Abstract
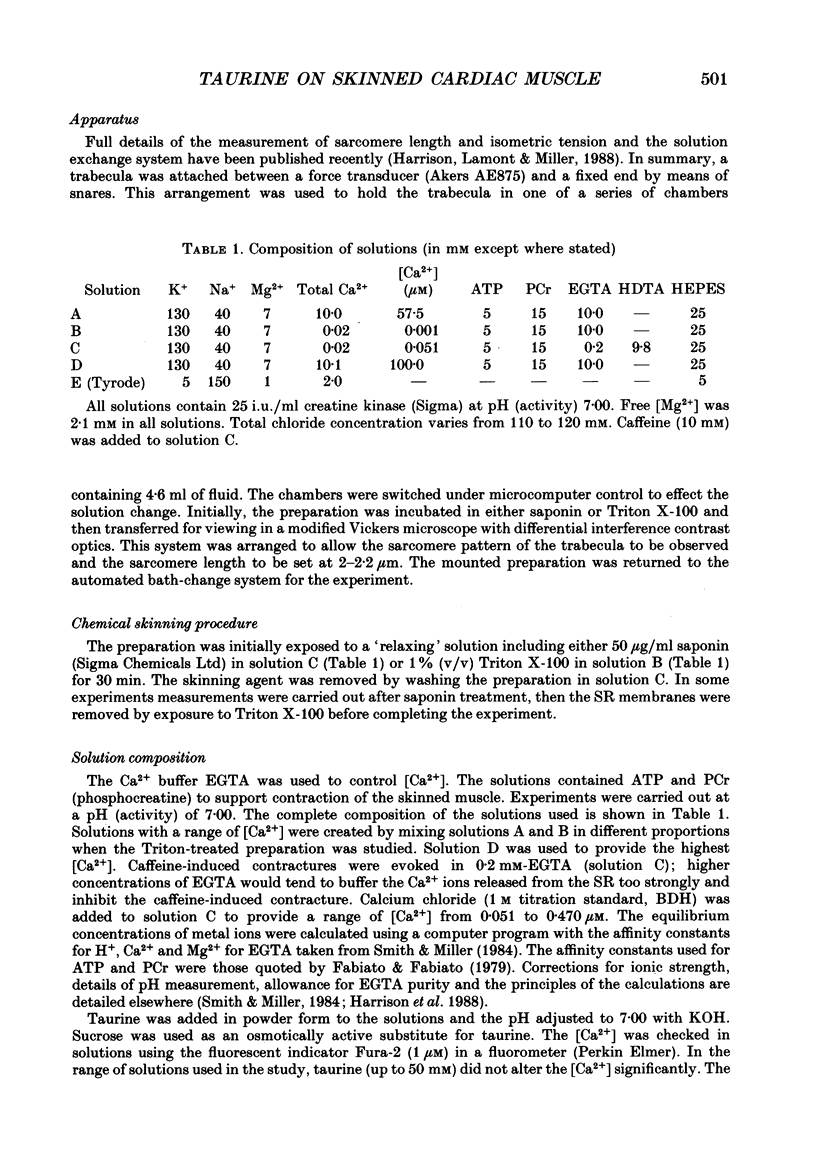
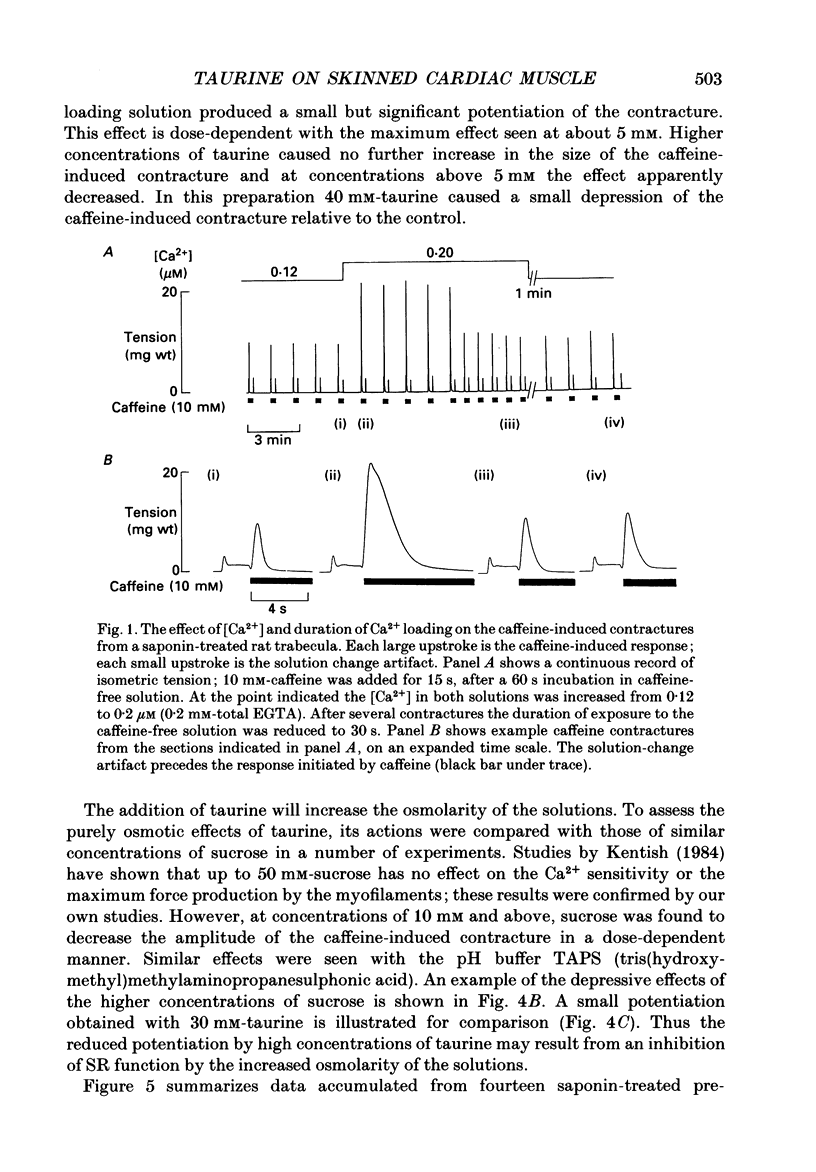
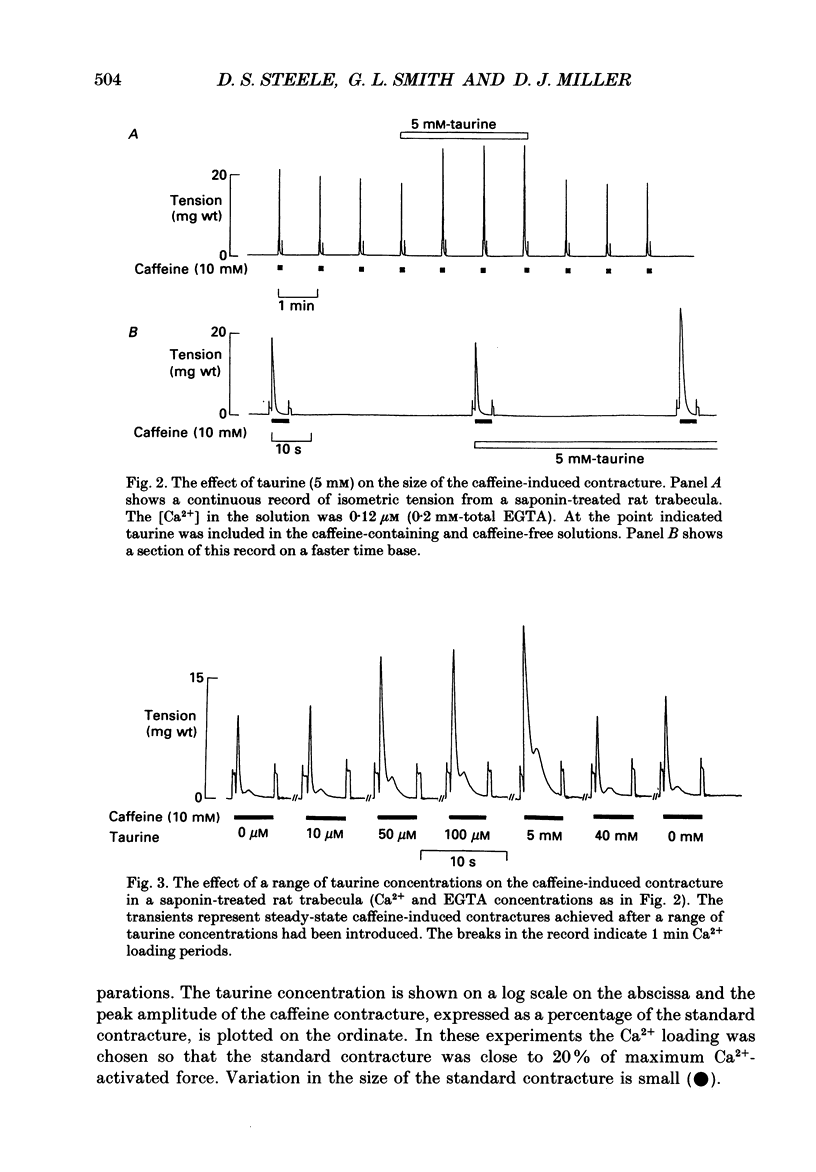
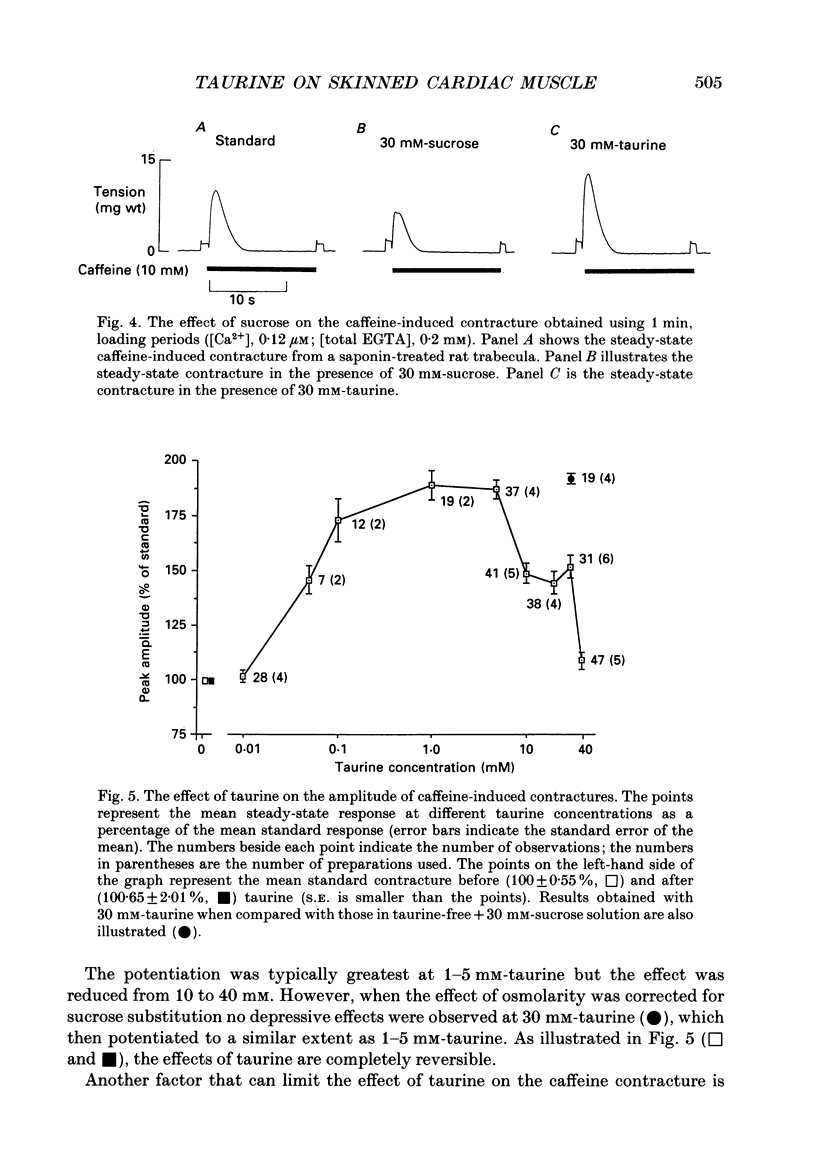
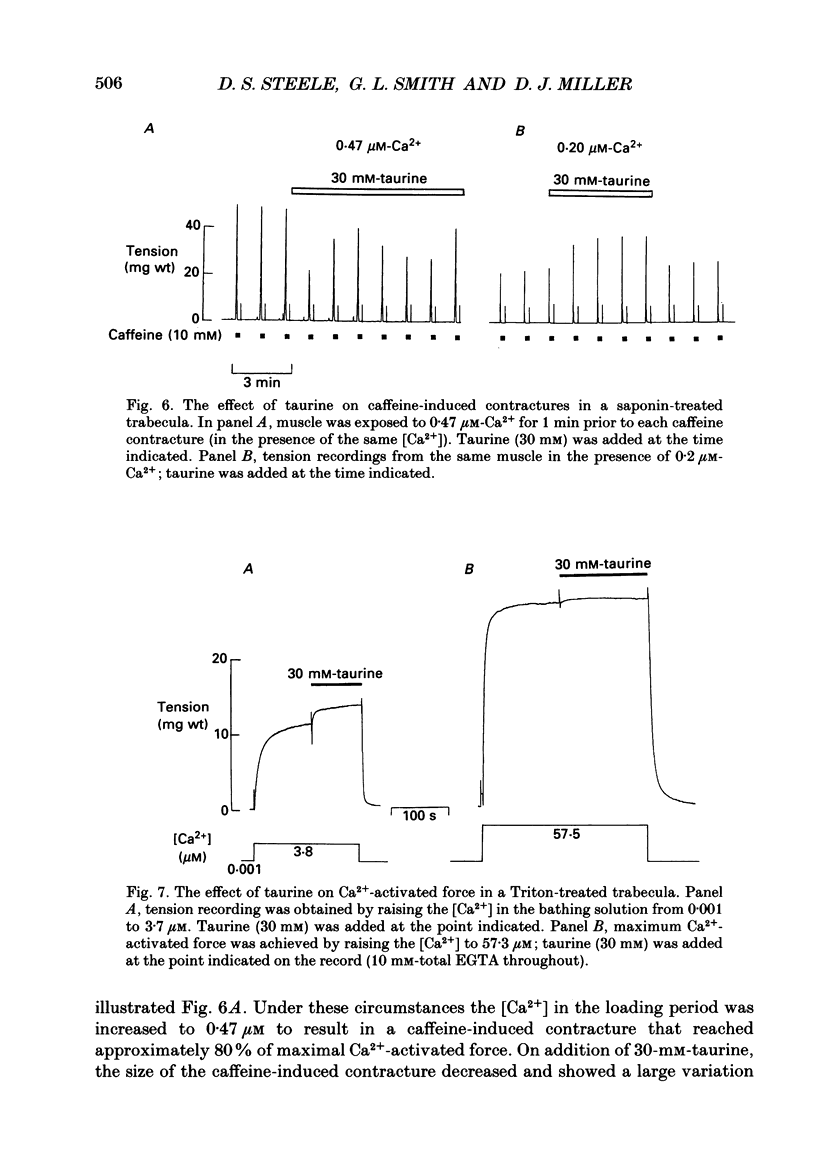
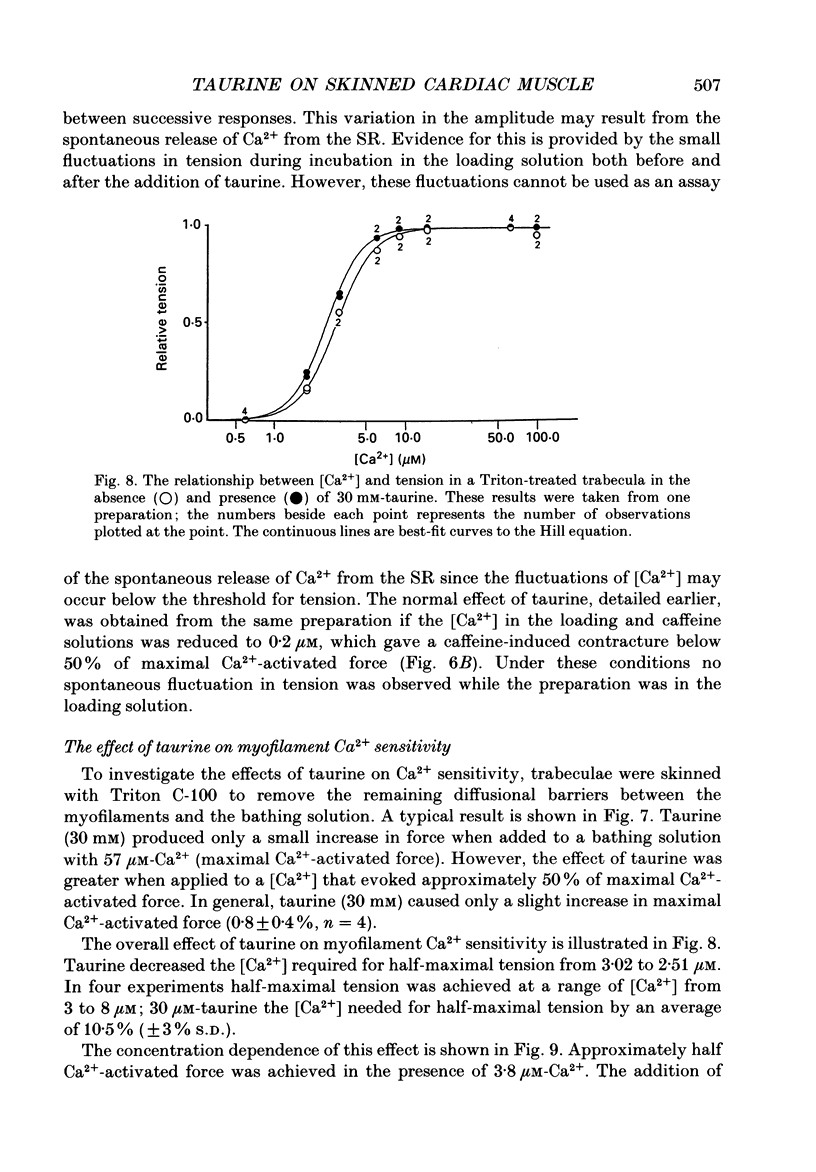
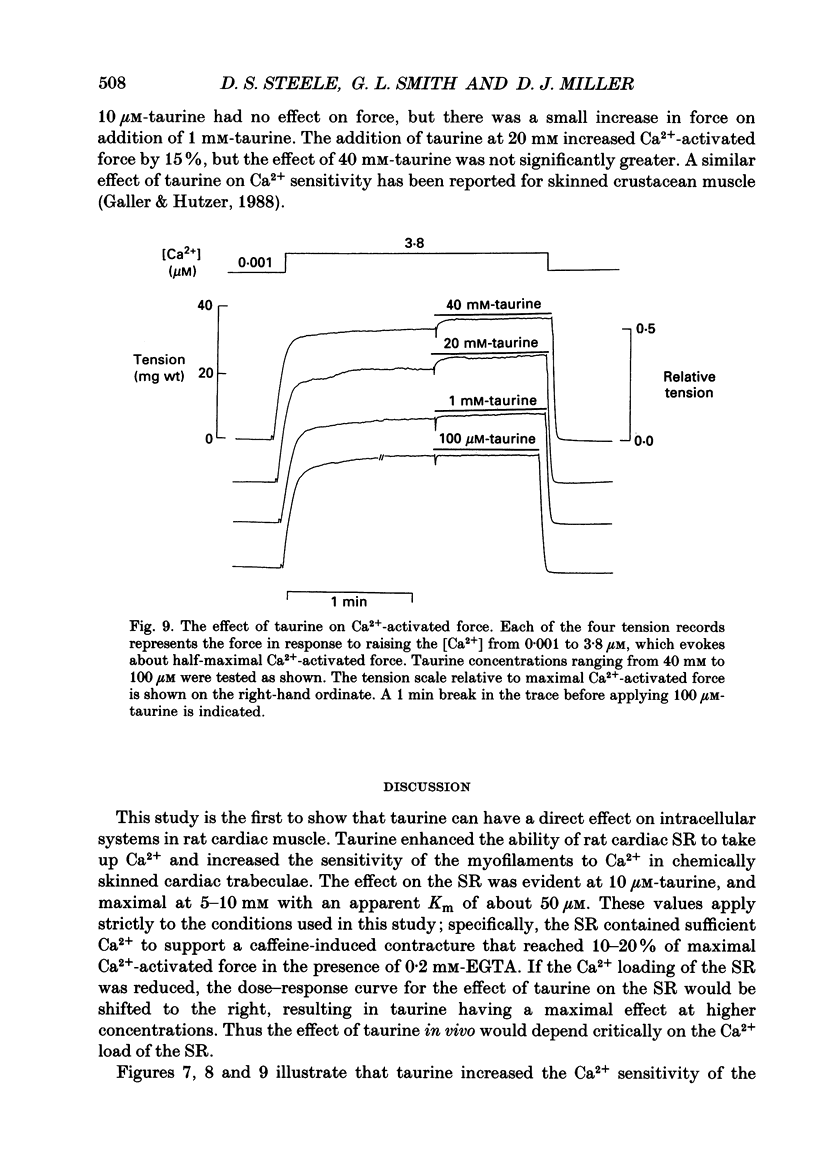
1. Caffeine (10 mM) induced a transient contracture in saponin-treated cardiac trabeculae as a result of Ca2+ release from the sarcoplasmic reticulum (SR). Regular cycles of uptake and release were repeated to stabilize responses. The SR accumulated Ca2+ during the period prior to the addition of caffeine and this was reflected in the size of the caffeine contracture. Increasing the time for Ca2+ loading between successive caffeine exposures resulted in an increase in the amplitude of the contracture. Similarly, the size of the contracture was a function of the calcium ion concentration [( Ca2+]) in the preceding loading period. 2. Taurine (microM-40-mM), when included in both loading and caffeine solutions, markedly potentiated the caffeine-induced contracture. The effect occurs even if taurine was included only in the loading solutions. The potentiating effect was ascribed to a direct action of taurine on the SR, since taurine did not significantly change the [Ca2+] in the loading solutions. 3. The maximal effect of taurine was produced at approximately 5 mM; higher taurine concentrations caused a lesser potentiation of the caffeine contracture. If the solutions were balanced with respect to osmolarity the effect of taurine did not decline at high concentrations. 4. If the [Ca2+] in the loading solutions was increased to produce a caffeine-induced contracture that peaked close to maximal Ca2(+)-activated force, taurine caused a fall in the size of contracture and a more variable response. This result could be explained by an increase in the spontaneous release of Ca2+ from the SR in the presence of taurine. 5. In Triton-skinned trabeculae, taurine (1 mM-40 mM) increased the Ca2+ sensitivity of the contractile proteins in a dose-dependent manner but had little effect on maximum Ca2(+)-activated force. The increase in Ca2+ sensitivity was small: in a typical experiment 30 mM-taurine reduced the [Ca2+] necessary for half-maximal activation from 3.02 to 2.56 microM, with no significant change in the shape of the relationship.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Eisner D. A., Pirolo J. S., Smith G. L. The relationship between intracellular calcium and contraction in calcium-overloaded ferret papillary muscles. J Physiol. 1985 Jul;364:169–182. doi: 10.1113/jphysiol.1985.sp015737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chovan J. P., Kulakowski E. C., Sheakowski S., Schaffer S. W. Calcium regulation by the low-affinity taurine binding sites of cardiac sarcolemma. Mol Pharmacol. 1980 May;17(3):295–300. [PubMed] [Google Scholar]
- Crass M. F., 3rd, Lombardini J. B. Loss of cardiac muscle taurine after acute left ventricular ischemia. Life Sci. 1977 Oct 1;21(7):951–958. doi: 10.1016/0024-3205(77)90261-2. [DOI] [PubMed] [Google Scholar]
- Dietrich J., Diacono J. Comparison between ouabain and taurine effects on isolated rat and guinea-pig hearts in low calcium medium. Life Sci I. 1971 May 1;10(9):499–507. doi: 10.1016/0024-3205(71)90212-8. [DOI] [PubMed] [Google Scholar]
- Dolara P., Agresti A., Giotti A., Pasquini G. Effect of taurine on calcium kinetics of guinea-pig heart. Eur J Pharmacol. 1973 Dec;24(3):352–358. doi: 10.1016/0014-2999(73)90162-3. [DOI] [PubMed] [Google Scholar]
- Entman M. L., Bornet E. P., Bressler R. The effect of taurine on cardiac sarcoplasmic reticulum. Life Sci. 1977 Aug 15;21(4):543–549. doi: 10.1016/0024-3205(77)90094-7. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Franconi F., Martini F., Stendardi I., Matucci R., Zilletti L., Giotti A. Effect of taurine on calcium levels and contractility in guinea-pig ventricular strips. Biochem Pharmacol. 1982 Oct 15;31(20):3181–3185. doi: 10.1016/0006-2952(82)90547-0. [DOI] [PubMed] [Google Scholar]
- Harrison S. M., Lamont C., Miller D. J. Hysteresis and the length dependence of calcium sensitivity in chemically skinned rat cardiac muscle. J Physiol. 1988 Jul;401:115–143. doi: 10.1113/jphysiol.1988.sp017154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable R., Bressler R. Effect of taurine on a muscle intracellular membrane. Biochim Biophys Acta. 1973 Nov 16;323(4):573–583. doi: 10.1016/0005-2736(73)90165-x. [DOI] [PubMed] [Google Scholar]
- Jacobsen J. G., Smith L. H. Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev. 1968 Apr;48(2):424–511. doi: 10.1152/physrev.1968.48.2.424. [DOI] [PubMed] [Google Scholar]
- Kentish J. C. The inhibitory effects of monovalent ions on force development in detergent-skinned ventricular muscle from guinea-pig. J Physiol. 1984 Jul;352:353–374. doi: 10.1113/jphysiol.1984.sp015296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer S. W., Chovan J. P., Werkman R. F. Dissociation of cAMP changes and myocardial contractility in taurine perfused rat heart. Biochem Biophys Res Commun. 1978 Mar 15;81(1):248–253. doi: 10.1016/0006-291x(78)91657-1. [DOI] [PubMed] [Google Scholar]



