Abstract
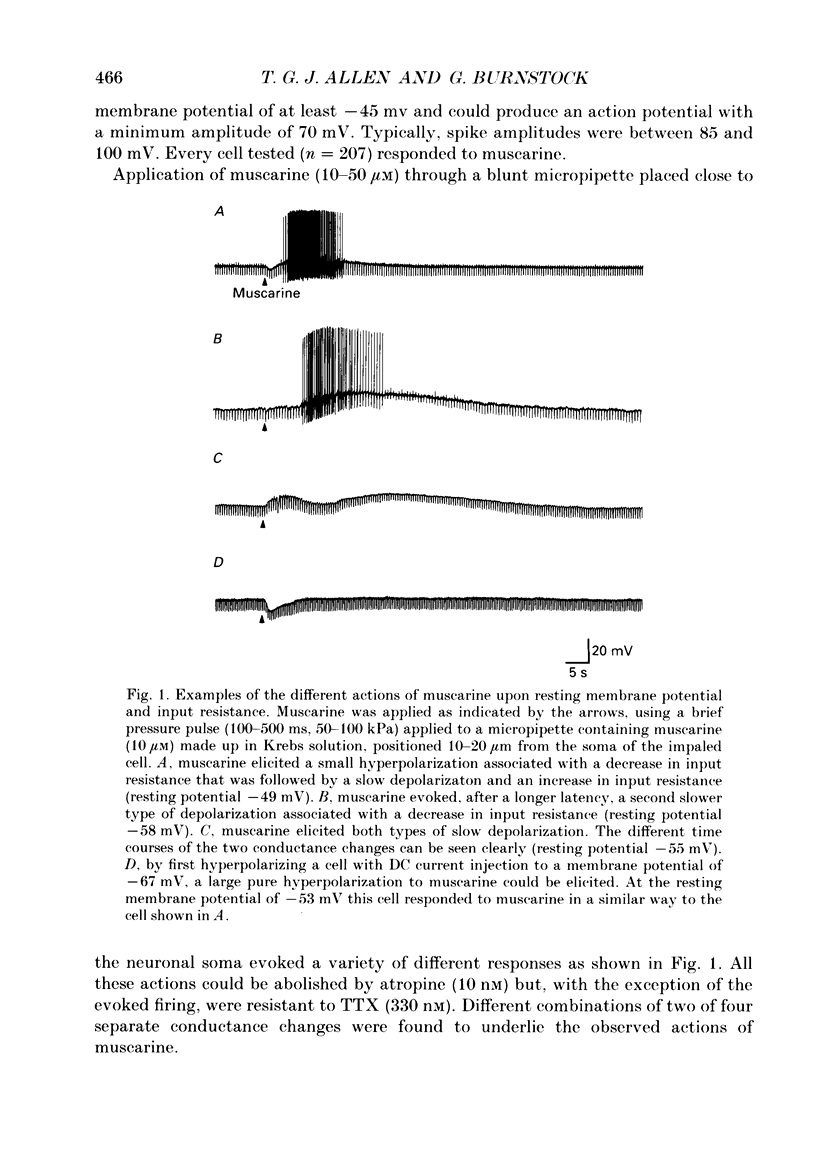
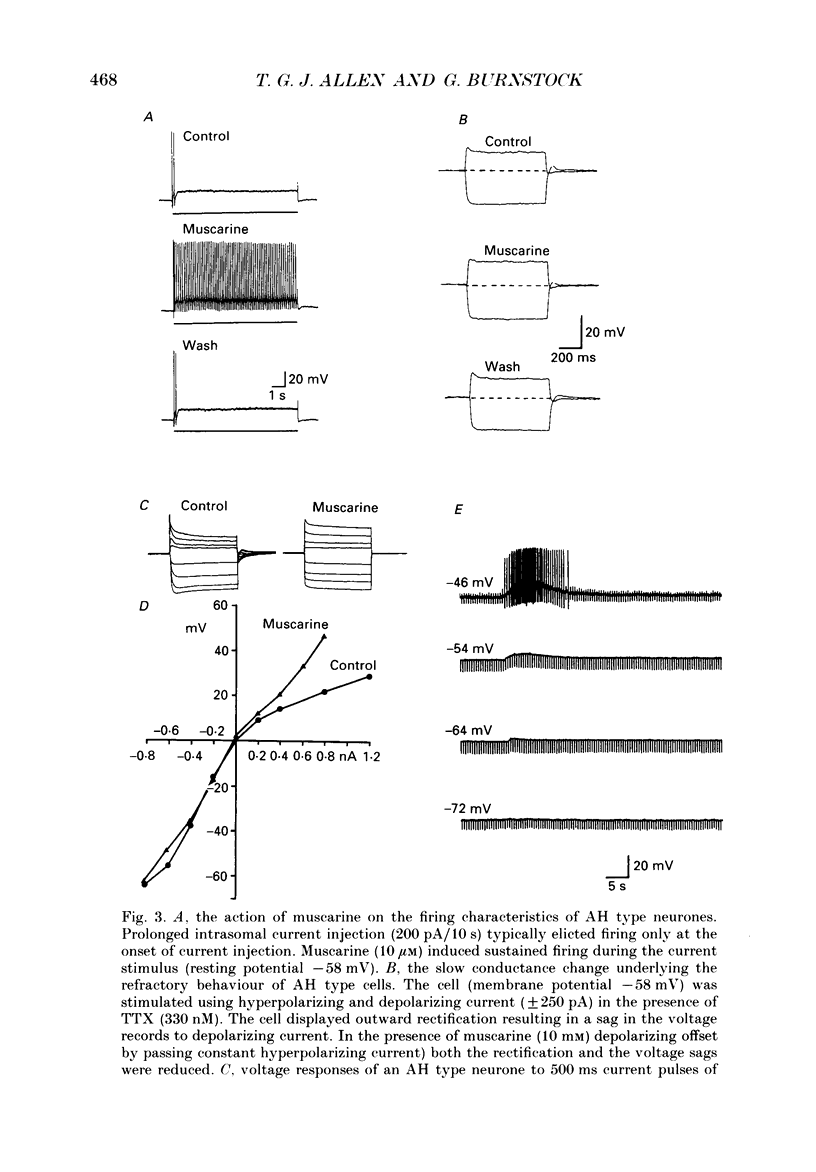
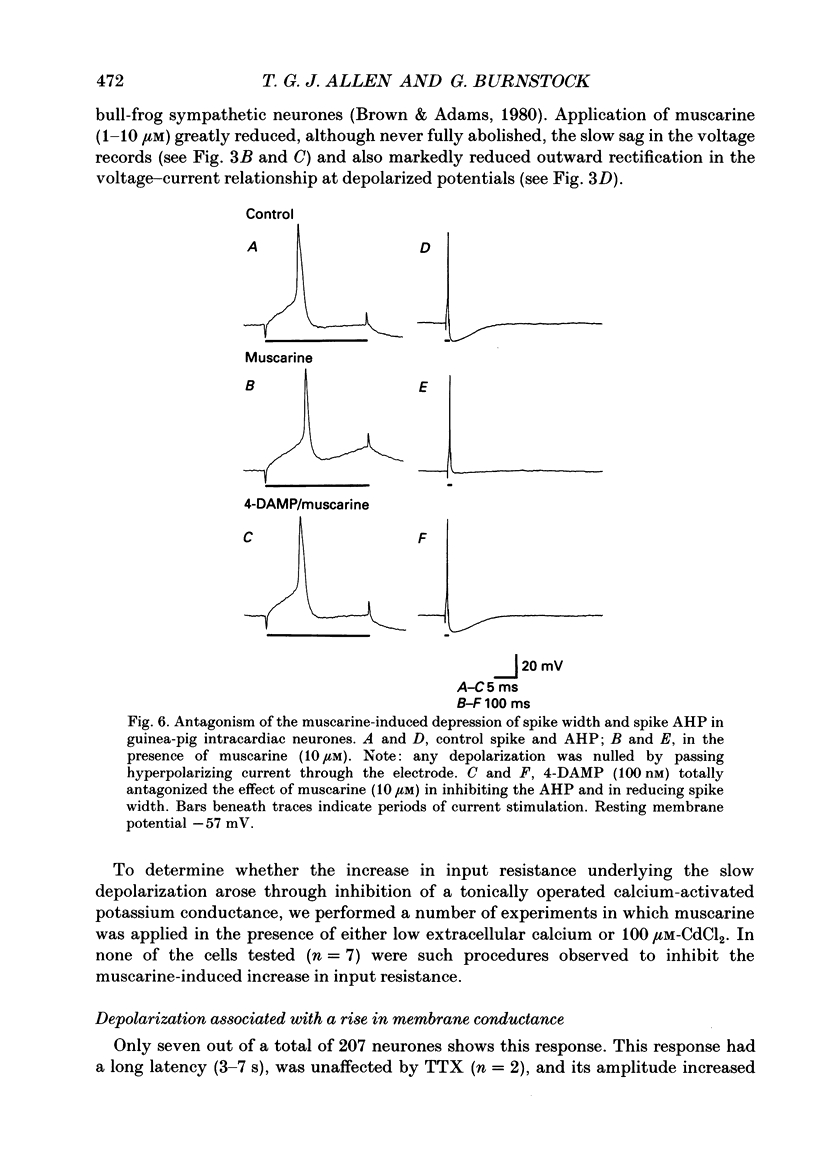
1. The effects of muscarine upon intracardiac neurones cultured from ganglia within the atria and interatrial septum of the newborn guinea-pig heart were studied using intracellular recording techniques. 2. Muscarine applied to the neuronal soma typically produced a biphasic change in membrane potential which consisted of a small hyperpolarization followed by a depolarization. In addition, muscarine (0.01-10 microM) inhibited the calcium-dependent, after-hyperpolarization (AHP) and greatly increased the number of action potentials that could be evoked by a given depolarizing current. 3. The hyperpolarization was associated with a decrease in input resistance and it reversed to become a depolarization at a potential of -86.5 mV. This response was antagonized by 4-diphenylacetoxy-N-methyl-piperidine (4-DAMP; 100 nM) and AF-DX 116 (500 nM), but was unaffected by pirenzepine (0.1-5 microM). 4. Two types of slow depolarization were observed in the presence of muscarine. The most common was associated with an increase in input resistance in the potential range -70 to -40 mV. Pirenzepine (100 nM) selectively antagonized this response, 4-DAMP (100 nM) similarly antagonized the response, but was non-selective. AF-DX 116 (0.5-5 microM) showed no antagonist effect. The less common depolarization (5% of cells) had a long latency and was associated with a decrease in input resistance. 5. Muscarine reduced the duration of the action potential and inhibited the AHP. Cadmium chloride (100 microM) mimicked these actions of muscarine. Application of muscarine immediately following a train of action potentials did not inhibit the AHP, suggesting that muscarine did not directly inhibit the calcium-activated potassium current (IK(Ca)). Muscarine-induced depression of the slow AHP was antagonized by 4-DAMP (100 nM) but was not antagonized by either pirenzepine (0.1-0.5 microM) or AF-DX 116 (0.5-5 microM). 6. It is concluded that the muscarine-induced depolarization of guinea-pig intracardiac neurones results from reduction of a potassium conductance similar to the M-conductance, through activation of M1 muscarinic receptors. The hyperpolarization results from an increase in potassium conductance, through activation of M2 muscarinic receptors.
Full text
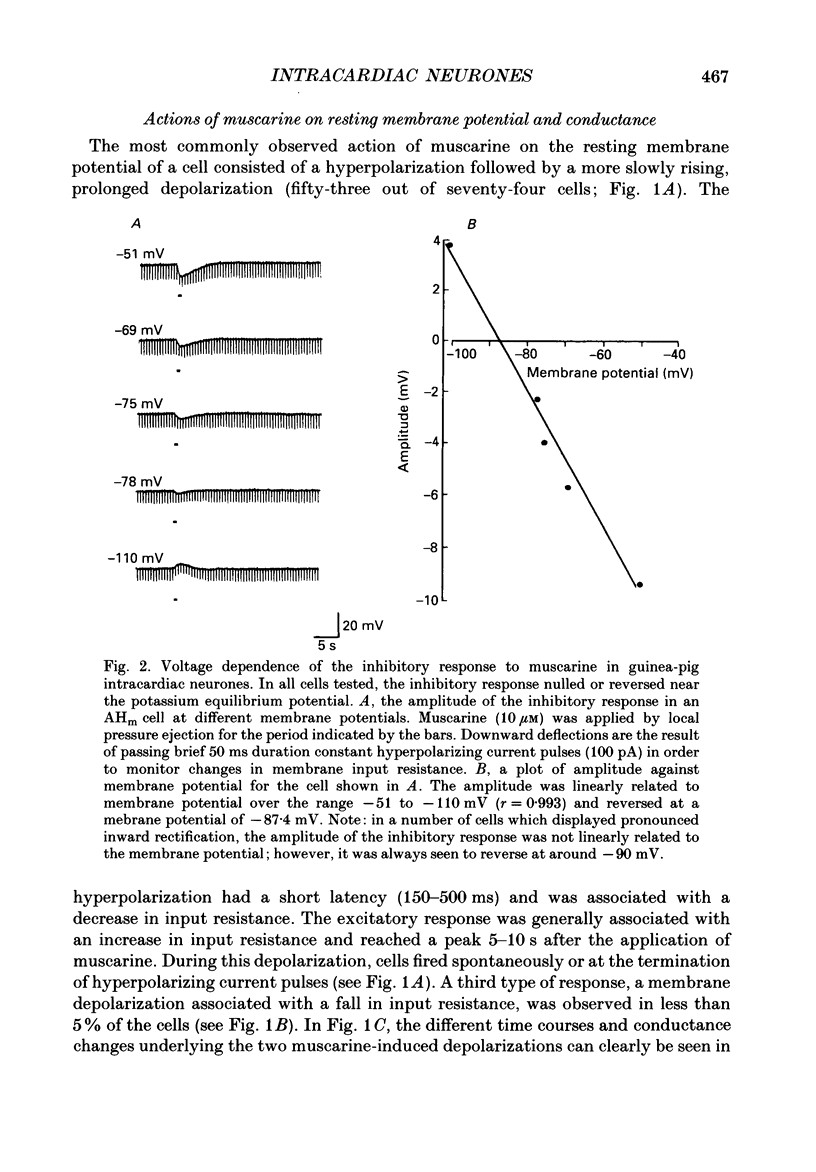
PDF



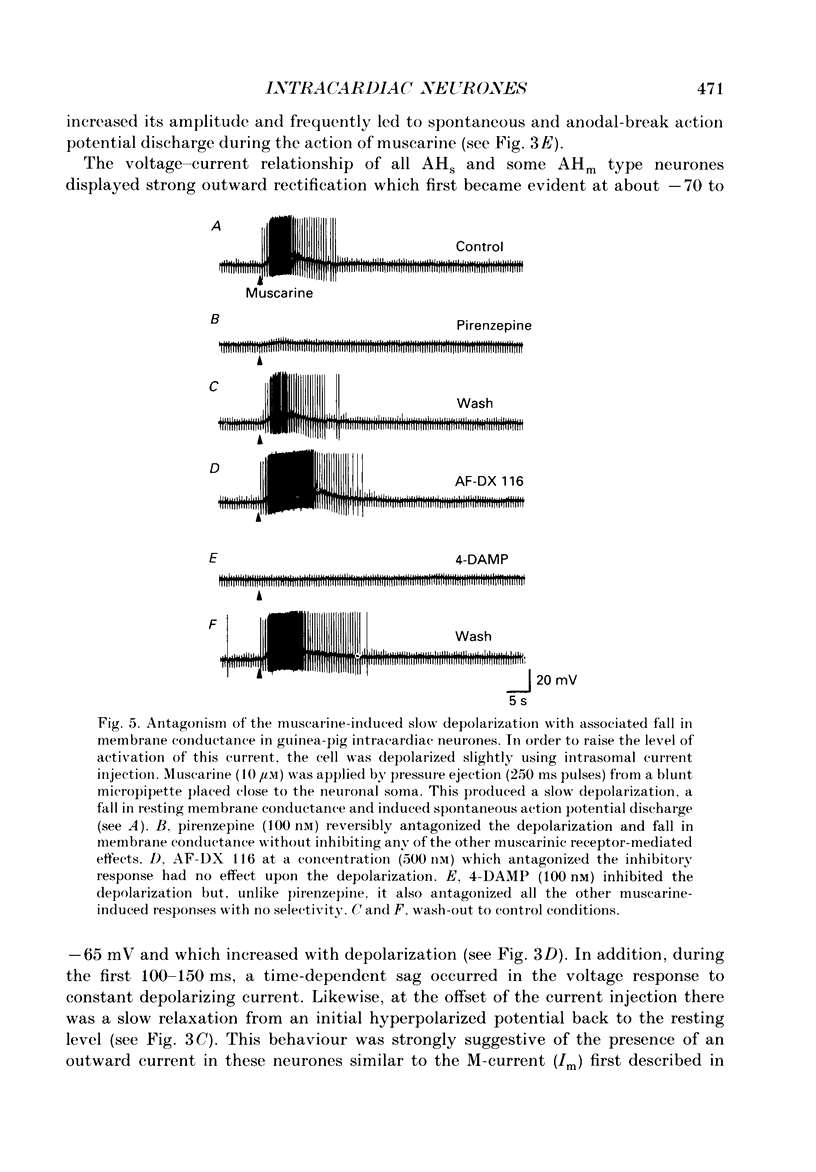
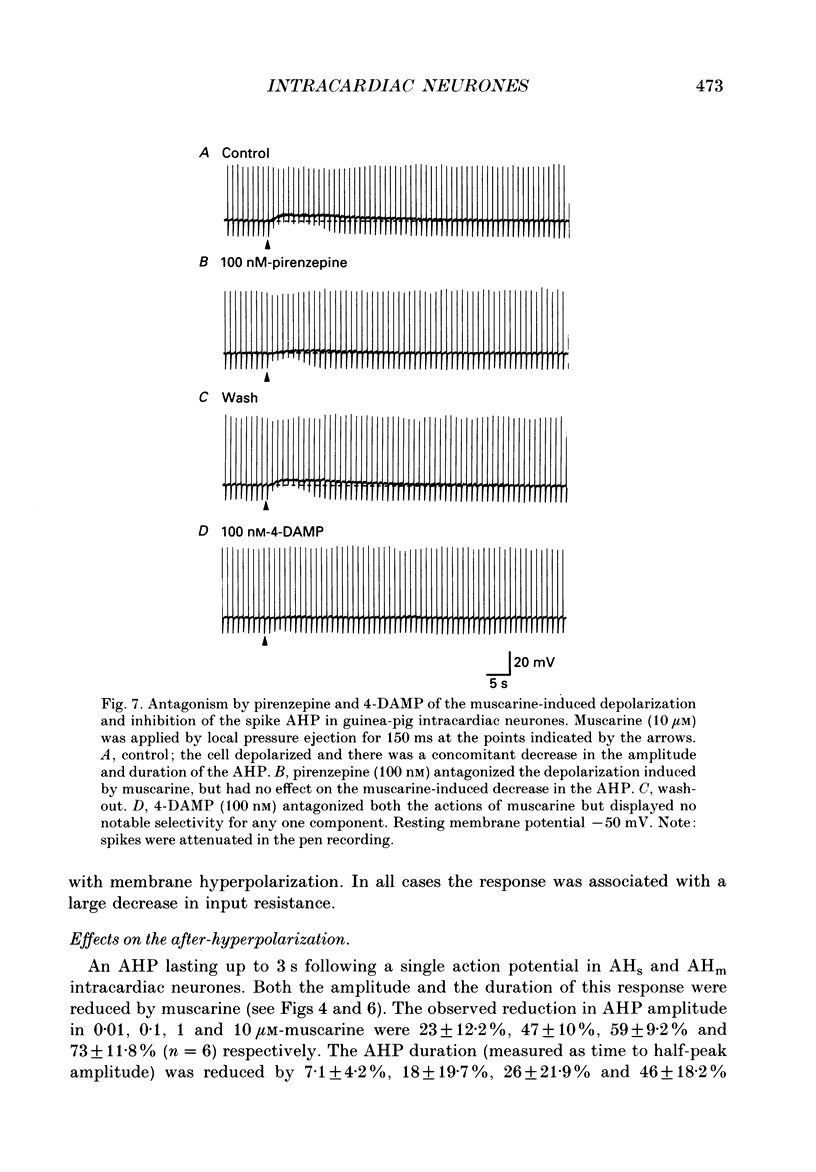
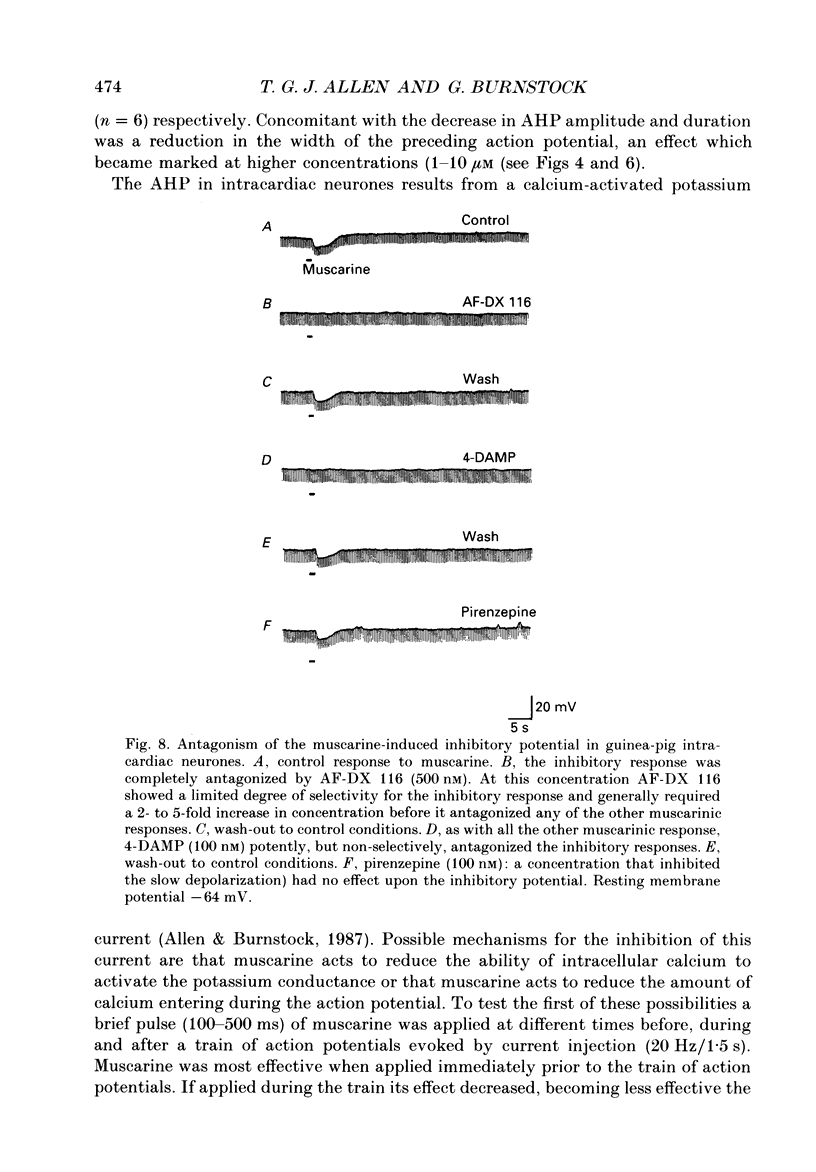






Selected References
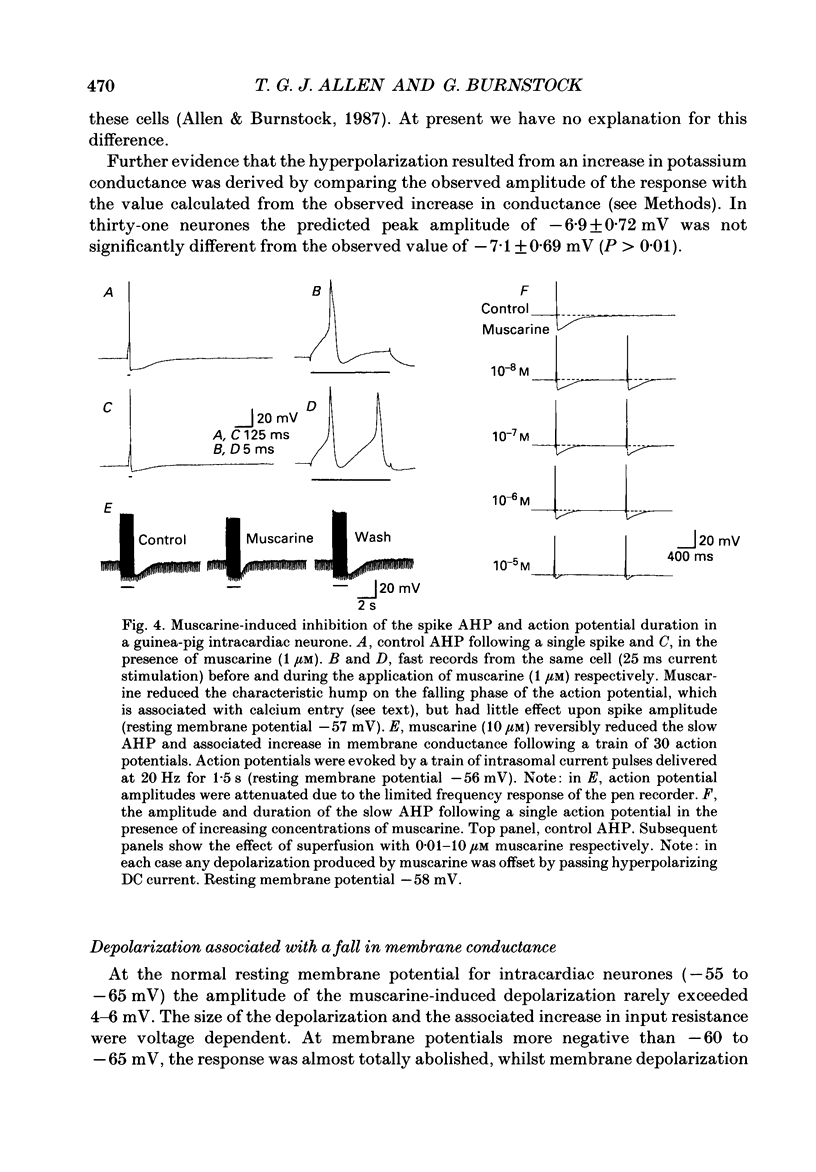
These references are in PubMed. This may not be the complete list of references from this article.
- Akasu T., Gallagher J. P., Koketsu K., Shinnick-Gallagher P. Slow excitatory post-synaptic currents in bull-frog sympathetic neurones. J Physiol. 1984 Jun;351:583–593. doi: 10.1113/jphysiol.1984.sp015264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T. G., Burnstock G. Intracellular studies of the electrophysiological properties of cultured intracardiac neurones of the guinea-pig. J Physiol. 1987 Jul;388:349–366. doi: 10.1113/jphysiol.1987.sp016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar-ul S., Gilani H., Cobbin L. B. The cardio-selectivity of himbacine: a muscarine receptor antagonist. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jan;332(1):16–20. doi: 10.1007/BF00633191. [DOI] [PubMed] [Google Scholar]
- Barlow R. B., Berry K. J., Glenton P. A., Nilolaou N. M., Soh K. S. A comparison of affinity constants for muscarine-sensitive acetylcholine receptors in guinea-pig atrial pacemaker cells at 29 degrees C and in ileum at 29 degrees C and 37 degrees C. Br J Pharmacol. 1976 Dec;58(4):613–620. doi: 10.1111/j.1476-5381.1976.tb08631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. Identification of delayed potassium and calcium currents in the rat sympathetic neurone under voltage clamp. J Physiol. 1985 Jan;358:109–129. doi: 10.1113/jphysiol.1985.sp015543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I. The molecular basis of muscarinic receptor diversity. Trends Neurosci. 1989 Apr;12(4):148–151. doi: 10.1016/0166-2236(89)90054-4. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Fatherazi S., Garthwaite J., White R. D. Muscarinic receptors in rat sympathetic ganglia. Br J Pharmacol. 1980 Dec;70(4):577–592. doi: 10.1111/j.1476-5381.1980.tb09777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Forward A., Marsh S. Antagonist discrimination between ganglionic and ileal muscarinic receptors. Br J Pharmacol. 1980;71(2):362–364. doi: 10.1111/j.1476-5381.1980.tb10948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Selyanko A. A. Two components of muscarine-sensitive membrane current in rat sympathetic neurones. J Physiol. 1985 Jan;358:335–363. doi: 10.1113/jphysiol.1985.sp015554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Sim J. A. Muscarinic receptors mediating suppression of the M-current in guinea-pig olfactory cortex neurones may be of the M2-subtype. Br J Pharmacol. 1987 Jan;90(1):3–5. doi: 10.1111/j.1476-5381.1987.tb16818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J., Dingledine R., Kelly J. S. The excitatory action of acetylcholine on hippocampal neurones of the guinea pig and rat maintained in vitro. Brain Res. 1981 Feb 23;207(1):109–127. doi: 10.1016/0006-8993(81)90682-x. [DOI] [PubMed] [Google Scholar]
- Dodd J., Horn J. P. Muscarinic inhibition of sympathetic C neurones in the bullfrog. J Physiol. 1983 Jan;334:271–291. doi: 10.1113/jphysiol.1983.sp014494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Classification of muscarinic responses in hippocampus in terms of receptor subtypes and second-messenger systems: electrophysiological studies in vitro. J Neurosci. 1988 Nov;8(11):4214–4224. doi: 10.1523/JNEUROSCI.08-11-04214.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan T. M., North R. A. Acetylcholine hyperpolarizes central neurones by acting on an M2 muscarinic receptor. 1986 Jan 30-Feb 5Nature. 319(6052):405–407. doi: 10.1038/319405a0. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Griffith W. H., Shinnick-Gallagher P. Cholinergic transmission in cat parasympathetic ganglia. J Physiol. 1982 Nov;332:473–486. doi: 10.1113/jphysiol.1982.sp014425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hammer R., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980 Jan 3;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- Hammer R., Giraldo E., Schiavi G. B., Monferini E., Ladinsky H. Binding profile of a novel cardioselective muscarine receptor antagonist, AF-DX 116, to membranes of peripheral tissues and brain in the rat. Life Sci. 1986 May 5;38(18):1653–1662. doi: 10.1016/0024-3205(86)90409-1. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassall C. J., Buckley N. J., Burnstock G. Autoradiographic localisation of muscarinic receptors on guinea pig intracardiac neurones and atrial myocytes in culture. Neurosci Lett. 1987 Feb 24;74(2):145–150. doi: 10.1016/0304-3940(87)90140-6. [DOI] [PubMed] [Google Scholar]
- Hassall C. J., Burnstock G. Intrinsic neurones and associated cells of the guinea-pig heart in culture. Brain Res. 1986 Jan 29;364(1):102–113. doi: 10.1016/0006-8993(86)90991-1. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Dodd J. Monosynaptic muscarinic activation of K+ conductance underlies the slow inhibitory postsynaptic potential in sympathetic ganglia. Nature. 1981 Aug 13;292(5824):625–627. doi: 10.1038/292625a0. [DOI] [PubMed] [Google Scholar]
- KING T. S., COAKLEY J. B. The intrinsic nerve cells of the cardiac atria of mammals and man. J Anat. 1958 Jul;92(3):353–376. [PMC free article] [PubMed] [Google Scholar]
- Koketsu K. Cholinergic synaptic potentials and the underlying ionic mechasims. Fed Proc. 1969 Jan-Feb;28(1):101–112. [PubMed] [Google Scholar]
- Koketsu K., Yamada M. Presynaptic muscarinic receptors inhibiting active acetylcholine release in the bullfrog sympathetic ganglion. Br J Pharmacol. 1982 Sep;77(1):75–82. doi: 10.1111/j.1476-5381.1982.tb09271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Pumain R., Renaud L. The mechanism of excitation by acetylcholine in the cerebral cortex. J Physiol. 1971 May;215(1):247–268. doi: 10.1113/jphysiol.1971.sp009467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Analysis of the slow excitatory postsynaptic potential in bullfrog sympathetic ganglion cells. Jpn J Physiol. 1976;26(6):651–669. doi: 10.2170/jjphysiol.26.651. [DOI] [PubMed] [Google Scholar]
- Libet B., Chichibu S., Tosaka T. Slow synaptic responses and excitability in sympathetic ganglia of the bullfrog. J Neurophysiol. 1968 May;31(3):383–395. doi: 10.1152/jn.1968.31.3.383. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984 Sep;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Acetylcholine induces burst firing in thalamic reticular neurones by activating a potassium conductance. 1986 Jan 30-Feb 5Nature. 319(6052):402–405. doi: 10.1038/319402a0. [DOI] [PubMed] [Google Scholar]
- Mochida S., Kobayashi H. Activation of M2 muscarinic receptors causes an alteration of action potentials by modulation of Ca entry in isolated sympathetic neurons of rabbits. Neurosci Lett. 1986 Dec 12;72(2):199–204. doi: 10.1016/0304-3940(86)90080-7. [DOI] [PubMed] [Google Scholar]
- Mochida S., Kobayashi H. Multiple muscarinic responses directly evoked in isolated neurones dissociated from rabbit sympathetic ganglia. J Auton Nerv Syst. 1986 Dec;17(4):289–301. doi: 10.1016/0165-1838(86)90095-0. [DOI] [PubMed] [Google Scholar]
- Mochida S., Kobayashi H. Three types of muscarinic conductance changes in sympathetic neurons discriminately evoked by the different concentrations of acetylcholine. Brain Res. 1986 Sep 24;383(1-2):299–304. doi: 10.1016/0006-8993(86)90028-4. [DOI] [PubMed] [Google Scholar]
- Neild T. O. Slowly-developing depolarization of neurones in the guinea-pig inferior mesenteric ganglion following repetitive stimulation of the preganglionic nerves. Brain Res. 1978 Jan 27;140(2):231–239. doi: 10.1016/0006-8993(78)90457-2. [DOI] [PubMed] [Google Scholar]
- North R. A., Tokimasa T. Depression of calcium-dependent potassium conductance of guinea-pig myenteric neurones by muscarinic agonists. J Physiol. 1983 Sep;342:253–266. doi: 10.1113/jphysiol.1983.sp014849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Tokimasa T. Muscarinic synaptic potentials in guinea-pig myenteric plexus neurones. J Physiol. 1982 Dec;333:151–156. doi: 10.1113/jphysiol.1982.sp014445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimasa T. Muscarinic agonists depress calcium-dependent gK in bullfrog sympathetic neurons. J Auton Nerv Syst. 1984 Apr;10(2):107–116. doi: 10.1016/0165-1838(84)90049-3. [DOI] [PubMed] [Google Scholar]
- Tokimasa T. Spontaneous muscarinic suppression of the Ca-activated K-current in bullfrog sympathetic neurons. Brain Res. 1985 Sep 30;344(1):134–141. doi: 10.1016/0006-8993(85)91197-7. [DOI] [PubMed] [Google Scholar]
- Wanke E., Ferroni A., Malgaroli A., Ambrosini A., Pozzan T., Meldolesi J. Activation of a muscarinic receptor selectively inhibits a rapidly inactivated Ca2+ current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4313–4317. doi: 10.1073/pnas.84.12.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M., Yamamura H. I., Roeske W. R. A unique regulatory profile and regional distribution of [3H]pirenzepine binding in the rat provide evidence for distinct M1 and M2 muscarinic receptor subtypes. Life Sci. 1983 Jun 27;32(26):3001–3011. doi: 10.1016/0024-3205(83)90652-5. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Votava J. Slow synaptic excitation in sympathetic ganglion cells: evidence for synaptic inactivation of potassium conductance. Science. 1970 Nov 13;170(3959):755–758. doi: 10.1126/science.170.3959.755. [DOI] [PubMed] [Google Scholar]


