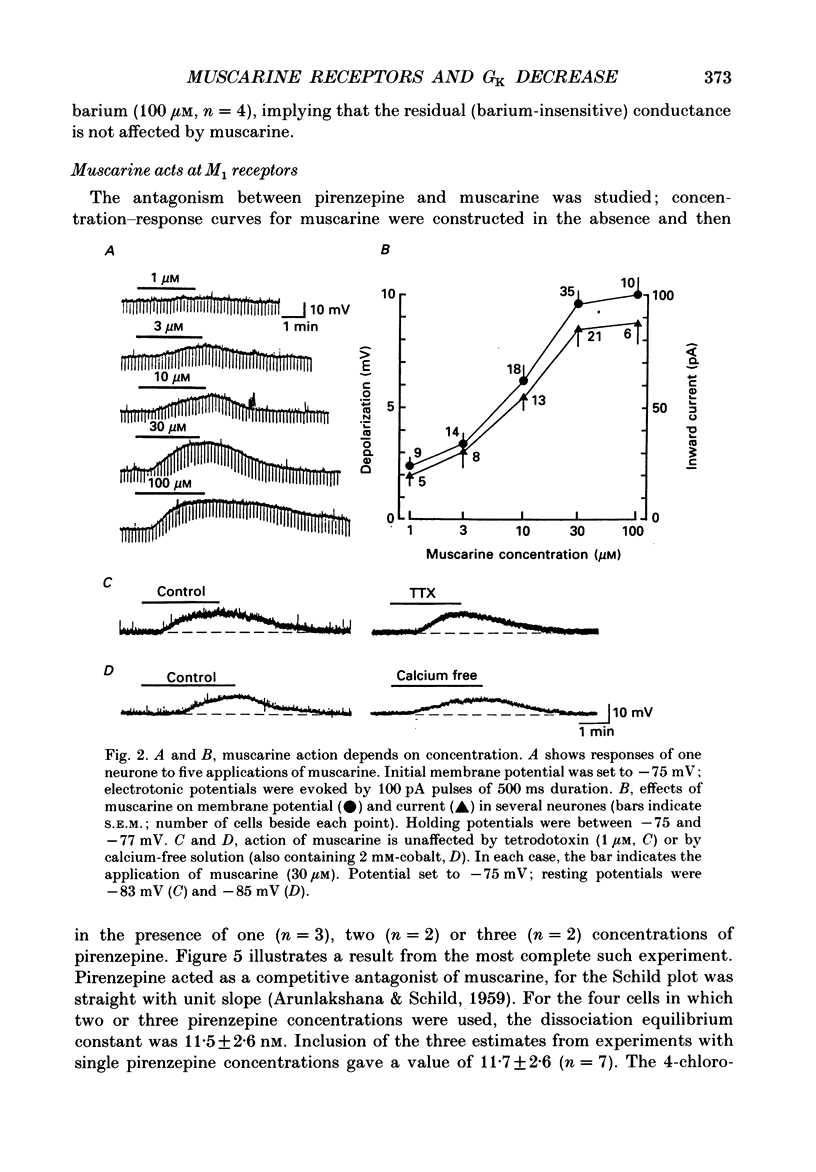
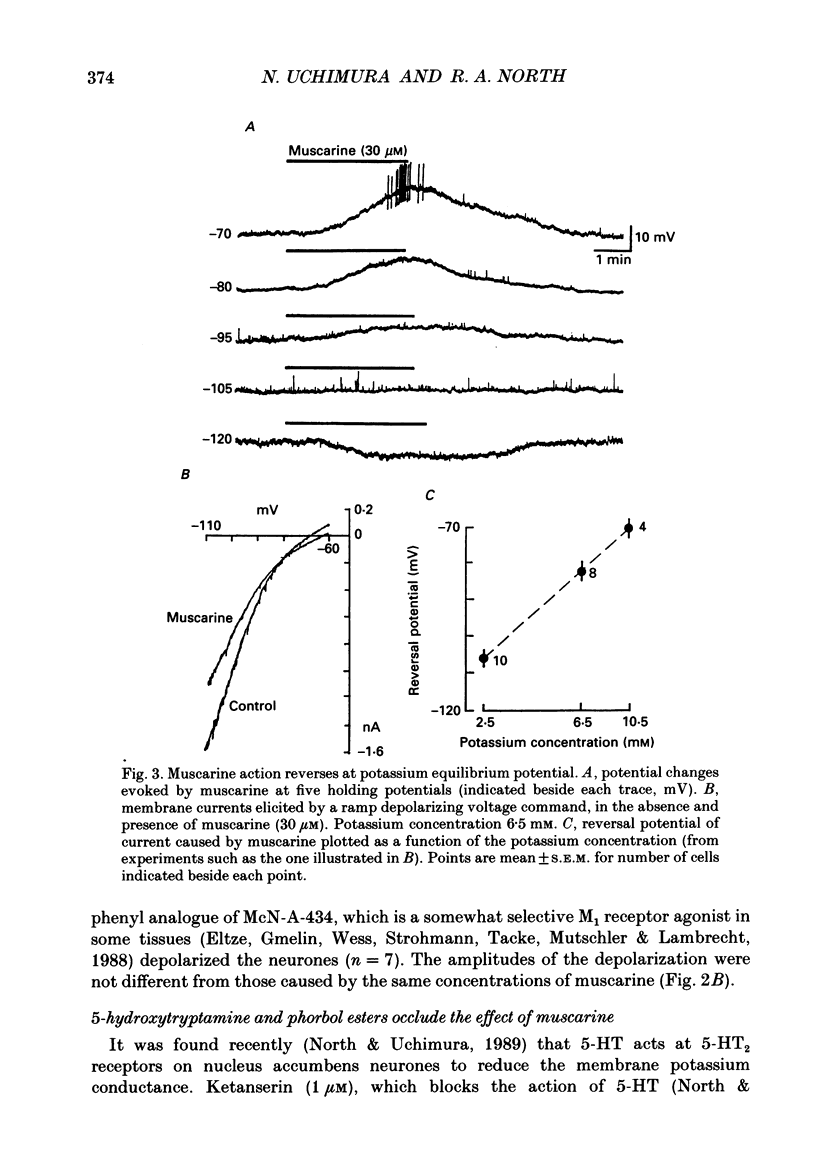
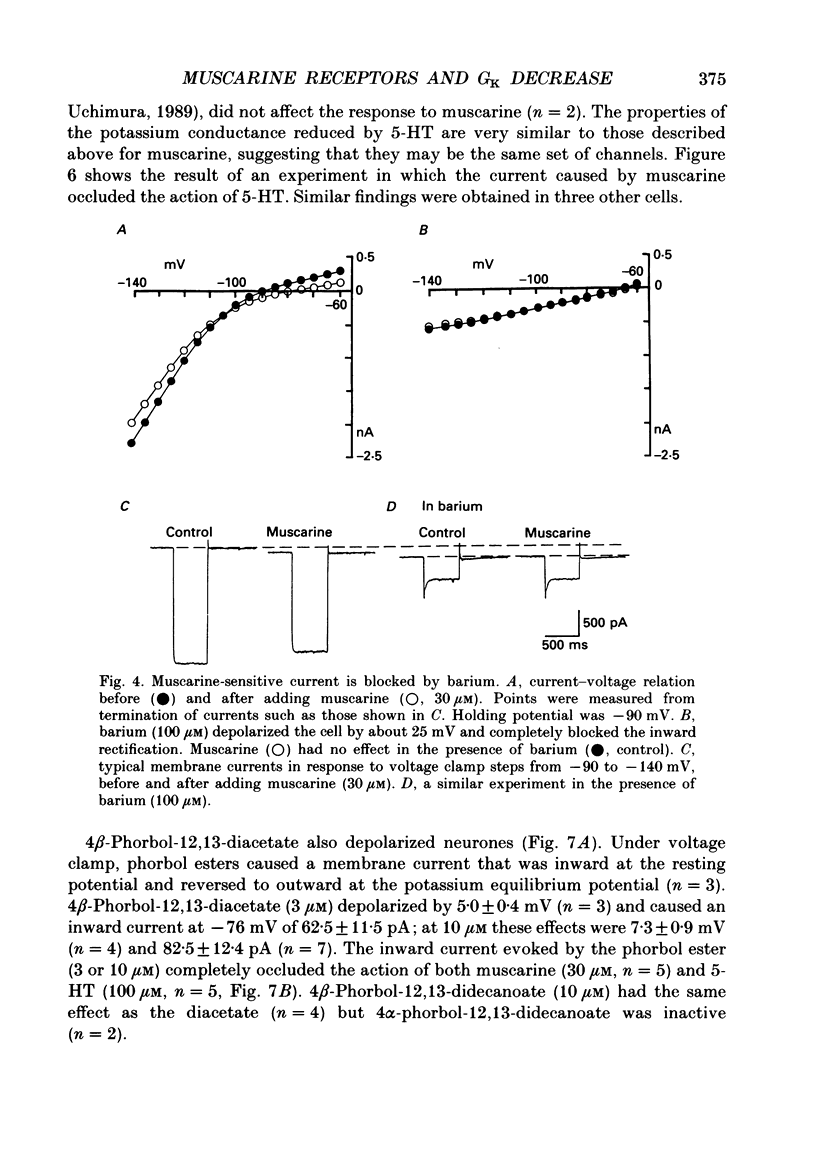
1. Intracellular recordings were made from neurones in the nucleus accumbens in slices from the rat brain maintained in vitro. 2. Muscarine (1-100 microM) depolarized 101 of 107 neurones; this was associated with an increase in the input resistance. The potential change reversed polarity with conditioning hyperpolarization and the reversal potential was linearly related to the logarithm of the extracellular potassium concentration. 3. The depolarization caused by muscarine was not changed by tetrodotoxin (1 microM) or by a solution that contained lower levels of calcium (0.24 instead of 2.4 mM), higher levels of magnesium (5 instead of 1.2 mM) and cobalt (2 mM). 4. Muscarine caused an inward current and a decrease in slope conductance when applied to neurones voltage clamped near their resting potential (-82 mV). The current caused by muscarine reversed polarity at the potassium equilibrium potential. The current-voltage relation of the neurones between -60 and -120 mV was well fitted by assuming a voltage-independent potassium conductance and an inward rectifier potassium conductance; muscarine reduced predominantly the inward rectifier conductance. 5. Phorbol-12,13-diacetate (3 microM) and 5-hydroxytryptamine mimicked the action of muscarine. The inward currents caused by muscarine or 5-hydroxytryptamine were occluded by the inward current evoked by the phorbol ester. 6. The depolarization caused by muscarine was competitively antagonized by pirenzepine; the dissociation constant of 11 nM suggested involvement of the M1 receptor. 7. It is concluded that muscarine acts at M1 receptors to reduce the membrane potassium conductance and that activation of protein kinase C may be an intermediate step.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
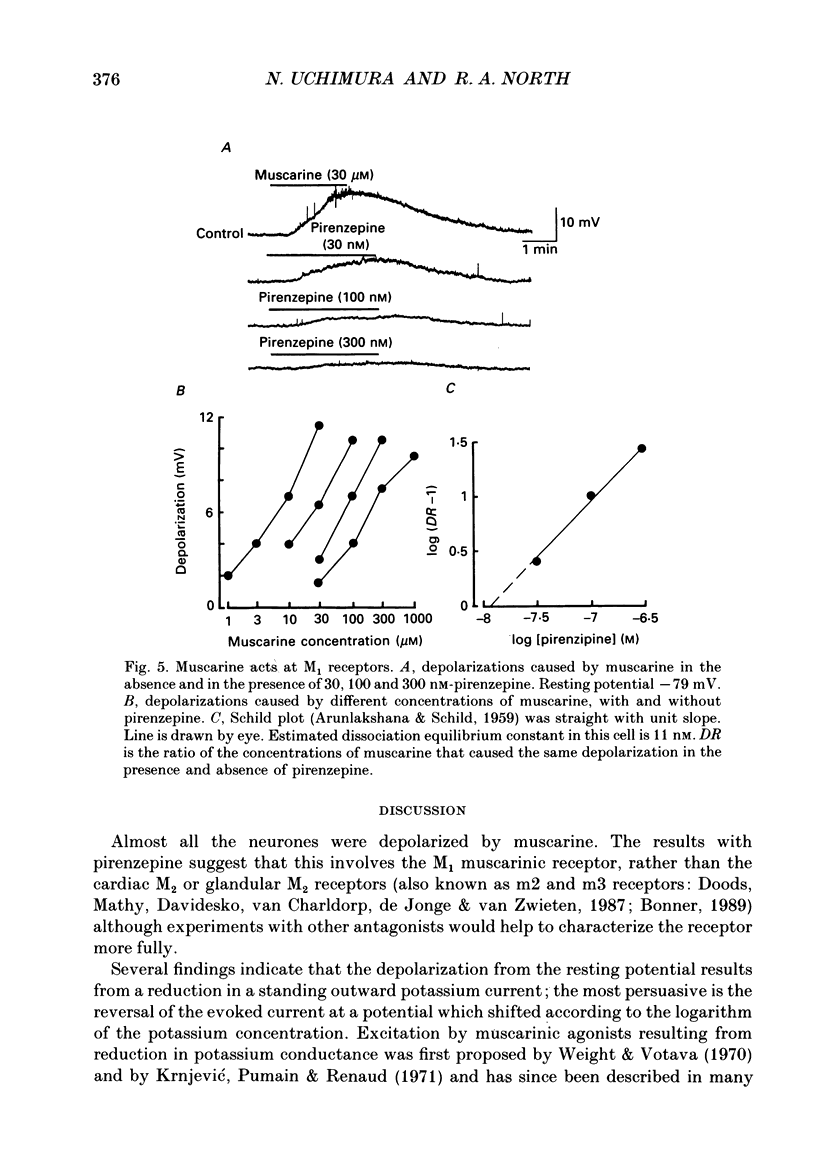
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin M. C., Kalivas P. W. The effect of cholinergic stimulation in the nucleus accumbens on locomotor behavior. Brain Res. 1988 Feb 16;441(1-2):209–214. doi: 10.1016/0006-8993(88)91400-x. [DOI] [PubMed] [Google Scholar]
- Bloom F. E., Costa E., Salmoiraghi G. C. Anesthesia and the responsiveness of individual neurons of the caudate nucleus of the cat to acetylcholine, norepinephrine and dopamine administered by microelectrophoresis. J Pharmacol Exp Ther. 1965 Nov;150(2):244–252. [PubMed] [Google Scholar]
- Bonner T. I. The molecular basis of muscarinic receptor diversity. Trends Neurosci. 1989 Apr;12(4):148–151. doi: 10.1016/0166-2236(89)90054-4. [DOI] [PubMed] [Google Scholar]
- Cassell J. F., McLachlan E. M. Muscarinic agonists block five different potassium conductances in guinea-pig sympathetic neurones. Br J Pharmacol. 1987 Jun;91(2):259–261. doi: 10.1111/j.1476-5381.1987.tb10279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés R., Palacios J. M. Muscarinic cholinergic receptor subtypes in the rat brain. I. Quantitative autoradiographic studies. Brain Res. 1986 Jan 8;362(2):227–238. doi: 10.1016/0006-8993(86)90448-8. [DOI] [PubMed] [Google Scholar]
- Dodt H. U., Misgeld U. Muscarinic slow excitation and muscarinic inhibition of synaptic transmission in the rat neostriatum. J Physiol. 1986 Nov;380:593–608. doi: 10.1113/jphysiol.1986.sp016304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doods H. N., Mathy M. J., Davidesko D., van Charldorp K. J., de Jonge A., van Zwieten P. A. Selectivity of muscarinic antagonists in radioligand and in vivo experiments for the putative M1, M2 and M3 receptors. J Pharmacol Exp Ther. 1987 Jul;242(1):257–262. [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Classification of muscarinic responses in hippocampus in terms of receptor subtypes and second-messenger systems: electrophysiological studies in vitro. J Neurosci. 1988 Nov;8(11):4214–4224. doi: 10.1523/JNEUROSCI.08-11-04214.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltz M., Gmelin G., Wess J., Strohmann C., Tacke R., Mutschler E., Lambrecht G. Presynaptic muscarinic receptors mediating inhibition of neurogenic contractions in rabbit vas deferens are of the ganglionic M1-type. Eur J Pharmacol. 1988 Dec 13;158(3):233–242. doi: 10.1016/0014-2999(88)90072-6. [DOI] [PubMed] [Google Scholar]
- Feldberg W., Vogt M. Acetylcholine synthesis in different regions of the central nervous system. J Physiol. 1948 Jun 25;107(3):372–381. doi: 10.1113/jphysiol.1948.sp004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibiger H. C., Lytle L. D., Campbell B. A. Cholinergic modulation of adrenergic arousal in the developing rat. J Comp Physiol Psychol. 1970 Sep;72(3):384–389. doi: 10.1037/h0029741. [DOI] [PubMed] [Google Scholar]
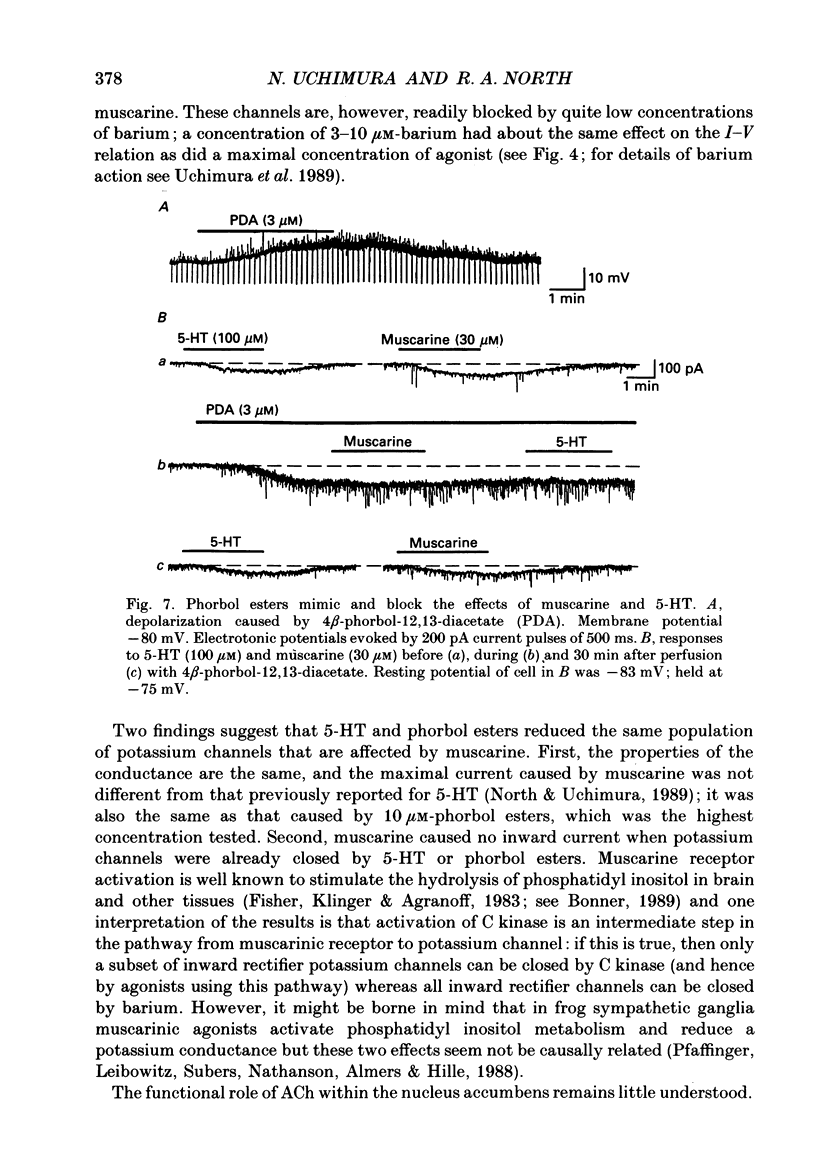
- Fisher S. K., Klinger P. D., Agranoff B. W. Muscarinic agonist binding and phospholipid turnover in brain. J Biol Chem. 1983 Jun 25;258(12):7358–7363. [PubMed] [Google Scholar]
- Graybiel A. M., Baughman R. W., Eckenstein F. Cholinergic neuropil of the striatum observes striosomal boundaries. Nature. 1986 Oct 16;323(6089):625–627. doi: 10.1038/323625a0. [DOI] [PubMed] [Google Scholar]
- HEBB C. O., SILVER A. Choline acetylase in the central nervous system of man and some other mammals. J Physiol. 1956 Dec 28;134(3):718–728. doi: 10.1113/jphysiol.1956.sp005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M., Edley S. M., Stuart J. Cell clusters in the nucleus accumbens of the rat, and the mosaic relationship of opiate receptors, acetylcholinesterase and subcortical afferent terminations. Neuroscience. 1984 Mar;11(3):561–593. doi: 10.1016/0306-4522(84)90045-9. [DOI] [PubMed] [Google Scholar]
- Herz A., Zieglgänsberger W. The influence of microelectrophoretically applied biogenic amines, cholinomimetics and procaine on synaptic excitation in the corpus striatum. Int J Neuropharmacol. 1968 May;7(3):221–230. doi: 10.1016/0028-3908(68)90029-4. [DOI] [PubMed] [Google Scholar]
- Higashi H., Inanaga K., Nishi S., Uchimura N. Enhancement of dopamine actions on rat nucleus accumbens neurones in vitro after methamphetamine pre-treatment. J Physiol. 1989 Jan;408:587–603. doi: 10.1113/jphysiol.1989.sp017478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. L., Mogenson G. J., Wu M. Injections of dopaminergic, cholinergic, serotoninergic and GABAergic drugs into the nucleus accumbens: effects on locomotor activity in the rat. Neuropharmacology. 1981 Jan;20(1):29–37. doi: 10.1016/0028-3908(81)90038-1. [DOI] [PubMed] [Google Scholar]
- Kelly P. H., Seviour P. W., Iversen S. D. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975 Sep 5;94(3):507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Pumain R., Renaud L. The mechanism of excitation by acetylcholine in the cerebral cortex. J Physiol. 1971 May;215(1):247–268. doi: 10.1113/jphysiol.1971.sp009467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J., Langer S. Z. The striatal cholinergic interneuron: synaptic target of dopaminergic terminals? Neuroscience. 1983 Dec;10(4):1105–1120. doi: 10.1016/0306-4522(83)90102-1. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., McGeer E. G., Fibiger H. C., Wickson V. Neostriatal choline acetylase and cholinesterase following selective brain lesions. Brain Res. 1971 Dec 10;35(1):308–314. doi: 10.1016/0006-8993(71)90625-1. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M., Mufson E. J., Levey A. I., Wainer B. H. Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Neuroscience. 1984 Jul;12(3):669–686. doi: 10.1016/0306-4522(84)90163-5. [DOI] [PubMed] [Google Scholar]
- Nastuk M. A., Graybiel A. M. Autoradiographic localization and biochemical characteristics of M1 and M2 muscarinic binding sites in the striatum of the cat, monkey, and human. J Neurosci. 1988 Mar;8(3):1052–1062. doi: 10.1523/JNEUROSCI.08-03-01052.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
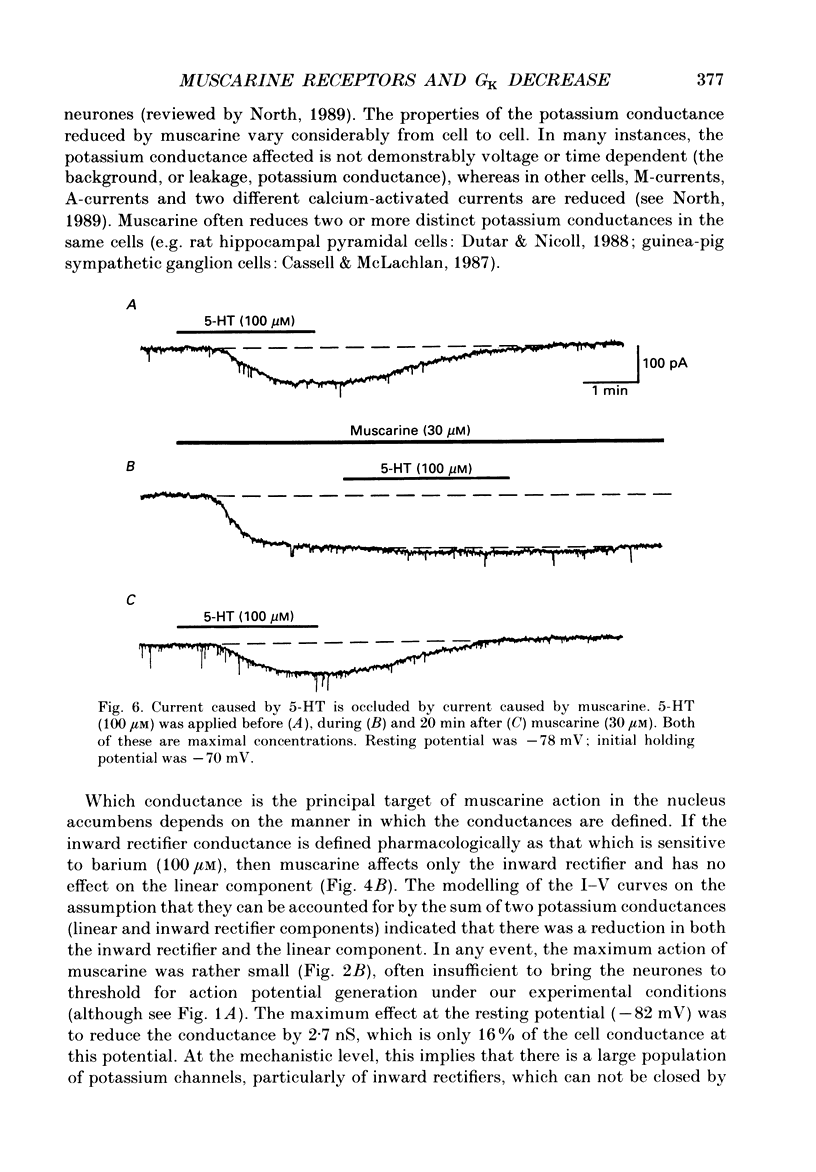
- North R. A., Uchimura N. 5-Hydroxytryptamine acts at 5-HT2 receptors to decrease potassium conductance in rat nucleus accumbens neurones. J Physiol. 1989 Oct;417:1–12. doi: 10.1113/jphysiol.1989.sp017786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffinger P. J., Leibowitz M. D., Subers E. M., Nathanson N. M., Almers W., Hille B. Agonists that suppress M-current elicit phosphoinositide turnover and Ca2+ transients, but these events do not explain M-current suppression. Neuron. 1988 Aug;1(6):477–484. doi: 10.1016/0896-6273(88)90178-x. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R., Nakajima Y., Yamaguchi K. Substance P raises neuronal membrane excitability by reducing inward rectification. Nature. 1985 Jun 6;315(6019):498–501. doi: 10.1038/315498a0. [DOI] [PubMed] [Google Scholar]
- Uchimura N., Higashi H., Nishi S. Hyperpolarizing and depolarizing actions of dopamine via D-1 and D-2 receptors on nucleus accumbens neurons. Brain Res. 1986 Jun 11;375(2):368–372. doi: 10.1016/0006-8993(86)90760-2. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Votava J. Slow synaptic excitation in sympathetic ganglion cells: evidence for synaptic inactivation of potassium conductance. Science. 1970 Nov 13;170(3959):755–758. doi: 10.1126/science.170.3959.755. [DOI] [PubMed] [Google Scholar]
- Woodruff G. N., McCarthy P. S., Walker R. J. Studies on the pharmacology of neurones in the nucleus accumbens of the rat. Brain Res. 1976 Oct 15;115(2):233–242. doi: 10.1016/0006-8993(76)90509-6. [DOI] [PubMed] [Google Scholar]


