ABSTRACT
This review explores the emerging term “gut-skin axis” (GSA), describing the bidirectional signaling that occurs between the skin and the gastrointestinal tract under both homeostatic and disease conditions. Central to GSA communication are the gut and skin microbiota, the microbial communities that colonize these barrier surfaces. By influencing diverse host pathways, including innate immune, vitamin D receptor, and Aryl hydrocarbon receptor signaling, a balanced microbiota contributes to both tissue homeostasis and host defense. In contrast, microbiota imbalance, or dysbiosis at one site, can lead to local barrier dysfunction, resulting in the activation of signaling pathways that can disrupt tissue homeostasis at the other site, potentially leading to inflammatory skin conditions such as atopic dermatitis and psoriasis, or gut diseases like Inflammatory Bowel Disease. To date, most research on the GSA has examined the impact of the gut microbiota and diet on skin health, but recent studies show that exposing the skin to ultraviolet B-light can beneficially modulate both the gut microbiome and intestinal health. Thus, despite the traditional focus of clinicians and researchers on these organ systems as distinct, the GSA offers new opportunities to better understand the pathogenesis of cutaneous and gastrointestinal diseases and promote health at both sites.
KEYWORDS: Gut-skin axis (GSA), ultraviolet B-light exposure, cutaneous diseases, gastrointestinal diseases, gut and skin microbiota
Introduction
The human body develops and grows while in constant contact with its external environment, primarily through two interfaces, namely the skin and the gastrointestinal (GI) tract. Both sites receive an array of environmental signals that are transduced to deeper tissues, usually eliciting beneficial and/or homeostatic responses. Unfortunately, they also represent vulnerable target sites for both biotic and abiotic threats. While their embryonic origins differ, with the skin arising from ectoderm and the gut forming from complex interactions between the endoderm and mesoderm, these organs share many fundamental structural and functional similarities.1,2 Both are lined by epithelial cells that serve as protective barriers, that are supported, and strengthened by signals from underlying stromal and immune cells.3 Furthermore, they are both extensively vascularized and innervated, allowing rapid communication with the immune and central nervous systems, thereby facilitating rapid responses to environmental threats.4 Notably, both surfaces host diverse communities of commensal microbes, collectively known as the microbiome.
Many studies have demonstrated significant immune cell transit and communication between distinct mucosal associated lymphoid tissues (MALT),5 such as the GI tract and the lungs, potentially as a means to broadly promote mucosal immunity and defense. In recent years, a growing body of research has also revealed significant signaling/cellular crosstalk between the skin and the gut, despite being apparently unrelated organ systems.6 Termed the gut-skin axis (GSA), the communication pathways connecting these two organs are mediated by an array of cytokines,7–10 microbial metabolites,11,12 hormones13,14 and neurotransmitters.15,16 These molecules have emerged as key players in maintaining tissue homeostasis and coordinating systemic responses to various stimuli. Among these molecules, hormones such as vitamin D, as well as key microbial metabolites, including Aryl hydrocarbon receptor (AhR) ligands, have been associated with modulating immune responses and metabolic processes, thereby influencing health outcomes throughout the body. Moreover, the effects of environmental factors, such as ultraviolet B (UVB) radiation on the skin, extend beyond those tissues that are directly affected, modulating the functions of distant organs, including the gut.17
This review provides a summary of recent findings that support the concept of bidirectional communication between the skin and the gut, as well as the roles played by various molecular players, including but not limited to microbial metabolites, signaling molecules, and environmental factors. By elucidating these pathways, we seek to deepen our understanding of how diverse environmental signals promote tissue homeostasis, as well as propose that UVB light may offer a novel therapeutic approach for the human GI tract, acting via the GSA.
Gut and skin barrier functions
The skin is composed of three layers: the hypodermis (deepest layer), dermis and epidermis.18 The epidermis, along with its appendage structures (i.e. hair follicles, sebaceous and sweat glands), is the first barrier encountered by the external environment and is colonized by, and constantly stimulated by an array of skin dwelling microorganisms including bacteria, fungi, viruses, and parasites.6 Likewise, the gut is protected by an epithelial barrier that safeguards the host by segregating potentially noxious factors such as pathogenic bacteria, viruses and food antigens inside the gut lumen.19 Among the cells that comprise this barrier, goblet cells (a subset of intestinal epithelial cells (IEC)) synthesize mucus that forms a protective physiochemical gel that overlies and supports the epithelium, while other secretory IEC release molecules that regulate immune responses, exert antimicrobial actions and aid in epithelial repair.20 The gut epithelial barrier is maintained through tight junction proteins like zonula occludens, claudins, and occludin, which play a crucial role in sealing the paracellular space and regulating IEC permeability.21 Disassembly of these proteins can result in increased intestinal permeability, also known as “leaky gut”, leading to the passage of luminal factors such as bacterial and dietary components into the gut mucosa and systemic circulation, thus triggering inflammation.21
Innate and adaptive immunity in the gut and skin
Consistent with their frequent exposure to environmental threats, the skin and gut both harbor an array of cells that can elicit protective immune and antimicrobial responses. Specifically within the dermis of the skin, several innate immune cell types are present, such as dendritic cells, macrophages, as well as γδ T lymphocytes and innate lymphoid cells (ILC).22 As the largest immune organ in the body, the GI tract contains a similar array of innate immune cells, but at higher densities.23 Moreover, IEC serve as an essential component of innate intestinal defense, expressing pattern-recognition receptors such as Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors, allowing them to rapidly respond to threats. For example, a subset of secretory IEC called “sentinel goblet cells” were recently shown to recognize pathogenic stimuli through TLRs, and respond by rapidly releasing mucus in an inflammasome dependent manner.24 Additionally, IEC actively protect against pathogen colonization through the production of antimicrobial peptides (AMPs), while infected IEC can undergo inflammasome-induced pyroptosis, expelling invading pathogens back into the gut lumen.25 Similarly, keratinocytes in the skin express various TLRs, enabling them to sense bacterial infections and produce AMPs that exert antimicrobial activity and chemokines that promote immune cell recruitment.26,27
Both the skin and gut also contain specialized subsets of dendritic cells that sample antigens from their surroundings and regulate immune responses. Correspondingly, diverse T cell subsets reside within these tissues, including regulatory T cells (Tregs), effector T cells, and memory T cells, which are crucial for immune regulation, surveillance, and the development of immune responses to pathogens.28 Moreover, tissue macrophages, which are essential for immune surveillance, inflammation, and tissue balance, are found in abundance in both organs, along with mast cells which are best known for their roles in host defense and allergic reactions.29,30
Microbiota in gut and skin health
The human gut and skin are colonized by diverse microbes, collectively termed the microbiota, with the skin carrying 104 -106 microbial cells per cm231 while microbial density in the gut can reach 1011 microbes per gram of fecal material.32 Under healthy conditions, the host and its microbiota symbiotically co-exist, with the host’s skin and gut providing a place to reside as well as nutrients for the microbes. The specific compositions of the gut and skin microbiotas, as well as their respective functions are outlined below.
The human skin is colonized at birth by an array of microorganisms such as bacteria, archaea, viruses and fungi, with the mode of delivery greatly impacting the infant’s initial skin microbiota profile.33–35 Neonates born vaginally initially carry a skin microbiota signature closely resembling their mother’s vaginal bacteria, predominately featuring Lactobacillus spp. Conversely, infants delivered by cesarean section often display lower skin microbial diversity,36–38 being mainly colonized by environmental microorganisms and those from their mother’s skin, including Staphylococcus, Corynebacterium, and Propionibacterium species.36 This initial colonization by commensal bacteria appears to play an important role in establishing immune tolerance,34 as the presence of skin bacteria causes Tregs to express the FOXP3 transcription factor, a critical step in developing their immunoregulatory functions.39 Within four to six weeks after birth, the infant’s skin microbiome increases in diversity, aligning more closely with their mother’s skin microbiome.38 The ability of these microbes to protect the infant’s skin from external threats is particularly important as children have underdeveloped immune systems and vulnerable skin barriers. Certain bacteria such as Staphylococcus epidermidis and Staphylococcus cohnii are known to protect infants from skin conditions like atopic dermatitis (AD) or eczema (an inflammatory disorder characterized by dry and scaly skin).40 While S. epidermidis is generally considered a beneficial commensal, its protective effects appear to be strain specific as recent studies have shown that certain strains of S. epidermidis can induce the expression of pro-inflammatory mediators, such as the cytokine IL-1β,41 which can explain the observed correlation between the higher abundance of S. epidermidis and the most severe cases of AD in both adults42,43 and infants.44,45
Throughout the infant’s development, commensal bacteria continue to influence the production of various cytokines and AMPs, thereby strengthening the skin’s defenses against pathogens. For example, bacteria like S. epidermidis can stimulate the production of calprotectin S100A8/9 from keratinocytes via interleukin (IL)-17 producing CD8+ T cells, and produce lipoteichoic acid that suppress inappropriate immune activation by inhibiting the production of the pro-inflammatory cytokines tumor necrosis factor (TNF)-α and IL-6.46,47 During adolescence, a significant shift occurs in the skin microbiome, with Firmicutes, Bacteroidetes, and Proteobacteria (β- and γ) giving way to the more lipophilic Actinobacteria. This shift can lead to an imbalance in the skin microbiota known as dysbiosis, and potentially contribute to skin disorders such as acne vulgaris.48 Ethnicity and geographical differences have also been described to influence skin properties, resulting in distinct skin microbiota compositions across populations.49,50 The most notable differences in skin bacterial profiles are associated with body site, as reflected by their different physiologic characteristics such as humidity, temperature, pH, lipid and sebum content and AMP expression.33,51–54 Interestingly, several skin sites including the forearm, palm, index finger, back of the knee, and the sole of the foot harbor microbiomes with higher phylogenetic diversity as compared to the gut microbiome.54 Hygiene practices, the type of clothing worn, diet, medications, and environmental conditions, including sunlight exposure, can also modulate skin bacterial communities over time, leading to an evolving microbiome composition.54,55
The human gut is also colonized by microbes immediately after birth and is influenced by the infant’s mode of delivery and subsequent diet (i.e. breastfeeding vs. formula). The GI tract, and especially the colon, harbor the largest bacterial load in the human body, with more than five million different genes carried by 1,000 to 1,500 distinct bacterial species that comprise the gut microbiota, belonging to the genera Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, Fusobacteria, Cyanobacteria, and Verrucomicrobia.56 The gut microbiota exert an array of functions, such as aiding in the digestion of food, promoting immune system development and defense against pathogens, vitamin biosynthesis, fat storage and angiogenesis while also producing important metabolites such as the short chain fatty acids (SCFA) acetate, propionate and butyrate.57 Strikingly, the gut microbiota also influences mood and behavior, as it has been linked to the pathogenesis of several neuropsychiatric disorders and abnormal behaviors in animal models.58,59
After their initial colonization, the community of gut microorganisms gradually develops into a diverse ecosystem as the host ages and grows. Microbes and the host immune system mature together through a cooperative relationship, where the host provides food, while commensal microbiota help protect the host against enteric pathogens and other noxious stimuli.60,61 Similar to the skin, gut bacteria such as Bacteroides fragilis and certain Clostridium species can trigger an increase in the local population of Foxp3+ Treg cells, thereby contributing to immune tolerance to commensal microbes.62 Additionally, macrophages in the intestinal lamina propria specialize in phagocytosing pathogens, exhibiting less pronounced pro-inflammatory responses as compared to macrophages at other tissue sites.63 This adaptation suggests that the host adjusts to the presence of a large population of commensal microbiota, by minimizing the risks of triggering unnecessary or exaggerated inflammatory responses.64
Microbiota in gut and skin disease
As noted above, microbial dysbiosis refers to an imbalanced microbiota community, often characterized by reduced bacterial diversity and stability. Gut microbiota dysbiosis has been implicated in the development and progression of various conditions, including atopic asthma, mental health disorders (depression, schizophrenia, addiction), obesity, type 2 diabetes, cardiovascular diseases, and autoimmune disorders like rheumatoid arthritis and multiple sclerosis (MS).65–67 Moreover, GI conditions, including inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS), have been associated with an altered gut microbiota profile in comparison with healthy subjects, as microbial dysbiosis can influence gut physiology, nutrient absorption, and the host’s immune system.
We suspect that in many cases, inflammation, whether originating in the intestine or the skin, may be initially triggered by a dysbiotic gut microbiota, providing a starting point for the GSA to negatively impact the skin.68 Notably, intestinal microbial dysbiosis often triggers systemic inflammation, in association with alterations in microbial metabolic capacity (i.e. nutrient metabolism, lipid metabolism, vitamins B and K synthesis, detoxification and hormone metabolism) which may affect other organ systems, including the skin, thus disrupting cutaneous homeostasis.69–74 Moreover, intestinal microbial dysbiosis is associated with T-cell activation as well as impaired production of immunosuppressive cytokines and Treg cells, which together, are essential for maintaining host tolerance to the microbiota.69 Whether skin microbial dysbiosis can initiate similar detrimental effects at distal sites, such as the GI tract has received less attention, but should be a priority for future studies.
The transition from microbial dysbiosis to the promotion of disease is complicated and involves multiple interconnected mechanisms. Disruptions in gut microbiota can compromise intestinal barriers, increasing permeability (“leaky gut”) and triggering systemic inflammation.75 This imbalance also promotes immune dysregulation, including to the overactivation of pro-inflammatory pathways (Th1, Th17), causing impaired immune tolerance.76 Furthermore, shifts in the levels of microbial metabolites, such as reduced SCFAs and increased harmful byproducts, can exacerbate inflammation and tissue damage.77–79 Genetic predisposition and environmental factors, such as diet, stress, and antibiotic use, can further contribute to disease progression.80,81 It should be noted that further studies are required to understand these relationships, as a prerequisite to developing targeted therapies to restore microbial balance and alleviate subsequent symptoms and pathology.
Dysbiosis may also significantly impact intestinal stem cells (ISCs), which are critical for maintaining gut integrity and function, and by extension, the gut-skin axis. A healthy microbiota supports ISC proliferation and differentiation, but dysbiosis can impair these functions, compromising gut barrier integrity and increasing permeability.82 This disruption can drive microbial translocation and subsequent systemic inflammation, which can exacerbate skin conditions.83 Microbes and their metabolic products can directly and/or indirectly interact with ISCs, influencing the stem cell niche and modulating epithelial function, regeneration, and repair.84 The link between dysbiosis and skin inflammation is highlighted by findings of elevated gut bacterial DNA in the bloodstream of patients with chronic skin disorders.85 As already mentioned, dysbiosis reduces the production of SCFAs, which normally play a critical role in supporting ISC function, epithelial barrier integrity, and in mitigating inflammation.11,68 In addition, dysbiosis can alter the regulation of ISCs through TLR and NOD pattern recognition receptors, which are influenced by the intestinal microbiota. Key microbial communities involved in these pathways include Fusobacteria, Proteobacteria, Firmicutes, Lachnospiraceae, Coriobacteria, Helicobacter pylori, and Vibrio cholerae. Specific bacteria, such as Serratia marcescens, Erwinia carotovora carotovora-15, and Pseudomonas entomophila are known to contribute to intestinal homeostasis, injury repair, and the maintenance of ISC function through these mechanisms.82
Associations between GI and cutaneous diseases are well-supported by previous studies,4 as depicted in Figure 1. Notably, 10% to 25% of patients with GI diseases such as IBD and Celiac disease also experience skin pathology, including psoriasis (characterized by scaly plaques and hypertrophic skin lesions), and cutaneous ulcers.4,86 Celiac disease, which is characterized by intestinal malabsorption, commonly co-presents with dermatitis and psoriasis as cutaneous manifestations, whereas patients with rosacea (a chronic inflammatory skin condition that causes reddened skin and rash) frequently exhibit small intestinal bacterial overgrowth (SIBO).87 Peutz -Jeghers Syndrome, which is characterized by GI polyposis and malignancy, often features perioral hyperpigmentation as a common cutaneous manifestation.86 Additionally, IBD patients frequently experience skin manifestations such as skin ulcers, vasculitis, hair loss, erythema folliculitis, and psoriasis,4,86 with some of these conditions linked to the severity of the patient’s GI inflammation.88 Notably, intestinal colonization with Malassezia restricta, a commensal fungus and frequent member of the skin microbiota, may contribute to the pathogenesis of Crohn’s disease (one form of IBD), in a subset of patients, with its presence correlating with increased disease severity.89 While these findings implicate a member of the skin microbiome in the pathogenesis of IBD, the underlying mechanisms have not yet been fully elucidated; but others have suggested that both the immune and endocrine systems play an important role in this interaction.69
Figure 1.
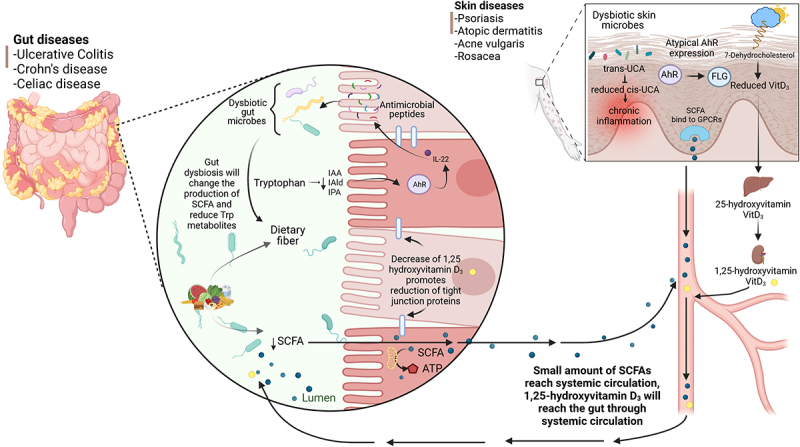
Links between GI disorders and cutaneous diseases. In high-latitude locations, reduced sunlight, especially during winter leads to decreased UVB light exposure and subsequently lower serum vitamin D levels. This decline leads to changes in the GI tract, including a reduction in epithelial tight junction proteins. Many patients with IBD are vitamin D insufficient or deficient, with a higher occurrence in CD than UC. Decreased UVB exposure also leads to reduced conversion of trans-urocanic acid (UCA) to cis-uca, which has been linked to chronic skin inflammation. In IBD patients, gut microbial dysbiosis leads to reduced short chain fatty acid (SCFA) levels, which is a key bacterial metabolite affecting both gut and skin health. Skin conditions such as psoriasis, acne, atopic dermatitis, and vitiligo are often associated with reduced AhR expression/activation. Several AhR ligands, including indole derivatives can impact skin health by modulating inflammation and oxidative stress. Patients with IBD exhibit decreased production of endogenous AhR ligands in their feces, likely as a result of microbial dysbiosis, which may contribute to their impaired mucosal healing. Created in BioRender.com.
Extensive pre-clinical research has shown that modulation of the gut microbiota can exert a positive impact on the cutaneous system, especially through the administration of beneficial microbes known as probiotics. Levkovich and colleagues90 found that oral supplementation of the probiotic Lactobacillus reuteri ATCC 6475 to aged mice resulted in improved skin health, including increased dermal thickness, enhanced folliculogenesis, a more acidic skin pH, and heightened sebum production in comparison with aged mice not given the probiotic. These benefits appeared to be immune mediated as probiotic-fed mice showed increased serum levels of the anti-inflammatory cytokine IL-10 and decreased levels of the pro-inflammatory cytokine IL-17. Another probiotic strain (Lactobacillus acidophilus KBL409) orally administered to mice was shown to attenuate AD and decrease the infiltration of immune cells into skin tissues.91 In addition to increasing IL-10 levels and Foxp3 expression in mice, probiotics have also been shown to modulate the gut microbiota’s production of metabolites such as SCFA and amino acids. The reduced dermatitis scores found in these studies demonstrate that probiotic bacteria can exert positive effects beyond the gut, through a coordinated modulation of both the immune system and the gut microbiota. For instance, Mendelian randomization (MR) studies reveal causal links between gut microbiota and skin disorders, including eczema, acne, psoriasis, and rosacea.92 For example, Eubacterium fissicatena is linked to psoriasis risk,93 while gut bacteria may influence skin fibrosis.94 These findings highlight the therapeutic potential of microbiota modulation.
Studies in humans also point to the immune system and the gut microbiota as key contributors to the overlap seen between gut and skin conditions. For example, a recent study found an association between gut microbial dysbiosis and AD. The authors showed that clinical improvement of the skin in response to the medication dupilumab, a monoclonal antibody that blocks interleukin 4 and interleukin 13, were due to dupilumab’s effects on gut bacterial function, by regulating the indole pathway of tryptophan metabolism.95 This effect is likely associated with dupilumab’s suppression of Th2 immune responses, leading to pleiotropic anti-inflammatory actions observed throughout the body including in both skin and gut.96,97 Moreover, several clinical trials have shown beneficial effects of oral probiotic administration on skin diseases such as AD,98 psoriasis99 and acne vulgaris.100 The potential benefits of oral probiotics have also been explored for the skin condition rosacea, with higher remission rates seen in patients that consumed a mixture of Bifidobacterium species in addition to the standard antibiotic treatment.101
The impact of gut microbiota composition and its metabolites has also been explored in allergic skin diseases. A study by Trompette and colleagues found that administration of dietary fiber or its byproducts ie. SCFAs and in particular butyrate, attenuated allergen-induced skin barrier breach in a mouse model that mimics AD-like skin inflammation in humans.102 This protection was associated with accelerated epidermal keratinocyte differentiation, which increased the production of key structural skin components such as proteins and lipids. Moreover, butyrate enhanced skin barrier function by directly shaping keratinocyte metabolism and eliciting a mitochondria-dependent differentiation program. These results support previous findings that butyrate can modulate immune responses in the skin,103,104 and that it is also reduced in children and infants diagnosed with AD,105,106 as well as in children that develop allergic reactions later in childhood.107
Communication pathways in the gut-skin axis
The relationship between the gut and skin is highlighted by the vital role of the microbiota in facilitating communication between these two systems. Further defining the communication pathways that underlie the GSA is key to understanding both its role in disease pathogenesis, and its potential therapeutic applications. Alongside the microbiome, various metabolites and micronutrients serve as essential components of the GSA dialogue, with the specifics outlined below.
Gut communication with the skin
The most common assumption regarding the GSA is that the communication largely occurs in only one direction, ie. the gut communicates with, and thereby impacts the skin. This assumption stems from the crucial roles played by the gut microbiota in an array of metabolic and immune functions that can impact the entire body and thus influence skin health. Members of the gut microbiota metabolize indigestible complex polysaccharides into essential nutrients, including vitamin K and the water soluble B vitamins, which are associated with wound healing and metabolic functions such as DNA replication, repair and methylation, respectively.108,109 Moreover, the gut microbiota produce their own (i.e. bacterial) metabolites such as SCFA, which have been described as important signaling factors in the GSA as they can alleviate skin inflammation.11
Diet is recognized as a major factor influencing the composition and function of the gut microbiota, highlighting the importance of dietary patterns and specific nutrients in the GSA. The western dietary pattern, which is rich in saturated fats as well as refined carbohydrates, is often associated with reduced microbial diversity and higher luminal concentrations of bacterial cell wall components such as lipopolysaccharides, which can negatively affect gut barrier function and trigger systemic inflammation.110 Additionally, a diet containing excessive levels of animal protein can favor the production of metabolites such as indoxyl sulfate, Trimethylamine-N-oxide (TMAO) and p-cresyl sulfate, that are linked to psoriatic arthritis, a chronic inflammatory disease affecting the joints and connective tissue that is commonly seen in patients with psoriasis.111,112 Conversely, a high-collagen diet has been shown to be beneficial with respect to skin aging and wound healing, emphasizing that different sources of protein may exert quite divergent effects on the skin.113,114 Moreover, a dietary pattern favoring oily fish and olive oil has been associated with a reduction in skin inflammation due to their high levels of omega 3 and mono-unsaturated fatty acids, respectively.115–119 In addition to macronutrients, the consumption of dietary fiber is largely considered beneficial for overall health. Fibers such as inulin and resistant starch cannot be digested by the human body but instead rely on the microbiota to be fermented, in a process that increases the population of beneficial gut microbes and their metabolites, such as SCFA, which can promote skin health.120
In addition to the indirect effects of diet on skin health via modulation of the gut microbiota, different ingested nutrients can directly support skin structure and function. While most studies focus on dietary compounds as supplements, it is important to note the effects elicited by whole foods on specific aspects of the GSA as a way to explore targeted dietary recommendations for skin conditions. The Mediterranean diet, rich in plant-based and healthy fat foods, is associated with a lower incidence of several chronic inflammatory diseases, including dermatological conditions.121 Plant-based foods such as vegetables, fruits, legumes and nuts are considered rich sources of bioactive compounds, including carotenoids, vitamins, and polyphenols, which play a key role in maintaining skin health.122,123
Vitamin C, which is abundant in many fruits and vegetables, plays a crucial role in collagen synthesis and acts as a potent antioxidant, protecting the skin from the damage caused by reactive oxygen species (ROS).124 Moreover, vitamin C works synergistically with vitamin E, a powerful antioxidant present in fruits such as avocado, that together can reduce skin aging and increase level of moisture. The effects of specific foods rich in vitamin C have also been studied, with mango, particularly the Ataulfo variety, reducing deep facial wrinkles in postmenopausal women after 16 weeks of intervention when consumed in moderation.125 However, portion sizes are key for dietary recommendations, as individuals who ingested 250 g of mango experienced an increase in wrinkles. This result could potentially be attributed to the sugar content of the fruit, as glucose and fructose can enhance the glycation process of collagen and elastin fibers, thereby compromising the structural integrity of the subcutaneous tissue that provides support to the skin.126
Polyphenols also seem to play an important role in skin health, with a study showing that cocoa flavanol supplementation reduced facial wrinkles and improved skin elasticity after only 24 weeks in photo-aged women.127 Additionally, supplementation with grape seed extract, rich in polyphenols such as anthocyanins, flavanols, and resveratrol,128–130 has also shown benefits in melasma, a common skin condition characterized by dark skin patches.131 Carotenoid-rich foods such as tomatoes, which are also rich in lycopene, exhibit robust antioxidant properties, offering protection against UV-induced skin erythema,132,133 while daily consumption of a carotenoid-rich kale extract was found to improve collagen and elastin levels, enhancing skin health.134
Intake of nuts such as almonds have also been associated with skin health benefits by contributing to antioxidant defense and reducing wrinkles.135 Additionally, legumes such as soybeans contain isoflavones, bioactive compounds that exhibits estrogen-like properties. As a result, their consumption can mitigate the reduced estrogen levels seen during menopause, thereby improving the typical skin wrinkling, dryness, and poor wound healing.136 Thus, a diverse diet, rich in nutrient-dense whole foods not only benefits the gut microbiota composition profile but also directly provides the essential nutrients and antioxidants needed to promote skin health and delay signs of aging.
Skin communication with the gut
In contrast to the well-studied roles that dietary components and the gut microbiome play in affecting skin health, the inverse direction of the GSA has received much less attention. We know that an array of external environmental factors such as pollution, smoking, climate, and sun exposure impact the skin directly.137 In this section, we will discuss how skin responses to specific stimuli impact the gut, highlighting the importance of vitamin D and tryptophan metabolites as key examples of the bidirectional communication between the GI tract and the skin. By focusing on these compounds, we seek to show how external factors, such as ultraviolet (UV) radiation, influence the skin and, in turn, affect gut health.
Vitamin D signaling
Calciferol, also known as vitamin D, is a fat-soluble vitamin that can be obtained from the diet as it is naturally found in oily fish and eggs, while breakfast cereals and milk are often fortified with vitamin D. In addition to dietary sources, vitamin D is also produced by the human body in response to sunlight; skin exposure to UVB light can lead to the production of vitamin D within the epidermis, from the precursor chromophore 7-dehydrocholesterol.138 Recently, exposing the skin to UVB light was shown to modulate the gut microbiota composition of vitamin D insufficient, but otherwise healthy women.17 The exact mechanisms by which vitamin D alters gut microbiota composition are unclear, but they likely include increased innate immune signaling, the downregulation of inflammation, and strengthening of intestinal barrier function.139
In both the skin and gut, vitamin D receptor (VDR) activation plays a critical role in regulating the production of AMPs such as cathelicidin, which help maintain microbiota homeostasis and provide protection against pathogens.140,141 Vitamin D itself also induces the expression of antimicrobial peptides in human keratinocytes, monocytes, and neutrophils,142–144 since the promoters of the human cathelicidin antimicrobial peptide (CAMP) and defensin beta-2 (DEFβ2) genes contain vitamin D response elements that regulate their transcription. Notably, vitamin D synthesized in the skin can activate VDR signaling in the gut, enhancing Paneth cell defensin production, with defensins acting as key regulators of gut microbiota and metabolic health.145 The VDR is expressed by various immune cells within the gut, including dendritic cells, macrophages, T cells (particularly regulatory T cells), and B cells, thus highlighting its role in immune regulation.146,147 Vitamin D modulates the function of regulatory T cells, which are crucial for maintaining immune tolerance and controlling inflammation.148,149 Additionally, it modulates dendritic cells to promote Treg development, regulates macrophage-mediated inflammation, and affects antibody production and immune regulation in B cells. This immune modulation is particularly relevant in IBD, where vitamin D deficiency is common and associated with increased disease severity.148,149
Vitamin D also plays an important role in maintaining mucosal barrier function by upregulating the expression of tight junction and adherent junction proteins in IEC, as well as suppressing IEC apoptosis. Furthermore, fermentation products from the gut microbiota, such as the SCFA butyrate, upregulate IEC expression of the VDR and suppress inflammation in a mouse colitis model.150 Together, these activities of vitamin D have potential downstream consequences for microbial composition change139
The links between vitamin D and gut microbiota are intriguing, as vitamin D deficiency has been linked to the increasing incidence of several dysbiosis-associated inflammatory diseases like IBD and MS. These conditions are common in northern countries where sunlight exposure is limited during winter, leading to vitamin D deficiency. For example, increasing IBD incidence has been associated with low sun exposure and high latitude, with the two forms of IBD, namely ulcerative colitis and Crohn’s disease being 40% and 80% higher, respectively, in northern Europe as compared to southern Europe. Additionally, regions in the United States with lower UV exposure exhibit increased incidence and severity of IBD, along with higher hospitalization rates.151 Vitamin D deficiency in IBD patients ranges from 16% to 95%, and is seen more commonly in CD than in UC patients.152–154
Notably, IBD patients often develop skin lesions that mirror the appearance and disappearance of their chronic relapsing gut inflammation. There are different cutaneous signs of IBD, such as fissures and fistulae, erythema nodosum (inflammation of subcutaneous fat tissue), pyoderma gangrenosum (an ulcerative disorder), pyostomatitis vegetans (pustular eruption of the mouth and skin folds), oral aphthous ulcers, cutaneous polyarteritis nodosa (type of vasculitis affecting medium-sized vessels in the skin), necrotizing vasculitis, and psoriasis.86 It has been reported that the serum vitamin D levels of IBD patients with psoriasis are significantly lower than those of patients without psoriasis. Moreover, after these patients received vitamin D supplementation, they showed clinical improvement over that of patients not receiving this therapy.155 Since vitamin D has such an important role in the immune system, it is not surprising that its deficiency has been implicated in several skin diseases beyond psoriasis, including AD (eczema),156 acne157 and vitiligo, a condition characterized by loss of skin pigmentation.158
Tryptophan metabolites and aryl hydrocarbon receptor signaling
Tryptophan is an essential amino acid and a precursor for the neurotransmitters kynurenines, serotonin, and melatonin.159 It also functions as an important chromophore when exposed to UVB light, resulting in the production of 6-formylindolo [3,2-b] carbazole (FICZ).159 This molecule is a potent activator of the AhR and promotes transcriptional regulation of a variety of downstream genes, including Il22 (which encodes IL-22, an innate immune cytokine and a key component of AhR-regulated intestinal immunity). The AhR is expressed in a variety of tissues, with very high expression seen in epithelial barriers and cells of the gut-associated immune system.160 Moreover, there are other endogenous host tryptophan-derived metabolites such as kynurenine, kynurenic acid, xanthurenic acid, and cinnabarinic acid, as well as a number of bacterial metabolites,161 including indole-3-aldehyde (IAld), indole-3-acetic-acid (IAA) and indole-3-propionic acid (IPA), skatole, and tryptamine, that can act as AhR ligands in the context of promoting intestinal immunity.162–164 Thus, an array of AhR activating tryptophan metabolites can be produced by the skin in response to UVB light as well as by commensal microbes in the gut.
AhR is an important regulator of the skin barrier as it is known to control immune-mediated responses in the skin.165 For example, the AhR is overexpressed in the epidermal lesions found in skin diseases, such as psoriasis, and AD.166 Moreover, indole-3-lactic acid mediated activation of AhR can influence both skin and gut health by reducing inflammation and increasing barrier function of the skin and gut epithelia.167,168 In patients with psoriasis and mouse models of skin disease, AhR activation has been shown to ameliorate inflammatory sequelae. For example, stimulation of affected skin zones with FICZ induces the expression of Cyp1a1, a gene downstream of the AhR pathway, and linked to the decreased expression of several pro-inflammatory chemokines involved in the psoriasis transcriptome.169 Another skin disease regulated by AhR pathway is vitiligo, as AhR is involved in melanogenesis through the induction of melanogenic genes.170 Correspondingly, two major therapies for vitiligo; psoralen (light-sensitive substance from plants that is sensitive to UVA light) and UV phototherapy, which promote the re-pigmentation of the skin171 both activate AhR signaling within the human skin.
Alterations in tryptophan metabolism have also been reported in several intestinal conditions such as IBD, IBS, and colorectal cancer.172–174 Studies using mouse models have determined that inflammation-induced gut microbial dysbiosis often involves the loss of tryptophan-metabolizing gut microbes such as Lactobacillus reuteri. This loss leads to a corresponding decrease in tryptophan metabolites, such as IAA, and a reduction in AhR activation. These microbiota changes impair activation of intestinal group 3 innate lymphoid cells (ILC3) and reduces their production of the tissue protective cytokine IL-22, a key component of AhR-regulated intestinal immunity, culminating in increased susceptibility to dextran sulfate sodium (DSS)-induced colitis.175 Similarly, fecal samples from patients with IBD often show reduced AhR activity and decreased levels of IAA.175 Correspondingly, studies have shown that AhR activation decreases cytokine (TNF, IFNγ, IL-7, IL-12, IL-17, and IL-6) production in the intestine under inflammatory conditions, while also promoting T cell differentiation by controlling key transcription factors such as Foxp3 for T regs and RORγT for T helper 17 (Th17)/ILC3 cells in a ligand-specific manner.176–179 These cells are crucial in promoting intestinal homeostasis as well as defending against invading pathogenic microbes, respectively.180
Despite the extensive assessments of AhR signaling in the gut or the skin, the potential for AhR signaling at one site to simultaneously modulate both organs is poorly defined. Memari et al. investigated the effects of UVB skin exposure on the induction of AhR signaling in peripheral tissues in vivo. They used a single 1.2 kJ/m2 dose of UVB to examine the expression of several AhR target genes in the blood, liver, and intestine, at 3 and 6 hours after UVB exposure. They showed that AhR target genes, including Il22 and Il23a (whose signaling lies upstream of Il-22) in the intestine, were upregulated following UVB exposure on the skin. These findings provide compelling evidence that moderate cutaneous UVB exposure induces endocrine signaling through AhR, a ligand-regulated environmental sensor.181 Given the emerging role of AhR signaling in the immune system in both mice and humans, particularly in barrier organs, this data further supports a role for cutaneous UVB exposure in mediating endocrine regulation of systemic immunity.
Skin wounding
Dermal injuries have been shown to initiate a complex interplay between the skin and gut, leading to significant disruptions in the intestinal microbiome and in immune balance. This phenomenon is particularly evident in mouse models, where skin injuries exacerbate gut microbial dysbiosis and alter immune responses. One mechanism by which skin injury impacts gut health is through the systemic circulation of inflammatory mediators. For example, Yokoyama et al. demonstrated that skin disruption induced by indomethacin treatment in mice resulted in elevated plasma levels of inflammatory mediators such as IgE and TNF-α.182 These mediators migrated to the gut, where they aggravated intestinal inflammation. Additionally, Gallo et al. highlight that localized skin injuries can compromise intestinal antimicrobial defenses and alter the gut microbiome, increasing susceptibility to DSS-induced colitis.183 Using a hyaluronan (HA) digestion model, they replicated the local release of HA fragments from the dermis, a process that occurs during skin wounding or in conditions like psoriasis.184 This model controlled for confounding factors commonly associated with skin inflammation. The study revealed that HA fragments disrupt the gut microbiome, resulting in heightened colitis susceptibility. Experiments further demonstrated that HA fragments enhanced Reg3 production in the mouse colon as well as in cultured human colonic epithelial cells, linking dermal inflammation to gut dysfunction.183
These findings demonstrate the close relationship between the skin and gut. Dermal injuries can disrupt the delicate balance of the intestinal microbiome and immune homeostasis, highlighting the need for further exploration into therapeutic strategies targeting this interplay when it becomes maladaptive.
Ultraviolet radiation – local and systemic effects
Building on the insights from the previous section, we know that UVB light/radiation can significantly affect both the skin and gut. UVB stimulates the production of vitamin D in the skin, which, among its many roles, helps sustain a healthy gut microbiome, as deficiencies have been linked to microbial dysbiosis and IBD.154 Moreover, UVB exposure influences the tryptophan pathway by promoting the synthesis of AhR ligands, which play a vital role in the GSA. In this section we will focus on the multifaceted impact of UVB radiation (largely from the sun) on both skin and gut health.
Sunlight is defined as a continuous spectrum of electromagnetic radiation divided into different wavelength ranges: Ultraviolet (UV), visible, and infrared. Specifically, the UV spectrum (200–400 nm) can be further subdivided into three wavelength ranges: UVC (200–290 nm), UVB (290–320 nm), and UVA (320–400 nm).185 Only UVA and UVB can penetrate the skin since UVC is absorbed by the atmosphere’s ozone layer.186 UV radiation can yield both beneficial and adverse effects on human health. Sunburns, immune suppression, cataracts, photo-aging and DNA damage that can lead to non-melanoma and melanoma skin cancers are some of the examples of unwanted UV effects. Among the positive aspects, a widely recognized outcome of UV radiation is the synthesis of vitamin D upon skin exposure.187 Moreover, specific skin pathologies such as vitiligo have been treated with phototherapy (sunlight) since 2000 B.C. in Egypt, Greece, and India. Starting in the 20th century, the UVA spectrum of light began to be frequently utilized in the treatment of morphea and eczema, while UVB is currently applied to address conditions such as psoriasis, vitiligo, and mycosis fungoides (the most common type of cutaneous T cell lymphoma).185,188–190
UV radiation as skin therapy
The effects of phototherapy depend on the depth of the light penetration into the skin. UVB light is typically absorbed in the epidermis and upper dermis, whereas UVA, due to its longer wavelengths, effectively penetrates the dermis and it can vasodilate arterial vasculature and lowers blood pressure.191–193 When UV light penetrates the skin, it interacts with molecules called chromophores, which absorb the light and undergo chemical reactions. UVB primarily targets nuclear DNA as its main chromophore,194 rapidly leading to the formation of DNA photoproducts as well as DNA damage, and the apoptosis of keratinocytes, immune cells, fibroblasts, and endothelial cells residing within the various layers of the skin.194 Delayed effects on skin health include the release of anti-inflammatory prostaglandins and cytokines. The overall outcome of UV light involves both localized and systemic immune suppression, changes in cytokine expression, and cell-cycle arrest, all contributing to the suppression of disease activity.
Studies have shown that certain wavelengths of UV light administered at specific doses can exhibit immunosuppressive effects. For example, treatment with the longer, non-erythemic and deepest penetrating wavelengths of UVA light, specifically the subclassification UVA1 (340–400 nm) can improve the symptoms suffered by patients with systemic lupus erythematosus (SLE). This benefit was attributed to UVA1 light reaching the deep dermal – epidermal junction, where it reduced systemic B cell and T cell activity, causing a decrease in the numbers of IFNγ-producing Th1 cells and cytotoxic T cells.195,196 In the case of psoriasis, UV radiation can exert divergent effects, either exacerbating or improving disease symptoms.190 Although excessive sun exposure can worsen existing psoriasis plaques, treatments such as narrow-band UVB and UVA combined with a plant substance called psoralen that increases skin photosensitization, have proven effective in treating moderate to severe cases of psoriasis.185 Psoriasis is believed to be triggered by elevated levels of Th1 and Th17 cell derived cytokines. UV radiation treatment modifies this cytokine profile by tilting the balance toward Th2 cell-associated cytokines, such as IL-4 and IL-13, thereby reducing the pro-inflammatory response and suppressing the IL-23–IL-17 axis197,198
Filaggrin is an important skin barrier protein, with AD often linked to loss of function mutations in the filaggrin gene (FLG), and thus AD may be caused by impaired epidermal barrier function.156 Even when normally expressed, filaggrin can be degraded by several “natural moisturizing factors” in the skin, including trans-urocanic acid (trans-UCA), a major UVB photon-absorbing chromophore.199 UVB phototherapy is commonly used to treat AD since it has been shown to increase filaggrin levels in patients after 12 weeks of treatment.200 This may reflect that upon absorption of UVB radiation, trans-UCA is converted to cis-urocanic acid (cis-UCA) which may initiate immunosuppression.201 Correspondingly, narrow-band UVB treatment in patients with AD has been shown to reduce skin-infiltrating lymphocytes, dendritic cells (DCs), and eosinophils, along with significant suppression of the Th2 and Th22 immune axes, along with a milder suppression of the Th1 axis.202 Clinical improvement is associated with a decrease in IL-22 levels,202 as well as the suppression of Th2-related cytokines such as IL-5, IL-13, and IL-31,203 and a reduction in IgE binding cell numbers.204
UV radiation has systemic effects
Localized beneficial effects of UV light on the human skin have been extensively studied185; however, beyond the skin, UV radiation can exert diverse systemic effects. Aside from inducing vitamin D synthesis, it also promotes immune system regulation,205 and metabolic processes such as the regulation of insulin levels and hepatic steatosis; moreover, it triggers the release of endorphins – natural pain-relievers and mood enhancers and helps regulate the body’s internal clock (circadian rhythm), affecting sleep patterns and hormonal release.206 Additionally, some studies suggest that UVA exposure may help regulate blood pressure through the release of nitric oxide, which can relax blood vessels.193 Likewise, there is emerging research suggesting that UV radiation might impact certain neurological processes, potentially affecting mood and cognitive function.207 While skin exposure to UV radiation is known to affect the resident skin microbiota, an exciting new area of research is the potential influence that UV radiation may exert on microbiota members at distant sites, such as those in the gut.
Gut and skin microbiota communication: modulation by UV radiation
As previously noted, many studies have linked a dysbiotic skin microbiota to cutaneous pathologies such as acne, AD, and psoriasis.208 Moreover, these conditions are often associated with concurrent GI diseases such as IBD, IBS and celiac disease. This raises the question of whether the ameliorative effects of UV radiation on these diseases may in part reflect UV driven-modification of the skin and gut microbiota. We suspect that exposure to UV radiation, depending on the exposure time and intensity, can directly modify skin microbial communities.205 One of the major effects of UV radiation on microbes is DNA damage. However, some bacteria and fungi show a selective tolerance to UV radiation at specific stages of their life cycles, but are often susceptible during sporulation, diffusion and infection.205 Thus, UV radiation could cause the loss of more susceptible microbes.209 Wang et al. revealed that porphyrins produced by Propionibacterium acnes (P. acnes) were decreased following UV treatment, providing evidence that skin bacteria are sensitive to UV exposure in patients with acne.210 AD patients have also been shown to carry increased S. aureus burdens on their skin, which were reduced after UVB treatment.211 In concert with any UV-induced changes in microbial composition, microbial metabolites should also be altered. In this section we describe some mediators that may underlie communication between the gut and skin microbiotas and their potential to being influenced by UV exposure (see Figure 2).
Figure 2.
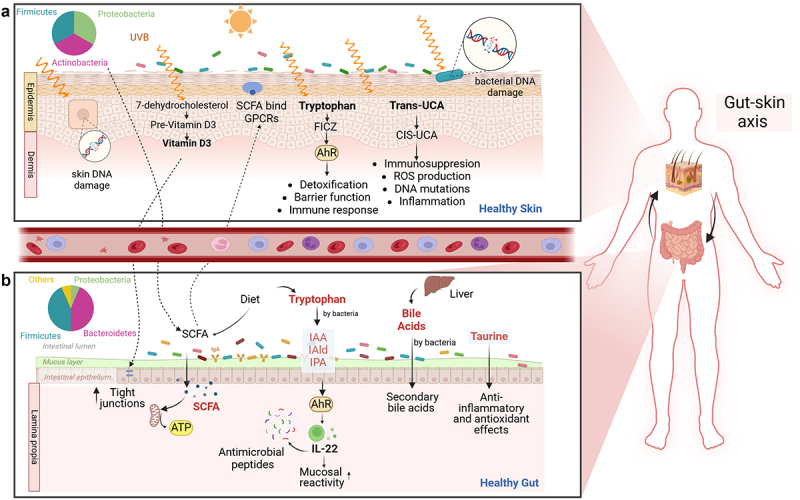
Effects on UVB on the skin and its potential impacts a) cutaneous exposure to UVB light can lead to the production of vitamin D from 7-dehydrocholesterol in the epidermis. Vitamin D also influences gut health by targeting three key components of the GI tract: the intestinal epithelial barrier, gut immunity, and gut microbiota. UVB light can cause DNA damage in skin cells and skin bacteria. UVB light also converts trans-uca to cis-uca in the skin, which generates ROS, resulting in immunosuppression, DNA mutations, and inflammation. Tryptophan acts as a chromophore when exposed to UVB light, leading to the production of 6-formylindolo [3,2-b] carbazole (FICZ), a potent activator of the AhR. AhR is involved in detoxication, barrier function, and immunity. b) exposing the skin to UVB light may impact distal organs such as the gut. UVB is reported to alter the gut microbiome composition and influence SCFA production. SCFA can be transported from the intestine to the skin, where they bind to G protein-coupled receptors (GPCRs) on skin cells, directly influencing tissue metabolism and function. In the gut, tryptophan can be metabolized by bacteria, producing AhR ligands that activate cells, such as ILC3 to produce IL-22. IL-22 regulates the release of antimicrobial peptides, thereby modulating microbial composition and affecting the balance between immunity and the microbiota. BAs, initially produced in the liver as primary BAs, can be metabolized in the colon by gut microbiota (e.g., Clostridium and eubacterium) into secondary BAs. BAs play fundamental roles in metabolism and immunological processes. Lastly, taurine can be degraded by sulfur-derived bacteria like Bilophila and Escherichia. Taurine plays roles in bile acid conjugation, osmoregulation, membrane stabilization, antioxidation, and modulation of calcium signaling. Created in Biorender.com
The importance of metabolites from the tryptophan pathway to both gut and skin health was previously addressed, but to what degree the production of these metabolites requires the microbiota is unclear. Recently, Patra et al. exposed germ-free (lacking a microbiota), disinfected mice (partially devoid of skin microbiota) and control mice with an intact microbiota to UVB radiation (one time exposure). Assaying skin biopsies from these mice, the authors showed that UVB exposure increased tryptophan metabolism, but only in control mice that possessed an intact microbiota.212 The authors highlight the difficulty in determining whether the increase in tryptophan metabolites caused by UV radiation was due to production by the microbiota or the host skin cells. Nevertheless, these results emphasize the importance of the skin microbiota in promoting a distinct metabolomics profile.
Another well-known product of gut bacteria are SCFA, which are produced through the fermentation of indigestible polysaccharides.213 Among the SCFA, butyrate is especially critical for gut homeostasis as it fuels colonocyte metabolism and acts on immune cells to reduce inflammation.214 SCFA have been shown to travel from the gut to distant organs and tissues via the peripheral circulation.215,216 SCFA bind to G protein-coupled receptors (GPCRs) expressed by skin cells, leukocytes, neutrophils, and other cell types, thereby directly impacting tissue metabolism and function.217,218 For example, many patients with AD exhibit low levels of fecal SCFAs, in keeping with a low abundance of SCFA-producing bacteria,219 a pattern also seen in animal models.220 Acne, an inflammatory skin condition caused by the overgrowth of P. acnes, can be alleviated by the probiotic Staphylococcus epidermidis .221,222 This probiotic inhibits P. acnes colonization and its ability to induce inflammation by producing SCFAs.223 In terms of GI diseases, IBD patients often carry lower levels of SCFA in their feces as compared to healthy controls.81 Human and murine studies indicate that multiple exposures to sub-erythemal UV radiation can enhance the diversity of the gut microbiota, potentially increasing the presence of SCFA-producing bacteria. It remains untested however, whether this results in a measurable increase in SCFA levels.17,224 In a recent study, mice were exposed to UVB radiation (75 mJ/cm2) three times per week for eight weeks to analyze the effects on the cecal microbiome. The study surprisingly found that UVB irradiation reduced bacteria involved in SCFA production such as Prevotella, Roseburia, Ruminococcus, and Akkermansia .225 These results contradict previous studies, but it is important to consider the intensity of the UVB light and the frequency of exposure. The longer exposure period of eight weeks in this study suggests that long-term UVB exposure may have a detrimental effect on SCFA-producing bacteria.
Bile acids (BAs) are crucial metabolites that also impact both the gut and skin and may play a role in communication between these two organs. Originating from cholesterol, these natural surfactants are produced in the liver and released into the small intestine, where they aid in digestion and absorption of fats.71 These primary BAs are not fully absorbed in the small intestine, so a portion reaches the colon, where the gut microbiota metabolizes these primary BAs through a process called deconjugation and dehydroxylation. This microbial conversion transforms primary BAs, such as chenodeoxycholic acid (CDCA) and cholic acid (CA), into secondary BAs like lithocholic acid (LCA) and deoxycholic acid (DCA). In this process, bacterial enzymes, specifically bile salt hydrolases (BSH), hydrolyze bile salts, releasing free bile acids. Subsequently, certain gut bacteria, predominantly from the Clostridium and Bacteroides genera, catalyze the removal of the hydroxyl group at the 7th position of the bile acid molecule, leading to the formation of secondary bile acids such as LCA and DCA.226–228
Beyond their primary functions in lipid digestion and cholesterol regulation, BAs play a role in innate immune regulation.229 Disruptions in BA balance, observed in conditions like psoriatic arthritis and IBD, can indicate an imbalance in the gut microbiota as well as abnormal immunity, leading to increased cytokine production.230,231 The amino acid taurine is essential for BA conjugation and has been shown to have therapeutic potential in treating UC and other diseases,232 for example taurine (0.05–0.15 g/day) effectively addresses psoriasis,233 eczema, and prevents irritative dermatitis.234 Studies in mice have shown that supplementing with BAs or using engineered bacteria that produce BAs will boost the numbers of colonic Tregs and decrease susceptibility to colitis.235,235 Patients with psoriasis often display dysfunctional BA profiles, with lower levels of conjugated primary and secondary BAs.236 Early clinical studies have indicated that oral supplements of dehydrocholic acid or ursodeoxycholic acid can treat psoriasis, likely by influencing gut microbiota or by inhibiting cutaneous phospholipase A2 activity.237,238 Moreover, in vitro studies have shown that BAs can directly suppress IL-17A production by T cells, suggesting a mechanistic link between BAs and their anti-psoriatic effects.239 Dietary changes and antimicrobial agents may help modulate the microbiota-BA axis,240 potentially treating various disease of the GI tract as well as skin conditions. Since studies have not yet addressed whether UVB exposure on the skin can alter BA metabolism, more research is needed to explore the potential connection between UV radiation-induced microbial shifts and BA metabolism.
Trimethylamine N-oxide (TMAO) is another gut microbial metabolite that can affect gut and skin health. Derived from dietary sources such as choline, betaine, and carnitine, increased levels of TMAO have been implicated in the pathogenesis of IBD and Hidradenitis suppurativa (HS),241 a highly disabling inflammatory skin disease associated with several cardiovascular and metabolic diseases such as metabolic syndrome, hypertriglyceridemia, diabetes mellitus, ischemic stroke and myocardial infarction.242 There is also an association between HS and IBD, indicating shared genetic susceptibility and immunological features between the two conditions,243 but TMAO was not studied as part of this association. The relationship between HS and IBD is influenced by shared risk factors like smoking, genetics, and common disease features like fistula formation. A study revealed that IBD (CD (0.8%) and UC (1.3%)) is significantly more prevalent among HS patients than in the general population (0.3%), with a higher risk of developing new-onset IBD.244 Additionally, a 2018 cohort study found that HS patients with IBD were predominantly younger African American females, more likely to be smokers, obese, and diabetic, with longer hospital stays and higher healthcare costs.245 This connection can be explained through the GSA. Both HS and IBD involve immune dysregulation, driven by key cytokines such as IL-1β, IL-17, and TNF-α, contributing to chronic inflammation in both the gut and skin.244,246,247 Disruptions in the gut microbiota in IBD may exacerbate systemic inflammation, affecting skin conditions like HS. The shared inflammatory environment in both diseases also explains common features like fistula formation due to the chronic, dysregulated immune responses that affect both the skin and gut. Targeted treatments, including TNF alpha inhibitors, have proven effective in both HS and IBD, showing their interconnected inflammatory pathways.244,246,247 Thus, acknowledging the GSA in HS patients with GI symptoms may warrant further evaluation for IBD, highlighting the importance of integrated care for both conditions. Interestingly, Vitamin D supplementation has shown promise in reducing TMAO levels, suggesting a novel therapeutic approach for both IBD and HS.248 Considering this, we suggest that exposure to UVB radiation, and its induction of Vitamin D should be studied for its potential to lower TMAO levels.
UV radiation as a possible therapy for conditions linked to the GSA
The GSA has emerged as a significant area of research in recent years, attracting growing interest for its focus on defining mechanisms of communication between organs in the human body, as well as its potential for developing therapies for both skin and gut disorders. Researchers have studied several therapies to modulate the gut microbiota, such as probiotics and prebiotics,249 dietary modifications,250,251 topical therapies,252 and fecal microbiota transplantation (FMT).253 In contrast, phototherapy has been primarily studied in the context of skin diseases.185,188–190 UV radiation has been investigated for its potential therapeutic effects on the skin, with studies suggesting that it can impact the cutaneous immune system through the GSA.205 An exciting concept is that sunlight, particularly UVB light, may offer protective properties against a range of immune-mediated disorders, extending beyond the conventional role of vitamin D.254 This discovery has sparked new discussions among scientists and reshaped our understanding of potential treatment approaches.
Phototherapy, a promising intervention, could provide benefits far beyond vitamin D regulation. Observations of SCFA production by the gut microbiome17,225 and activation of targeted AhR genes181 in the gut after UVB exposure to the skin suggest that this effect may extend to bile acids, TMAO, and other metabolites regulated by the microbiota. Evidence indicates that exposing the skin to UV radiation can promote changes in gut microbiota,17,225 although more studies are needed to confirm that these changes can yield gut health benefits. By further exploring the complex interactions within the GSA, future studies can pave the way for personalized and effective treatments that leverage the power of the microbiota to promote gut-skin health.
Conclusions
In conclusion, our review highlights the dynamic communication in the skin and gut axis. While the various organs interact, these two organs are uniquely exposed to the environment, and play a crucial role in maintaining homeostasis, and balancing between beneficial and detrimental effects of environmental factors. We have shown that the GSA involves both microbiota-dependent and independent mediators, emphasizing the complexity of this communication. By understanding these interactions, we can anticipate advancements in therapeutic approaches and precision medicine. Ultimately, further research will help to clarify how environmental stimuli such as UV treatment can control the complex signaling networks that control human health and disease, offering prospects for improved patient outcomes and enhanced healthcare practices.
Funding Statement
This work was supported by grants from the Canadian Institutes of Health Research (CIHR), from Crohn’s and Colitis Canada (CCC) and from Natural Sciences and Engineering Research Council of Canada to B.A.V. CONAHCYT PhD grant to M.J.S.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing not applicable – no new data generated.
References
- 1.Hu MS, Borrelli MR, Hong WX, Malhotra S, Cheung ATM, Ransom RC, Rennert RC, Morrison SD, Lorenz HP, Longaker MT.. Embryonic skin development and repair. Organogenesis. 2018. Jan 2. 14(1):46–27. doi: 10.1080/15476278.2017.1421882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kostouros A, Koliarakis I, Natsis K, Spandidos DA, Tsatsakis A, Tsiaoussis J. Large intestine embryogenesis: molecular pathways and related disorders (review). Int J Mol Med. 2020. Jul 1. 46(1):27–57. doi: 10.3892/ijmm.2020.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Günther J, Seyfert HM. The first line of defence: insights into mechanisms and relevance of phagocytosis in epithelial cells. Semin Immunopathol. 2018. Nov 1. 40(6):555–565. doi: 10.1007/s00281-018-0701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Neill CA, Monteleone G, McLaughlin JT, Paus R. The gut-skin axis in health and disease: a paradigm with therapeutic implications. BioEssays. 2016. Nov 1. 38(11):1167–1176. doi: 10.1002/bies.201600008. [DOI] [PubMed] [Google Scholar]
- 5.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005. Apr. 11(4):S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 6.De Pessemier B, Grine L, Debaere M, Maes A, Paetzold B, Callewaert C. Gut–skin axis: current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms. 2021. Feb. 9(2):353. doi: 10.3390/microorganisms9020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alhendi A, Naser SA. The dual role of interleukin-6 in Crohn’s disease pathophysiology. Front Immunol [Internet]. 2023. Dec 1 [accessed 2024 Jul 17]. 14. https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2023.1295230/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakatsuji T, Chen TH, Two AM, Chun KA, Narala S, Geha RS, Hata TR, Gallo RL. Staphylococcus aureus exploits epidermal barrier defects in atopic dermatitis to trigger cytokine expression. J Invest Dermatol. 2016. Nov 1. 136(11):2192–2200. doi: 10.1016/j.jid.2016.05.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharya N, Sato WJ, Kelly A, Ganguli-Indra G, Indra AK. Epidermal lipids: key mediators of atopic dermatitis pathogenesis. Trends Mol Med. 2019. June. 25(6):551–562. doi: 10.1016/j.molmed.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009. Sep 1. 183(5):3170–3176. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao X, Hu X, Yao J, Cao W, Zou Z, Wang L, Qin H, Zhong D, Li Y, Xue P, et al. The role of short-chain fatty acids in inflammatory skin diseases. Front Microbiol. 2023. Feb 2. 13:1083432. doi: 10.3389/fmicb.2022.1083432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong JMW, de Souza R, Kendall CWC, Emam A, Jenkins DJA. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006. Mar. 40(3):235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Sadiq A, Shah A, Jeschke MG, Belo C, Qasim Hayat M, Murad S, Amini-Nik S. The role of serotonin during skin healing in post-thermal injury. Int J Mol Sci. 2018. Apr. 19(4):1034. doi: 10.3390/ijms19041034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terry N, Margolis KG. Serotonergic mechanisms regulating the GI Tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:319–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Micci MA, Murthy KS, Pasricha PJ. Substance P is essential for maintaining gut muscle contractility: a novel role for coneurotransmission revealed by botulinum toxin. Am J Physiol-Gastrointestinal Liver Physiol. 2014. May 15. 306(10):G839–48. doi: 10.1152/ajpgi.00436.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi H, Kim DJ, Nam S, Lim S, Hwang JS, Park KS, Hong HS, Won Y, Shin MK, Chung E, et al. Substance P restores normal skin architecture and reduces epidermal infiltration of sensory nerve fiber in tncb-induced atopic dermatitis-like lesions in NC/Nga mice. J Dermatological Sci. 2018. Mar 1. 89(3):248–257. doi: 10.1016/j.jdermsci.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Bosman ES, Albert AY, Lui H, Dutz JP, Vallance BA. Skin exposure to narrow band ultraviolet (UVB) light modulates the human intestinal microbiome. Front Microbiol. 2019;10(OCT). doi: 10.3389/fmicb.2019.02410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The dynamic anatomy and patterning of skin - Wong - 2016 - experimental dermatology - Wiley Online Library [Internet]. [accessed 2023 Mar 18]. doi: 10.1111/exd.12832. [DOI] [PubMed] [Google Scholar]
- 19.The gut barrier and chronic diseases [Internet]. [accessed 2023 Aug 30. https://oce.ovid.com/article/00075197-202205000-00009/PDF.
- 20.Melhem H, Regan-Komito D, Niess JH. Mucins dynamics in physiological and pathological conditions. IJMS. 2021. Dec 20. 22(24):13642. doi: 10.3390/ijms222413642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckley A, Turner JR. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol. 2018. Jan. 10(1):a029314. doi: 10.1101/cshperspect.a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallegos-Alcalá P, Jiménez M, Cervantes-García D, Salinas E. The keratinocyte as a crucial cell in the predisposition, onset, progression, therapy and study of the atopic dermatitis. IJMS. 2021. Oct 1. 22(19):10661. doi: 10.3390/ijms221910661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chassaing B, Kumar M, Baker MT, Singh V, Vijay-Kumar M. Mammalian gut immunity. Biomed J. 2014;37(5):246–258. doi: 10.4103/2319-4170.130922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birchenough GMH, Nyström EEL, Johansson MEV, Hansson GC. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science. 2016. June 24. 352(6293):1535–1542. doi: 10.1126/science.aaf7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Churchill MJ, Mitchell PS, Rauch I. Epithelial pyroptosis in host defense. J Mol Biol. 2022. Feb 28. 434(4):167278. doi: 10.1016/j.jmb.2021.167278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christophers E, Schröder JM. Evolution of innate defense in human skin. Exp Dermatol. 2022. Mar. 31(3):304–311. doi: 10.1111/exd.14482. [DOI] [PubMed] [Google Scholar]
- 27.Mempel M, Voelcker V, Köllisch G, Plank C, Rad R, Gerhard M, Schnopp C, Fraunberger P, Walli AK, Ring J, et al. Toll-like receptor expression in human keratinocytes: nuclear factor κB controlled gene activation by Staphylococcus aureus is toll-like receptor 2 but not toll-like receptor 4 or platelet activating factor receptor dependent. J Invest Dermatol. 2003. Dec. 121(6):1389–1396. doi: 10.1111/j.1523-1747.2003.12630.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Yao Z. Immune cell trafficking: a novel perspective on the gut-skin axis. Inflammation Regener. 2024. Apr 24. 44(1):21. doi: 10.1186/s41232-024-00334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coates M, Lee MJ, Norton D, MacLeod AS. The skin and intestinal microbiota and their specific innate immune systems. Front Immunol. 2019. Dec 17. 10:2950. doi: 10.3389/fimmu.2019.02950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baran J, Sobiepanek A, Mazurkiewicz-Pisarek A, Rogalska M, Gryciuk A, Kuryk L, Abraham SN, Staniszewska M. Mast cells as a target—a comprehensive review of recent therapeutic approaches. Cells. 2023. Jan. 12(8):1187. doi: 10.3390/cells12081187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grice EA, Kong HH, Renaud G, Young AC, NISC Comparative Sequencing Program, Bouffard GG, Blakesley RW, Wolfsberg TG, Turner ML, Segre JA. A diversity profile of the human skin microbiota. Genome Res. 2008. Jul. 18(7):1043–1050. doi: 10.1101/gr.075549.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016. Jan 28. 164(3):337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Bouffard GG, Blakesley RW, Murray PR, Green ED, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009. May 29. 324(5931):1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol Med. 2015. Feb. 21(2):109–117. doi: 10.1016/j.molmed.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serghiou IR, Webber MA, Hall LJ. An update on the current understanding of the infant skin microbiome and research challenges. Curr Opin Microbiol. 2023. Oct 1. 75:102364. doi: 10.1016/j.mib.2023.102364. [DOI] [PubMed] [Google Scholar]
- 36.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010. June 29. 107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scholz CFP, Kilian M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov. Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int J Syst Evol Microbiol. 2016. Nov. 66(11):4422–4432. doi: 10.1099/ijsem.0.001367. [DOI] [PubMed] [Google Scholar]
- 38.Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017. Mar. 23(3):314–326. doi: 10.1038/nm.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scharschmidt TC, Vasquez KS, Truong HA, Gearty SV, Pauli ML, Nosbaum A, Gratz I, Otto M, Moon J, Liese J, et al. A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity. 2015. Nov 17. 43(5):1011–1021. doi: 10.1016/j.immuni.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy EA, Connolly J, Hourihane JO, Fallon PG, McLean WHI, Murray D, Jo J-H, Segre JA, Kong HH, Irvine AD. Skin microbiome before development of atopic dermatitis: early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J Allergy Clin Immunol. 2017. Jan. 139(1):166–172. doi: 10.1016/j.jaci.2016.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simanski M, Erkens AS, Rademacher F, Harder J. Staphylococcus epidermidis-induced interleukin-1 beta and human beta-defensin-2 expression in human keratinocytes is regulated by the host molecule A20 (TNFAIP3). Acta Derm Venereol. 2019. Feb 1. 99(2):181–187. doi: 10.2340/00015555-3073. [DOI] [PubMed] [Google Scholar]
- 42.Ochlich D, Rademacher F, Drerup KA, Gläser R, Harder J. The influence of the commensal skin bacterium Staphylococcus epidermidis on the epidermal barrier and inflammation: implications for atopic dermatitis. Exp Dermatol. 2023. Apr. 32(4):555–561. doi: 10.1111/exd.14727. [DOI] [PubMed] [Google Scholar]
- 43.Williams MR, Bagood MD, Enroth TJ, Bunch ZL, Jiang N, Liu E, Almoughrabie S, Khalil S, Li F, Brinton S, et al. Staphylococcus epidermidis activates keratinocyte cytokine expression and promotes skin inflammation through the production of phenol-soluble modulins. Cell Rep. 2023. Sep 26. 42(9):113024. doi: 10.1016/j.celrep.2023.113024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Totté JEE, Pardo LM, Fieten KB, Vos MC, van den Broek TJ, Schuren FHJ, Pasmans SGMA. Nasal and skin microbiomes are associated with disease severity in paediatric atopic dermatitis. Br J Dermatol. 2019. Oct. 181(4):796–804. doi: 10.1111/bjd.17755. [DOI] [PubMed] [Google Scholar]
- 45.Hon KL, Tsang YCK, Pong NH, Leung TF, Ip M. Exploring Staphylococcus epidermidis in atopic eczema: friend or foe? Clin Exp Dermatol. 2016. Aug. 41(6):659–663. doi: 10.1111/ced.12866. [DOI] [PubMed] [Google Scholar]
- 46.Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, et al. Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015. Apr 2. 520(7545):104–108. doi: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, Wu Z-R, Hooper LV, Schmidt RR, von Aulock S, et al. Commensal bacteria regulate Toll-like receptor 3–dependent inflammation after skin injury. Nat Med. 2009. Dec. 15(12):1377–1382. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates - dréno - 2018. J Eur Acad Dermatol Venereology - Wiley Online Lib [Internet]. [cited 2023 Jul 24. doi: 10.1111/jdv.15043. [DOI] [PubMed] [Google Scholar]
- 49.Leung MHY, Wilkins D, Lee PKH. Insights into the pan-microbiome: skin microbial communities of Chinese individuals differ from other racial groups. Sci Rep. 2015. Jul 16. 5(1):11845. doi: 10.1038/srep11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shibagaki N, Suda W, Clavaud C, Bastien P, Takayasu L, Iioka E, Kurokawa R, Yamashita N, Hattori Y, Shindo C, et al. Aging-related changes in the diversity of women’s skin microbiomes associated with oral bacteria. Sci Rep. 2017. Sep 5. 7(1):10567. doi: 10.1038/s41598-017-10834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belkaid Y, Tamoutounour S. The influence of skin microorganisms on cutaneous immunity. Nat Rev Immunol. 2016. June. 16(6):353–366. doi: 10.1038/nri.2016.48. [DOI] [PubMed] [Google Scholar]
- 52.Nakatsuji T, Chiang HI, Jiang SB, Nagarajan H, Zengler K, Gallo RL. The microbiome extends to subepidermal compartments of normal skin. Nat Commun. 2013;4(1):1431. doi: 10.1038/ncomms2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao Z, Tseng CH, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci USA. 2007. Feb 20. 104(8):2927–2932. doi: 10.1073/pnas.0607077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009. Dec 18. 326(5960):1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilkins D, Leung MHY, Lee PKH. Microbiota fingerprints lose individually identifying features over time. Microbiome. 2017. Jan 9. 5(1):1. doi: 10.1186/s40168-016-0209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D’Argenio V, Salvatore F. The role of the gut microbiome in the healthy adult status. Clinica (Rome) Acta. 2015. Dec. 451:97–102. doi: 10.1016/j.cca.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Thursby E, Juge N. Introduction to the human gut microbiota. Biochemical J. 2017. June 1. 474(11):1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterology & Motil. 2011;23(3):187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 59.Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, MacQueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011. Mar 1. 60(3):307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- 60.Fulde M, Hornef MW. Maturation of the enteric mucosal innate immune system during the postnatal period. Immunol Rev. 2014. Jul. 260(1):21–34. doi: 10.1111/imr.12190. [DOI] [PubMed] [Google Scholar]
- 61.Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013. Jul. 14(7):685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010. Jul 6. 107(27):12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005. Jan. 115(1):66–75. doi: 10.1172/JCI200519229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010. Mar. 10(3):159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 65.Durack J, Lynch SV. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med. 2019. Jan 7. 216(1):20–40. doi: 10.1084/jem.20180448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luca M, Chattipakorn SC, Sriwichaiin S, Luca A. Cognitive-behavioural correlates of dysbiosis: a review. Int J Mol Sci. 2020. Jul 8. 21(14):4834. doi: 10.3390/ijms21144834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nesci A, Carnuccio C, Ruggieri V, D’Alessandro A, Di Giorgio A, Santoro L, Gasbarrini A, Santoliquido A, Ponziani FR. Gut microbiota and cardiovascular disease: evidence on the metabolic and inflammatory background of a complex relationship. Int J Mol Sci. 2023. May 22. 24(10):9087. doi: 10.3390/ijms24109087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salem I, Ramser A, Isham N, Ghannoum MA. The gut microbiome as a Major regulator of the gut-skin Axis. Front Microbiol. 2018. Jul 10. 9:1459. doi: 10.3389/fmicb.2018.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arck P, Handjiski B, Hagen E, Pincus M, Bruenahl C, Bienenstock J, Paus R. Is there a ‘gut–brain–skin axis’? Exp Dermatol. 2010;19(5):401–405. doi: 10.1111/j.1600-0625.2009.01060.x. [DOI] [PubMed] [Google Scholar]
- 70.Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007. Feb. 73(4):1073–1078. doi: 10.1128/AEM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jia W, Xie G, Jia W. Bile acid–microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol & Hepatol. 2018. Feb 1. 15(2):111–128. doi: 10.1038/nrgastro.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wibowo S, Pramadhani A, Wibowo S, Pramadhani A. Vitamin B, role of gut microbiota and gut Health. In: Vitamin B and Vitamin E - Pleiotropic and nutritional benefits [Internet]. IntechOpen; 2024. [cited 2024 Jul 18]. https://www.intechopen.com/chapters/86651. [Google Scholar]
- 73.Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016. May 4. 2(1):16003. doi: 10.1038/npjbiofilms.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hussain T, Murtaza G, Kalhoro DH, Kalhoro MS, Metwally E, Chughtai MI, Mazhar MU, Khan SA. Relationship between gut microbiota and host-metabolism: emphasis on hormones related to reproductive function. Anim nutr. 2021. Mar 1. 7(1):1–10. doi: 10.1016/j.aninu.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahmad R, Sorrell MF, Batra SK, Dhawan P, Singh AB. Gut permeability and mucosal inflammation: bad, good or context dependent. Mucosal Immunol. 2017. Mar. 10(2):307–317. doi: 10.1038/mi.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Omenetti S, Pizarro TT. The Treg/Th17 Axis: a dynamic balance regulated by the gut microbiome. Front Immunol. 2015. Dec 17. 6:639. doi: 10.3389/fimmu.2015.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu XF, Shao JH, Liao YT, Wang LN, Jia Y, Dong PJ, Liu Z-Z, He D-D, Li C, Zhang X. Regulation of short-chain fatty acids in the immune system. Front Immunol. 2023. May 5. 14:1186892. doi: 10.3389/fimmu.2023.1186892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kling JJ, Wright RL, Moncrief JS, Wilkins TD. Cloning and characterization of the gene for the metalloprotease enterotoxin of Bacteroides fragilis. FEMS Microbiol Lett. 1997. Jan 1. 146(2):279–284. doi: 10.1111/j.1574-6968.1997.tb10205.x. [DOI] [PubMed] [Google Scholar]
- 79.Wexler HM. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev. 2007. Oct. 20(4):593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis. 2016. May. 22(5):1137–1150. doi: 10.1097/MIB.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chong AC, Visitsunthorn K, Ong PY. Genetic/Environmental contributions and immune dysregulation in children with atopic dermatitis. J Asthma Allergy. 2022. Nov 23. 15:1681–1700. doi: 10.2147/JAA.S293900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma N, Chen X, Johnston LJ, Ma X. Gut microbiota-stem cell niche crosstalk: a new territory for maintaining intestinal homeostasis. iMeta. 2022;1(4):e54. doi: 10.1002/imt2.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014. Jan. 15(1):19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 84.Pédron T, Mulet C, Dauga C, Frangeul L, Chervaux C, Grompone G, Sansonetti PJ. A crypt-specific core microbiota resides in the mouse colon. mBio. 2012. May 22. 3(3). doi: 10.1128/mBio.00116-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lelonek E, Bouazzi D, Jemec GBE, Szepietowski JC. Skin and gut microbiome in Hidradenitis Suppurativa: a systematic review. Biomedicines. 2023. Aug 16. 11(8):2277. doi: 10.3390/biomedicines11082277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thrash B, Patel M, Shah KR, Boland CR, Menter A. Cutaneous manifestations of gastrointestinal disease: part II. J Am Acad Dermatol. 2013;68(2):.e211.1–.e211.33. doi: 10.1016/j.jaad.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 87.Drago F, Col ED, Agnoletti AF, Schiavetti I, Savarino V, Rebora A, Paolino S, Cozzani E, Parodi A. The role of small intestinal bacterial overgrowth in rosacea: a 3-year follow-up. J Am Acad Dermatol. 2016. Sep 1. 75(3):e113–5. doi: 10.1016/j.jaad.2016.01.059. [DOI] [PubMed] [Google Scholar]
- 88.Greuter T, Navarini A, Vavricka SR. Skin manifestations of inflammatory bowel disease. Clin Rev Allergy Immunol. 2017. Dec. 53(3):413–427. doi: 10.1007/s12016-017-8617-4. [DOI] [PubMed] [Google Scholar]
- 89.Limon JJ, Tang J, Li D, Wolf AJ, Michelsen KS, Funari V, Gargus M, Nguyen C, Sharma P, Maymi VI, et al. Malassezia is associated with Crohn’s disease and exacerbates colitis in mouse models. Cell Host And Microbe. 2019;25(3):377–388.e6. doi: 10.1016/j.chom.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Levkovich T, Poutahidis T, Smillie C, Varian BJ, Ibrahim YM, Lakritz JR, Alm EJ, Erdman SE. Probiotic bacteria induce a ‘glow of health’. PLoS One. 2013. Jan 16. 8(1):e53867. doi: 10.1371/journal.pone.0053867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ki KW, Jang YJ, Park S, Gyu MS, Kwon H, Jo MJ, Ko G. Lactobacillus acidophilus KBL409 ameliorates atopic dermatitis in a mouse model. J Microbiol [Internet]. 2024. Feb 2262(2):91–99 [cited 2024 Apr 4]. doi: 10.1007/s12275-024-00104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Long J, Gu J, Yang J, Chen P, Dai Y, Lin Y, Wu M, Wu Y. Exploring the association between gut microbiota and inflammatory skin diseases: a two-sample Mendelian randomization analysis. Microorganisms. 2023. Oct. 11(10):2586. doi: 10.3390/microorganisms11102586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zang C, Liu J, Mao M, Zhu W, Chen W, Wei B. Causal associations between gut microbiota and psoriasis: a Mendelian randomization study. Dermatol Ther (Heidelb). 2023. Oct. 13(10):2331–2343. doi: 10.1007/s13555-023-01007-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao Z, Xu Z, Lv D, Rong Y, Hu Z, Yin R, Dong Y, Cao X, Tang B. Impact of the gut microbiome on skin fibrosis: a Mendelian randomization study. Front Med. 2024;11:1380938. doi: 10.3389/fmed.2024.1380938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang L, Li D, Sun S, Liu D, Wang Y, Liu X, Zhou B, Nie W, Li L, Wang Y, et al. Dupilumab therapy improves gut microbiome dysbiosis and tryptophan metabolism in Chinese patients with atopic dermatitis. Int Immunopharmacol. 2024. Mar 16. 131:111867. doi: 10.1016/j.intimp.2024.111867. [DOI] [PubMed] [Google Scholar]
- 96.McCann MR, Kosloski MP, Xu C, Davis JD, Kamal MA. Dupilumab: mechanism of action, clinical, and translational science. Clin Transl Sci. 2024. Aug. 17(8):e13899. doi: 10.1111/cts.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harb H, Chatila TA. Mechanisms of Dupilumab. Clin Exp Allergy. 2020. Jan. 50(1):5–14. doi: 10.1111/cea.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Navarro-López V, Ramírez-Boscá A, Ramón-Vidal D, Ruzafa-Costas B, Genovés-Martínez S, Chenoll-Cuadros E, Carrión-Gutiérrez M, Horga de la Parte J, Prieto-Merino D, Codoñer-Cortés FM. Effect of oral administration of a mixture of Probiotic strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2018. Jan 1. 154(1):37–43. doi: 10.1001/jamadermatol.2017.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Navarro-López V, Martínez-Andrés A, Ramírez-Boscá A, Ruzafa-Costas B, Núñez-Delegido E, Carrión-Gutiérrez MA, Prieto-Merino D, Codoñer-Cortés F, Ramón-Vidal D, Genovés-Martínez S, et al. Efficacy and safety of oral administration of a mixture of probiotic strains in patients with psoriasis: a randomized controlled clinical trial. Acta Derm Venereol. 2019. Nov 1. 99(12):1078–1084. doi: 10.2340/00015555-3305. [DOI] [PubMed] [Google Scholar]
- 100.Jung GW, Tse JE, Guiha I, Rao J. Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J Cutan Med Surg. 2013;17(2):114–122. doi: 10.2310/7750.2012.12026. [DOI] [PubMed] [Google Scholar]
- 101.Buianova I, Girnyk G, Senyshyn N, Hepburn IS. Gut-skin connection: role of intestinal biome in Rosacea: 216. Off J Am Coll Gastroenterol | ACG. 2018. Oct. 113(Supplement):S126. doi: 10.14309/00000434-201810001-00216. [DOI] [Google Scholar]
- 102.Trompette A, Pernot J, Perdijk O, Alqahtani RAA, Domingo JS, Camacho-Muñoz D, Wong NC, Kendall AC, Wiederkehr A, Nicod LP, et al. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal Immunol. 2022. Aug 1. 15(5):908–926. doi: 10.1038/s41385-022-00524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keshari S, Balasubramaniam A, Myagmardoloonjin B, Herr DR, Negari IP, Huang CM. Butyric acid from probiotic Staphylococcus epidermidis in the skin microbiome down-regulates the ultraviolet-induced Pro-inflammatory IL-6 Cytokine via short-chain fatty acid receptor. Int J Mol Sci. 2019. Sep 11. 20(18):4477. doi: 10.3390/ijms20184477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schwarz A, Bruhs A, Schwarz T. The short-chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J Invest Dermatol. 2017. Apr. 137(4):855–864. doi: 10.1016/j.jid.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 105.Ta LDH, Chan JCY, Yap GC, Purbojati RW, Drautz-Moses DI, Koh YM, Tay CJX, Huang C-H, Kioh DYQ, Woon JY, et al. A compromised developmental trajectory of the infant gut microbiome and metabolome in atopic eczema. Gut Microbes. 2020. Nov 9. 12(1):1–22. doi: 10.1080/19490976.2020.1801964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Song H, Yoo Y, Hwang J, Na YC, Kim HS. Faecalibacterium prausnitzii subspecies–level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. 2016. Mar. 137(3):852–860. doi: 10.1016/j.jaci.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 107.Cait A, Cardenas E, Dimitriu PA, Amenyogbe N, Dai D, Cait J, Sbihi H, Stiemsma L, Subbarao P, Mandhane PJ, et al. Reduced genetic potential for butyrate fermentation in the gut microbiome of infants who develop allergic sensitization. J Allergy Clin Immunol. 2019. Dec. 144(6):1638–1647.e3. doi: 10.1016/j.jaci.2019.06.029. [DOI] [PubMed] [Google Scholar]
- 108.Pazyar N, Houshmand G, Yaghoobi R, Hemmati AA, Zeineli Z, Ghorbanzadeh B. Wound healing effects of topical Vitamin K: a randomized controlled trial. Indian J Pharmacol. 2019;51(2):88–92. doi: 10.4103/ijp.IJP_183_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013. Apr 1. 24(2):160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 110.Morales P, Fujio S, Navarrete P, Ugalde JA, Magne F, Carrasco-Pozo C, Tralma K, Quezada M, Hurtado C, Covarrubias N, et al. Impact of dietary lipids on colonic function and microbiota: an experimental approach involving orlistat-induced fat malabsorption in human volunteers. Clin Transl Gastroenterol. 2016. Apr 7. 7(4):e161. doi: 10.1038/ctg.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coras R, Kavanaugh A, Boyd T, Huynh D, Lagerborg KA, Xu YJ, Rosenthal SB, Jain M, Guma M. Choline metabolite, trimethylamine N-oxide (TMAO), is associated with inflammation in psoriatic arthritis. Clin Exp Rheumatol. 2019;37(3):481–484. [PMC free article] [PubMed] [Google Scholar]
- 112.Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu TH, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017. Apr 8. 15(1):73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sangsuwan W, Asawanonda P. Four-weeks daily intake of oral collagen hydrolysate results in improved skin elasticity, especially in sun-exposed areas: a randomized, double-blind, placebo-controlled trial. J Dermatological Treat. 2021. Nov 17. 32(8):991–996. doi: 10.1080/09546634.2020.1725412. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Z, Wang J, Ding Y, Dai X, Li Y. Oral administration of marine collagen peptides from Chum salmon skin enhances cutaneous wound healing and angiogenesis in rats. J Sci Food Agric. 2011;91(12):2173–2179. doi: 10.1002/jsfa.4435. [DOI] [PubMed] [Google Scholar]
- 115.Mataix J, Ochoa JJ, Quiles JL. Olive oil and mitochondrial oxidative stress. Int J Vitam Nutr Res. 2006. Jul. 76(4):178–183. doi: 10.1024/0300-9831.76.4.178. [DOI] [PubMed] [Google Scholar]
- 116.Latreille J, Kesse-Guyot E, Malvy D, Andreeva V, Galan P, Tschachler E, Hercberg S, Guinot C, Ezzedine K. Dietary Monounsaturated fatty acids intake and risk of skin photoaging. PLOS ONE. 2012. Sep 6. 7(9):e44490. doi: 10.1371/journal.pone.0044490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Horrobin DF. Essential fatty acid metabolism and its modification in atopic eczema. Am J Clin Nutr. 2000. Jan. 71(1):367S–372S. doi: 10.1093/ajcn/71.1.367S. [DOI] [PubMed] [Google Scholar]
- 118.Boelsma E, Hendriks HF, Roza L. Nutritional skin care: health effects of micronutrients and fatty acids. Am J Clin Nutr. 2001. May. 73(5):853–864. doi: 10.1093/ajcn/73.5.853. [DOI] [PubMed] [Google Scholar]
- 119.Orengo IF, Black HS, Wolf JE. Influence of fish oil supplementation on the minimal erythema dose in humans. Arch Dermatol Res. 1992;284(4):219–221. doi: 10.1007/BF00375797. [DOI] [PubMed] [Google Scholar]
- 120.Mahmud MDR, Akter S, Tamanna SK, Mazumder L, Esti IZ, Banerjee S, Akter S, Hasan MR, Acharjee M, Hossain MS, et al. Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 2022. Dec 31. 14(1):2096995. doi: 10.1080/19490976.2022.2096995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Di Salvo E, Gangemi S, Genovese C, Cicero N, Casciaro M. Polyphenols from Mediterranean plants: biological activities for skin photoprotection in atopic dermatitis, psoriasis, and chronic urticaria. Plants (Basel). 2023. Oct 15. 12(20):3579. doi: 10.3390/plants12203579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meinke MC, Friedrich A, Tscherch K, Haag SF, Darvin ME, Vollert H, Groth N, Lademann J, Rohn S. Influence of dietary carotenoids on radical scavenging capacity of the skin and skin lipids. Eur J Pharm Biopharm. 2013. June. 84(2):365–373. doi: 10.1016/j.ejpb.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 123.Richelle M, Sabatier M, Steiling H, Williamson G. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br J Nutr. 2006. Aug. 96(2):227–238. doi: 10.1079/BJN20061817. [DOI] [PubMed] [Google Scholar]
- 124.Dattola A, Silvestri M, Bennardo L, Passante M, Scali E, Patruno C, Nisticò SP. Role of vitamins in skin health: a systematic review. Curr Nutr Rep. 2020. Sep. 9(3):226–235. doi: 10.1007/s13668-020-00322-4. [DOI] [PubMed] [Google Scholar]
- 125.Fam VW, Holt RR, Keen CL, Sivamani RK, Hackman RM. Prospective evaluation of mango fruit intake on facial wrinkles and erythema in postmenopausal women: a randomized clinical Pilot study. Nutrients. 2020. Nov 4. 12(11):3381. doi: 10.3390/nu12113381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Danby FW. Nutrition and aging skin: sugar and glycation. Clin Dermatol. 2010;28(4):409–411. doi: 10.1016/j.clindermatol.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 127.Yoon HS, Kim JR, Park GY, Kim JE, Lee DH, Lee KW, Chung JH. Cocoa flavanol supplementation influences skin conditions of photo-aged women: a 24-week double-blind, randomized, controlled trial. J Nutr. 2016. Jan. 146(1):46–50. doi: 10.3945/jn.115.217711. [DOI] [PubMed] [Google Scholar]
- 128.Singh CK, Liu X, Ahmad N. Resveratrol, in its natural combination in whole grape, for health promotion and disease management. Ann N Y Acad Sci. 2015. Aug. 1348(1):150–160. doi: 10.1111/nyas.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Costa E, Cosme F, Jordão AM, Mendes-Faia A. Anthocyanin profile and antioxidant activity from 24 grape varieties cultivated in two Portuguese wine regions. OENO One. 2014. Jan 31. 48(1):51–62. doi: 10.20870/oeno-one.2014.48.1.1661. [DOI] [Google Scholar]
- 130.Kim MJ, Jun JG, Park SY, Choi MJ, Park E, Kim JI, Kim M-J. Antioxidant activities of fresh grape juices prepared using various household processing methods. Food Sci Biotechnol. 2017. Jul 12. 26(4):861–869. doi: 10.1007/s10068-017-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yamakoshi J, Sano A, Tokutake S, Saito M, Kikuchi M, Kubota Y, Kawachi Y, Otsuka F. Oral intake of proanthocyanidin-rich extract from grape seeds improves chloasma. Phytother Res. 2004. Nov. 18(11):895–899. doi: 10.1002/ptr.1537. [DOI] [PubMed] [Google Scholar]
- 132.Toma RB, Frank GC, Nakayama K, Tawfik E. Lycopene content in raw tomato varieties and tomato products. J Foodserv. 2008;19(2):127–132. doi: 10.1111/j.1745-4506.2008.00094.x. [DOI] [Google Scholar]
- 133.Friedman M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J Agric Food Chem. 2013. Oct 9. 61(40):9534–9550. doi: 10.1021/jf402654e. [DOI] [PubMed] [Google Scholar]
- 134.Meinke MC, Nowbary CK, Schanzer S, Vollert H, Lademann J, Darvin ME. Influences of orally taken carotenoid-rich curly Kale extract on collagen I/Elastin index of the skin. Nutrients. 2017. Jul 19. 9(7):775. doi: 10.3390/nu9070775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Latreille J, Kesse-Guyot E, Malvy D, Andreeva V, Galan P, Tschachler E, Hercberg S, Guinot C, Ezzedine K. Association between dietary intake of n-3 polyunsaturated fatty acids and severity of skin photoaging in a middle-aged Caucasian population. J Dermatol Sci. 2013. Dec. 72(3):233–239. doi: 10.1016/j.jdermsci.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 136.McCue P, Shetty K. Health benefits of soy isoflavonoids and strategies for enhancement: a review. Crit Rev Food Sci Nutr. 2004;44(5):361–367. doi: 10.1080/10408690490509591. [DOI] [PubMed] [Google Scholar]
- 137.Vierkötter A, Krutmann J. Environmental influences on skin aging and ethnic-specific manifestations. Dermatoendocrinol. 2012. Jul 1. 4(3):227–231. doi: 10.4161/derm.19858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Solar UV doses of Young Americans and vitamin D3 production |. Environ Health Perspectives. [cited 2023 Apr 27]. 120(1) [Internet]. 10.1289/ehp.1003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Waterhouse M, Hope B, Krause L, Morrison M, Protani MM, Zakrzewski M, Neale RE. Vitamin D and the gut microbiome: a systematic review of in vivo studies. Eur J Nutr. 2019. Oct. 58(7):2895–2910. doi: 10.1007/s00394-018-1842-7. [DOI] [PubMed] [Google Scholar]
- 140.Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate–induced Colitis1, 2, 3. The J Nutr. 2013. Oct 1. 143(10):1679–1686. doi: 10.3945/jn.113.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008. Aug 1. 122(2):261–266. doi: 10.1016/j.jaci.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Braff MH, Zaiou M, Fierer J, Nizet V, Gallo RL. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005. Oct. 73(10):6771–6781. doi: 10.1128/IAI.73.10.6771-6781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006;306:91–110. [DOI] [PubMed] [Google Scholar]
- 144.Antal AS, Dombrowski Y, Koglin S, Ruzicka T, Schauber J. Impact of vitamin D3 on cutaneous immunity and antimicrobial peptide expression. Dermatoendocrinol. 2011;3(1):18–22. doi: 10.4161/derm.3.1.14616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lu R, Zhang YG, Xia Y, Zhang J, Kaser A, Blumberg R, Sun J. Paneth cell alertness to pathogens maintained by vitamin D receptors. Gastroenterology. 2021. Mar. 160(4):1269–1283. doi: 10.1053/j.gastro.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ooi JH, Chen J, Cantorna MT. Vitamin D regulation of immune function in the gut: why do T cells have vitamin D receptors? Mol Aspects Med. 2012. Feb 1. 33(1):77–82. doi: 10.1016/j.mam.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Barragan M, Good M, Kolls JK. Regulation of dendritic cell function by Vitamin D. Nutrients. 2015. Sep. 7(9):8127–8151. doi: 10.3390/nu7095383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cantorna MT, McDaniel K, Bora S, Chen J, James J. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp Biol Med (Maywood). 2014. Nov 1. 239(11):1524–1530. doi: 10.1177/1535370214523890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tabatabaeizadeh SA, Tafazoli N, Ferns GA, Avan A, Ghayour-Mobarhan M. Vitamin D, the gut microbiome and inflammatory bowel disease. J Res Med Sci. 2018;23(1):75. doi: 10.4103/jrms.JRMS_606_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu S, Zhang YG, Lu R, Xia Y, Zhou D, Petrof EO, Claud EC, Chen D, Chang EB, Carmeliet G, et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut. 2015. Jul 1. 64(7):1082–1094. doi: 10.1136/gutjnl-2014-307436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bora S, Cantorna MT. The role of UVR and Vitamin D on T cells and inflammatory bowel disease. Photochem Photobiol Sci. 2017;16(3):347–353. doi: 10.1039/c6pp00266h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sadeghian M, Saneei P, Siassi F, Esmaillzadeh A. Vitamin D status in relation to Crohn’s disease: meta-analysis of observational studies. Nutrition. 2016. May 1. 32(5):505–514. doi: 10.1016/j.nut.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 153.Siffledeen JS, Siminoski K, Steinhart H, Greenberg G, Fedorak RN. The frequency of vitamin D deficiency in adults with Crohn’s disease. Can J Gastroenterol. 2003. Jan 1. 17(8):473–478. doi: 10.1155/2003/391308. [DOI] [PubMed] [Google Scholar]
- 154.Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, Issa M. Vitamin D deficiency in patients with inflammatory bowel disease. J Parenteral Enter Nutr. 2011;35(3):308–316. doi: 10.1177/0148607110381267. [DOI] [PubMed] [Google Scholar]
- 155.Wilchowski SM, Lareau T. Psoriasis: are your patients D-pleted? A brief literature review on vitamin D deficiency and its role in psoriasis. J Clin Aesthet Dermatol. 2022. Mar. 15(3 Suppl 1):S30–3. [PMC free article] [PubMed] [Google Scholar]
- 156.Wei J, Jaleel T, MacLeod AS, Ji JS. Inverted U-shaped relationship between vitamin D and ever-reported eczema in US adults. Allergy. 2019;74(5):964–975. doi: 10.1111/all.13708. [DOI] [PubMed] [Google Scholar]
- 157.Lim SK, Ha JM, Lee YH, Lee Y, Seo YJ, Kim CD, Lee J-H, Im M. Comparison of vitamin D levels in patients with and without acne: a case-control study combined with a randomized controlled trial. PLoS One. 2016. Aug 25. 11(8):e0161162. doi: 10.1371/journal.pone.0161162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bae JM, Jung HM, Hong BY, Lee JH, Choi WJ, Lee JH, Kim GM. Phototherapy for vitiligo: a systematic review and meta-analysis. JAMA Dermatol. 2017. Jul 1. 153(7):666–674. doi: 10.1001/jamadermatol.2017.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Da S, Mello G, De P, Cardoso A, Oliveira EWRS, Siqueira AB. Tryptophan: a proposal of the mechanism of thermal decomposition. J Therm Anal Calorim. 2015. Dec. 122(3):1395–1401. doi: 10.1007/s10973-015-4916-2. [DOI] [Google Scholar]
- 160.Martins LRL, Grzech-Leśniak K, Castro dos Santos N, Suárez LJ, Giro G, Bastos MF, Shibli JA. Transcription factor AhR, cytokines IL-6 and IL-22 in subjects with and without peri-implantitis: a case control-study. Int J Environ Res Public Health. 2022. June 17. 19(12):7434. doi: 10.3390/ijerph19127434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host & Microbe. 2018. June 13. 23(6):716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 162.Peters JC. Tryptophan nutrition and metabolism: an overview. In: Schwarcz R, Young SBrown R, editors. Kynurenine and serotonin pathways: progress in tryptophan research [internet]. Boston (MA): Springer New York; 1991. [cited 2023 Apr 28]. p. 345–358. (Advances in Experimental Medicine and Biology). 10.1007/978-1-4684-5952-4_32. [DOI] [PubMed] [Google Scholar]
- 163.Badawy AAB. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci Rep. 2015. Oct 30. 35(5):e00261. doi: 10.1042/BSR20150197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Korecka A, Dona A, Lahiri S, Tett AJ, Al-Asmakh M, Braniste V, D’Arienzo R, Abbaspour A, Reichardt N, Fujii-Kuriyama Y, et al. Bidirectional communication between the aryl hydrocarbon receptor (AhR) and the microbiome tunes host metabolism. NPJ Biofilms Microbiomes. 2016. Aug 24. 2(1):16014. doi: 10.1038/npjbiofilms.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fernández-Gallego N, Sánchez-Madrid F, Cibrian D. Role of AHR ligands in skin homeostasis and cutaneous inflammation. Cells. 2021. Nov 15. 10(11):3176. doi: 10.3390/cells10113176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim JE, Kim HR, Kang SY, Jung MJ, Heo NH, Lee HJ, Ryu A, Kim HO, Park CW, Chung BY. Aryl hydrocarbon receptor and autophagy-related protein microtubule-associated protein light chain 3 expression in Psoriasis. Ann Dermatol. 2021. Apr 1. 33(2):138–146. doi: 10.5021/ad.2021.33.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim K, Kim H, Sung GY. Effects of indole-3-lactic acid, a metabolite of tryptophan, on IL-4 and IL-13-Induced human skin-equivalent atopic dermatitis models. Int J Mol Sci. 2022. Nov 4. 23(21):13520. doi: 10.3390/ijms232113520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yu K, Li Q, Sun X, Peng X, Tang Q, Chu H, Zhou L, Wang B, Zhou Z, Deng X, Yang J, Lv J, Liu R, Miao C, Zhao W, Yao Z, Wang Q. Bacterial indole-3-lactic acid affects epithelium–macrophage crosstalk to regulate intestinal homeostasis. Proceedings of the National Academy of Sciences; U. S. A; Vol. 120(45):2023. Nov 7; p. e2309032120. 10.1073/pnas.2309032120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Di Meglio P, Duarte JH, Ahlfors H, Owens NDL, Li Y, Villanova F, Tosi I, Hirota K, Nestle F, Mrowietz U, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014. June 19. 40(6):989–1001. doi: 10.1016/j.immuni.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Furue M, Takahara M, Nakahara T, Uchi H. Role of AhR/ARNT system in skin homeostasis. Arch Dermatol Res. 2014. Nov 1. 306(9):769–779. doi: 10.1007/s00403-014-1481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Haarmann-Stemmann T, Esser C, Krutmann J. The Janus-faced role of aryl hydrocarbon receptor signaling in the skin: consequences for prevention and treatment of skin disorders. J Invest Dermatol. 2015. Nov 1. 135(11):2572–2576. doi: 10.1038/jid.2015.285. [DOI] [PubMed] [Google Scholar]
- 172.Wang S, van Schooten FJ, Jin H, Jonkers D, Godschalk R. The involvement of intestinal tryptophan metabolism in inflammatory bowel disease identified by a meta-analysis of the transcriptome and a systematic review of the metabolome. Nutrients. 2023. Jan. 15(13):2886. doi: 10.3390/nu15132886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sun XZ, Zhao DY, Zhou YC, Wang QQ, Qin G, Yao SK. Alteration of fecal tryptophan metabolism correlates with shifted microbiota and may be involved in pathogenesis of colorectal cancer. World J Gastroenterol. 2020. Dec 7. 26(45):7173–7190. doi: 10.3748/wjg.v26.i45.7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chojnacki C, Błońska A, Konrad P, Chojnacki M, Podogrocki M, Poplawski T. Changes in tryptophan metabolism on serotonin and Kynurenine pathways in patients with irritable bowel syndrome. Nutrients. 2023. Jan. 15(5):1262. doi: 10.3390/nu15051262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016. June. 22(6):598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nguyen NT, Hanieh H, Nakahama T, Kishimoto T. The roles of aryl hydrocarbon receptor in immune responses. Int Immunol. 2013. June 1. 25(6):335–343. doi: 10.1093/intimm/dxt011. [DOI] [PubMed] [Google Scholar]
- 177.Kiss EA, Diefenbach A. Role of the aryl hydrocarbon receptor in controlling maintenance and functional programs of RORγt+ innate lymphoid cells and intraepithelial lymphocytes. Front Immunol [Internet]. 2012. May 31 [cited 2024 Jul 17]. 3. https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2012.00124/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang Q, Zhu Y, Lv C, Fang Y, Liao M, Xia Y, Wei Z, Dai Y. AhR activation promotes treg cell generation by enhancing Lkb1-mediated fatty acid oxidation via the Skp2/K63-ubiquitination pathway. Immunology. 2023;169(4):412–430. doi: 10.1111/imm.13638. [DOI] [PubMed] [Google Scholar]
- 179.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008. May. 453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 180.Hubbard TD, Murray IA, Perdew GH. Indole and Tryptophan metabolism: endogenous and dietary routes to ah receptor activation. Drug Metab Dispos. 2015. Oct 1. 43(10):1522–1535. doi: 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Memari B, Nguyen-Yamamoto L, Salehi-Tabar R, Zago M, Fritz JH, Baglole CJ, Goltzman D, White JH. Endocrine aryl hydrocarbon receptor signaling is induced by moderate cutaneous exposure to ultraviolet light. Sci Rep. 2019. June 11. 9(1):8486. doi: 10.1038/s41598-019-44862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yokoyama S, Hiramoto K, Koyama M, Ooi K. Skin disruption is associated with indomethacin-induced small intestinal injury in mice. Exp Dermatol. 2014. Sep. 23(9):659–663. doi: 10.1111/exd.12499. [DOI] [PubMed] [Google Scholar]
- 183.Dokoshi T, Chen Y, Cavagnero KJ, Rahman G, Hakim D, Brinton S, Schwarz H, Brown EA, O’Neill A, Nakamura Y, et al. Dermal injury drives a skin to gut axis that disrupts the intestinal microbiome and intestinal immune homeostasis in mice. Nat Commun. 2024. Apr 8. 15(1):3009. doi: 10.1038/s41467-024-47072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Muto J, Morioka Y, Yamasaki K, Kim M, Garcia A, Carlin AF, Varki A, Gallo RL. Hyaluronan digestion controls DC migration from the skin. J Clin Invest. 2014. Mar 3. 124(3):1309–1319. doi: 10.1172/JCI67947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vangipuram R, Feldman SR. Ultraviolet phototherapy for cutaneous diseases: a concise review. Oral Dis. 2016. May 1. 22(4):253–259. doi: 10.1111/odi.12366. [DOI] [PubMed] [Google Scholar]
- 186.D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013. June. 14(6):12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Holick MF, MacLaughlin JA, Clark MB, Holick SA, Potts JT, Anderson RR, Blank IH, Parrish JA, Elias P. Photosynthesis of previtamin D 3 in human skin and the physiologic consequences. Science. 1980;210(4466):203–205. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- 188.Majid I. Efficacy of targeted narrowband ultraviolet B therapy in Vitiligo. Indian J Dermatol. 2014;59(5):485–489. doi: 10.4103/0019-5154.139892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gara S, Litaiem N, Bacha T, Jmour Y, Zeglaoui F. Maintenance phototherapy for the treatment of early-stage mycosis Fungoides. J Clin Aesthet Dermatol. 2021. Oct. 14(10):25–26. [PMC free article] [PubMed] [Google Scholar]
- 190.Codoñer FM, Ramírez-Bosca A, Climent E, Carrión-Gutierrez M, Guerrero M, Pérez-Orquín JM, Horga de la Parte J, Genovés S, Ramón D, Navarro-López V, et al. Gut microbial composition in patients with psoriasis. Sci Rep. 2018;8(1). doi: 10.1038/s41598-018-22125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Baron ED, Suggs AK. Introduction to photobiology. Dermatologic Clinics. 2014. Jul 1. 32(3):255–266. doi: 10.1016/j.det.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 192.Weichenthal M, Schwarz T. Phototherapy: how does UV work? Photodermatol, Photoimmunol & Photomed. 2005;21(5):260–266. doi: 10.1111/j.1600-0781.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 193.Liu D, Fernandez BO, Hamilton A, Lang NN, Gallagher JMC, Newby DE, Feelisch M, Weller RB. UVA irradiation of human skin vasodilates arterial Vasculature and lowers blood pressure independently of nitric oxide synthase. J Invest Dermatol. 2014. Jul. 134(7):1839–1846. doi: 10.1038/jid.2014.27. [DOI] [PubMed] [Google Scholar]
- 194.Bulat V, Situm M, Dediol I, Ljubicić I, Bradić, L. The mechanisms of action of phototherapy in the treatment of the most common dermatoses. Coll Antropol. 2011;35 Suppl 2:147–51. PMID: 22220423. [PubMed] [Google Scholar]
- 195.McGrath H. Ultraviolet-A1 irradiation therapy for systemic lupus erythematosus. Lupus. 2017. Oct. 26(12):1239–1251. doi: 10.1177/0961203317707064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Szegedi A, Simics E, Aleksza M, Horkay I, Gaál K, Sipka S, Hunyadi J, Kiss E. Ultraviolet-A1 phototherapy modulates Th1/Th2 and Tc1/Tc2 balance in patients with systemic lupus erythematosus. Rheumatol (Oxford). 2005. Jul. 44(7):925–931. doi: 10.1093/rheumatology/keh643. [DOI] [PubMed] [Google Scholar]
- 197.Bernard JJ, Gallo RL, Krutmann J. Photoimmunology: how ultraviolet radiation affects the immune system. Nat Rev Immunol. 2019. Nov. 19(11):688–701. doi: 10.1038/s41577-019-0185-9. [DOI] [PubMed] [Google Scholar]
- 198.Johnson-Huang LM, Suárez-Fariñas M, Sullivan-Whalen M, Gilleaudeau P, Krueger JG, Lowes MA. Effective narrow-band UVB radiation therapy suppresses the IL-23/IL-17 axis in normalized psoriasis plaques. J Invest Dermatol. 2010. Nov. 130(11):2654–2663. doi: 10.1038/jid.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hoober JK, Eggink LL. The discovery and function of Filaggrin. Int J Mol Sci. 2022. Jan. 23(3):1455. doi: 10.3390/ijms23031455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tintle S, Shemer A, Suárez-Fariñas M, Fujita H, Gilleaudeau P, Sullivan-Whalen M, Johnson-Huang L, Chiricozzi A, Cardinale I, Duan S, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011. Sep. 128(3):583–593.e1–4. doi: 10.1016/j.jaci.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Walterscheid JP, Nghiem DX, Kazimi N, Nutt LK, McConkey DJ, Norval M, Ullrich, S.E. Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proceedings of the National Academy of Sciences U. S. A.; Vol. 103(46):2006. Nov 14; p. 17420–17425. doi: 10.1073/pnas.0603119103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Suárez-Fariñas M, Gittler JK, Shemer A, Cardinale I, Krueger JG, Guttman-Yassky E. Residual genomic signature of atopic dermatitis despite clinical resolution with narrow-band UVB. J Allergy Clin Immunol. 2013. Feb. 131(2):577–579. doi: 10.1016/j.jaci.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 203.Gambichler T, Kreuter A, Tomi NS, Othlinghaus N, Altmeyer P, Skrygan M. Gene expression of cytokines in atopic eczema before and after ultraviolet A1 phototherapy. Br J Dermatol. 2008. May. 158(5):1117–1120. doi: 10.1111/j.1365-2133.2008.08498.x. [DOI] [PubMed] [Google Scholar]
- 204.Grabbe J, Welker P, Humke S, Grewe M, Schöpf E, Henz BM, Krutmann J. High-dose ultraviolet A1 (UVA1), but not UVA/UVB therapy, decreases IgE-binding cells in lesional skin of patients with atopic eczema. J Invest Dermatol. 1996. Sep. 107(3):419–422. doi: 10.1111/1523-1747.ep12363402. [DOI] [PubMed] [Google Scholar]
- 205.Patra V, Byrne SN, Wolf P. The skin microbiome: Is it affected by UV-induced immune suppression? Front Microbiol [Internet]. 2016. Aug 10 [cited 2022 Nov 28]. 7. http://journal.frontiersin.org/Article/10.3389/fmicb.2016.01235/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dakup P, Gaddameedhi S. Impact of the circadian clock on UV-Induced DNA damage response and Photocarcinogenesis. Photochem Photobiol. 2017. Jan. 93(1):296–303. doi: 10.1111/php.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Slominski AT, Zmijewski MA, Plonka PM, Szaflarski JP, Paus R. How UV light touches the brain and endocrine system through skin, and why. Endocrinology. 2018. May 1. 159(5):1992–2007. doi: 10.1210/en.2017-03230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zeeuwen PLJM, Kleerebezem M, Timmerman HM, Schalkwijk J. Microbiome and skin diseases. Curr Opin Allergy & Clin Immunol. 2013. Oct. 13(5):514–520. doi: 10.1097/ACI.0b013e328364ebeb. [DOI] [PubMed] [Google Scholar]
- 209.Rothschild LJ. The influence of UV radiation on protistan evolution. J Eukaryotic Microbiol. 1999. Sep. 46(5):548–555. doi: 10.1111/j.1550-7408.1999.tb06074.x. [DOI] [PubMed] [Google Scholar]
- 210.Wang Y, Zhu W, Shu M, Jiang Y, Gallo RL, Liu YT, Huang C-M. The response of human skin commensal bacteria as a reflection of UV radiation: UV-B decreases porphyrin production. PLoS One. 2012;7(10):e47798. doi: 10.1371/journal.pone.0047798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Influence of narrow‐band UVB phototherapy on cutaneous microbiota of children with atopic dermatitis - Silva - 2006 -. Journal of the European Academy Of Dermatology And Venereology - Wiley Online Library [Internet]. [cited 2023 Feb 27. https://onlinelibrary.wiley.com/doi/10.1111/j.1468-3083.2006.01748.x. [DOI] [PubMed]
- 212.Patra V, Bordag N, Clement Y, Köfeler H, Nicolas JF, Vocanson M, Ayciriex S, Wolf P. Ultraviolet exposure regulates skin metabolome based on the microbiome. Sci Rep. 2023. May 3. 13(1):7207. doi: 10.1038/s41598-023-34073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Miller TL, Wolin MJ. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol. 1996. May. 62(5):1589–1592. doi: 10.1128/aem.62.5.1589-1592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman S. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011. May 4. 13(5):517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015. Oct. 11(10):577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 216.van der Hee B, Wells JM. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021. Aug. 29(8):700–712. doi: 10.1016/j.tim.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 217.Krejner A, Bruhs A, Mrowietz U, Wehkamp U, Schwarz T, Schwarz A. Decreased expression of G-protein-coupled receptors GPR43 and GPR109a in psoriatic skin can be restored by topical application of sodium butyrate. Arch Dermatol Res. 2018. Nov. 310(9):751–758. doi: 10.1007/s00403-018-1865-1. [DOI] [PubMed] [Google Scholar]
- 218.Schlatterer K, Peschel A, Kretschmer D. Short-chain fatty acid and FFAR2 activation – a new option for treating infections? Front Cell Infect Microbiol [Internet]. 2021. Dec 2 [cited 2024 Jul 19]. 11. https://www.frontiersin.org/journals/cellular-and-infection-microbiology/articles/10.3389/fcimb.2021.785833/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Reddel S, Del Chierico F, Quagliariello A, Giancristoforo S, Vernocchi P, Russo A, Fiocchi A, Rossi P, Putignani L, El Hachem M. Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci Rep. 2019. Mar 21. 9(1):4996. doi: 10.1038/s41598-019-41149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kim JH, Kim K, Kim W. Cream cheese-derived Lactococcus chungangensis CAU 28 modulates the gut microbiota and alleviates atopic dermatitis in BALB/c mice. Sci Rep. 2019. Jan 24. 9(1):446. doi: 10.1038/s41598-018-36864-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wu J, Guo R, Chai J, Xiong W, Tian M, Lu W, Xu X. The protective effects of cath-mh with Anti-Propionibacterium Acnes and Anti-inflammation functions on acne vulgaris. Front Pharmacol. 2021. Dec 9. 12:788358. doi: 10.3389/fphar.2021.788358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kong HH, Segre JA. Skin microbiome: looking back to move forward. J Invest Dermatol. 2012. Mar. 132(3):933–939. doi: 10.1038/jid.2011.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang Y, Kao MS, Yu J, Huang S, Marito S, Gallo RL, Huang C-M. A precision microbiome approach using sucrose for selective augmentation of Staphylococcus epidermidis fermentation against Propionibacterium acnes. Int J Mol Sci. 2016. Nov 9. 17(11):1870. doi: 10.3390/ijms17111870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ghaly S, Kaakoush NO, Hart PH. Effects of UVR exposure on the gut microbiota of mice and humans. Photochem Photobiol Sci. 2020;19(1):20–28. doi: 10.1039/c9pp00443b. [DOI] [PubMed] [Google Scholar]
- 225.Seo E, Song HH, Kim H, Kim BY, Park S, Suh HJ, Ahn Y. Oral administration of mixed probiotics improves photoaging by modulating the cecal microbiome and MAPK pathway in UVB-Irradiated hairless mice. Mol Nutr & Food Res. 2023;67(12):2200841. doi: 10.1002/mnfr.202200841. [DOI] [PubMed] [Google Scholar]
- 226.Chiang JYL. Bile acid metabolism and signaling. Compr Physiol. 2013. Jul. 3(3):1191–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wise JL, Cummings BP. The 7-α-dehydroxylation pathway: an integral component of gut bacterial bile acid metabolism and potential therapeutic target. Front Microbiol [Internet]. 2023. Jan 9 [cited 2024 Sep 21]. 13. https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2022.1093420/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bourgin M, Kriaa A, Mkaouar H, Mariaule V, Jablaoui A, Maguin E, Rhimi M. Bile salt hydrolases: At the crossroads of microbiota and human health. Microorganisms. 2021. May 22. 9(6):1122. doi: 10.3390/microorganisms9061122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, Zheng M, Zhang X, Xia D, Ke Y, et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. 2016. Oct 18. 45(4):802–816. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 230.Heinken A, Ravcheev DA, Baldini F, Heirendt L, Fleming RMT, Thiele I. Systematic assessment of secondary bile acid metabolism in gut microbes reveals distinct metabolic capabilities in inflammatory bowel disease. Microbiome. 2019. May 15. 7(1):75. doi: 10.1186/s40168-019-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Paine A, Brookes PS, Bhattacharya S, Li D, De La Luz Garcia-Hernandez M, Tausk F, Ritchlin C. Dysregulation of bile acids, lipids, and nucleotides in psoriatic arthritis revealed by unbiased profiling of serum metabolites. Arthritis Rheumatol. 2023. Jan. 75(1):53–63. doi: 10.1002/art.42288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Shimizu M, Zhao Z, Ishimoto Y, Satsu H. Dietary Taurine Attenuates Dextran Sulfate Sodium (DSS)-induced Experimental Colitis in Mice. In: Azuma J, Schaffer SIto T, editors. Taurine 7. New York (NY): Springer; 2009. p. 265–271. (Advances in Experimental Medicine and Biology). [DOI] [PubMed] [Google Scholar]
- 233.Stapleton PP, Molloy AM, Rogers S, Bloomfield FJ. Neutrophil taurine in psoriasis. IJMS. 1996. Jul 1. 165(3):173–176. doi: 10.1007/BF02940245. [DOI] [PubMed] [Google Scholar]
- 234.Yoshimura T, Manabe C, Nagumo JI, Nagahama T, Sato T, Murakami S. Taurine accelerates the synthesis of ceramides and hyaluronic acid in cultured epidermis and dermal fibroblasts. Exp Ther Med. 2023. Sep 20. 26(5):512. doi: 10.3892/etm.2023.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, Mai C, Jin W-B, Guo C-J, Violante S, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature. 2020. May. 581(7809):475–479. doi: 10.1038/s41586-020-2193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sorokin AV, Domenichiello AF, Dey AK, Yuan ZX, Goyal A, Rose SM, Playford MP, Ramsden CE, Mehta NN. Bioactive lipid mediator profiles in human psoriasis skin and blood. J Invest Dermatol. 2018. Jul. 138(7):1518–1528. doi: 10.1016/j.jid.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gyurcsovics K, Bertók L. Pathophysiology of psoriasis: coping endotoxins with bile acid therapy. Pathophysiology. 2003. Dec 1. 10(1):57–61. doi: 10.1016/j.pathophys.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 238.Itoh S, Kono M, Akimoto T. Psoriasis treated with ursodeoxycholic acid: three case reports. Clin Exp Dermatol. 2007. Jul. 32(4):398–400. doi: 10.1111/j.1365-2230.2007.02401.x. [DOI] [PubMed] [Google Scholar]
- 239.Shi Z, Wu X, Wu CY, Singh SP, Law T, Yamada D, Huynh M, Liakos W, Yang G, Farber JM, et al. Bile acids improve Psoriasiform dermatitis through inhibition of IL-17A expression and CCL20-CCR6–Mediated trafficking of T cells. J Invest Dermatol. 2022. May. 142(5):1381–1390.e11. doi: 10.1016/j.jid.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Trefflich I, Marschall HU, Giuseppe RD, Ståhlman M, Michalsen A, Lampen A, Abraham K, Weikert C. Associations between dietary patterns and bile acids—results from a cross-sectional study in vegans and omnivores. Nutrients. 2019. Dec 23. 12(1):47. doi: 10.3390/nu12010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Barrea L, Muscogiuri G, Pugliese G, de Alteriis G, Maisto M, Donnarumma M, Tenore GC, Colao A, Fabbrocini G, Savastano S. Association of Trimethylamine N-Oxide (TMAO) with the clinical severity of hidradenitis suppurativa (acne inversa). Nutrients. 2021. June 10. 13(6):1997. doi: 10.3390/nu13061997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Cartron A, Driscoll MS. Comorbidities of hidradenitis suppurativa: a review of the literature. Int J Womens Dermatol. 2019. Jul 2. 5(5):330–334. doi: 10.1016/j.ijwd.2019.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chen WT, Chi CC. Association of hidradenitis Suppurativa with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2019. Sep 1. 155(9):1022–1027. doi: 10.1001/jamadermatol.2019.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Egeberg A, Jemec GBE, Kimball AB, Bachelez H, Gislason GH, Thyssen JP, Mallbris L. Prevalence and risk of inflammatory bowel disease in patients with Hidradenitis Suppurativa. J Invest Dermatol. 2017. May. 137(5):1060–1064. doi: 10.1016/j.jid.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 245.Ramos-Rodriguez AJ, Timerman D, Khan A, Bonomo L, Hunjan MK, Lemor A. The in-hospital burden of hidradenitis suppurativa in patients with inflammatory bowel disease: a decade nationwide analysis from 2004 to 2014. Int J Dermatol. 2018. May. 57(5):547–552. doi: 10.1111/ijd.13932. [DOI] [PubMed] [Google Scholar]
- 246.Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018. Jan. 16(1):26–42. doi: 10.5217/ir.2018.16.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis Suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi: 10.3389/fimmu.2018.02965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wang X, Li X, Dong Y, Charles L. Vitamin D decreases plasma trimethylamine-N-oxide level in mice by regulating gut microbiota. Biomed Res Int. 2020. Oct 5. 2020(1):9896743. doi: 10.1155/2020/9896743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Yu Y, Dunaway S, Champer J, Kim J, Alikhan A. Changing our microbiome: probiotics in dermatology. Br J Dermatol. 2020;182(1):e28–e28. doi: 10.1111/bjd.18659. [DOI] [PubMed] [Google Scholar]
- 250.Katta R, Desai SP. Diet and dermatology: the role of dietary intervention in skin disease. J Clin & Aesthetic Dermatol. 2014. Jul. 7(7):46–51. [PMC free article] [PubMed] [Google Scholar]
- 251.Phillips M, Quintero MA, Martinez A, Kerman D, Deshpande AR, Damas O, Pignac-Kobinger J, Santaolalla R, Knight K, Rodriguez V, et al. Challenges of conducting a randomized, standardized, outpatient study of dietary intervention in IBD: 643. Off J Am Coll Gastroenterol | ACG. 2017. Oct. 112:S357. doi: 10.14309/00000434-201710001-00643. [DOI] [Google Scholar]
- 252.Marcinkiewicz J, Wojas-Pelc A, Walczewska M, Lipko-Godlewska S, Jachowicz R, Maciejewska A, Białecka A, Kasprowicz A. Topical taurine bromamine, a new candidate in the treatment of moderate inflammatory acne vulgaris: a pilot study. Eur J Dermatol. 2008;18(4):433–439. doi: 10.1684/ejd/2008.0460. [DOI] [PubMed] [Google Scholar]
- 253.Sokol H. Toward rational donor selection in faecal microbiota transplantation for IBD. J Crohn’s Colitis. 2016. Apr 1. 10(4):375–376. doi: 10.1093/ecco-jcc/jjw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Breuer J, Schwab N, Schneider-Hohendorf T, Marziniak M, Mohan H, Bhatia U, Gross CC, Clausen BE, Weishaupt C, Luger TA, et al. Ultraviolet B light attenuates the systemic immune response in central nervous system autoimmunity. Ann Neurol. 2014;75(5):739–758. doi: 10.1002/ana.24165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable – no new data generated.


