Abstract
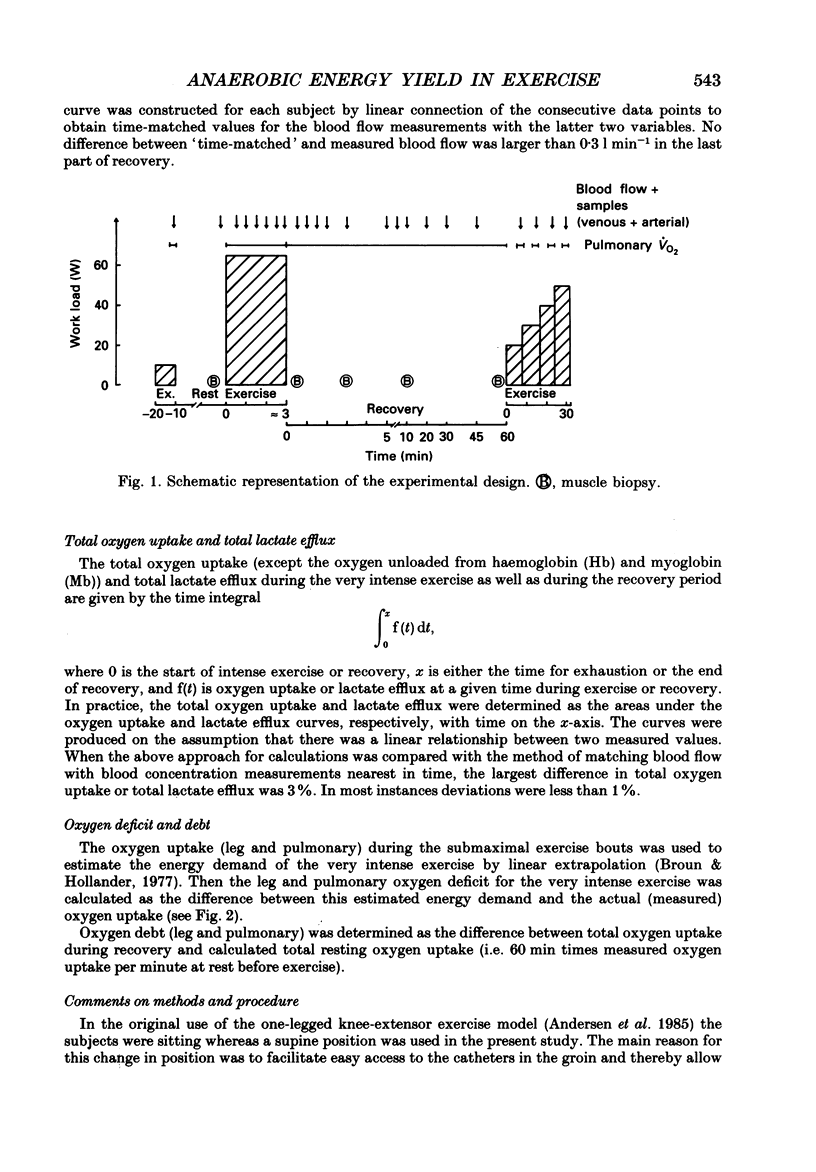
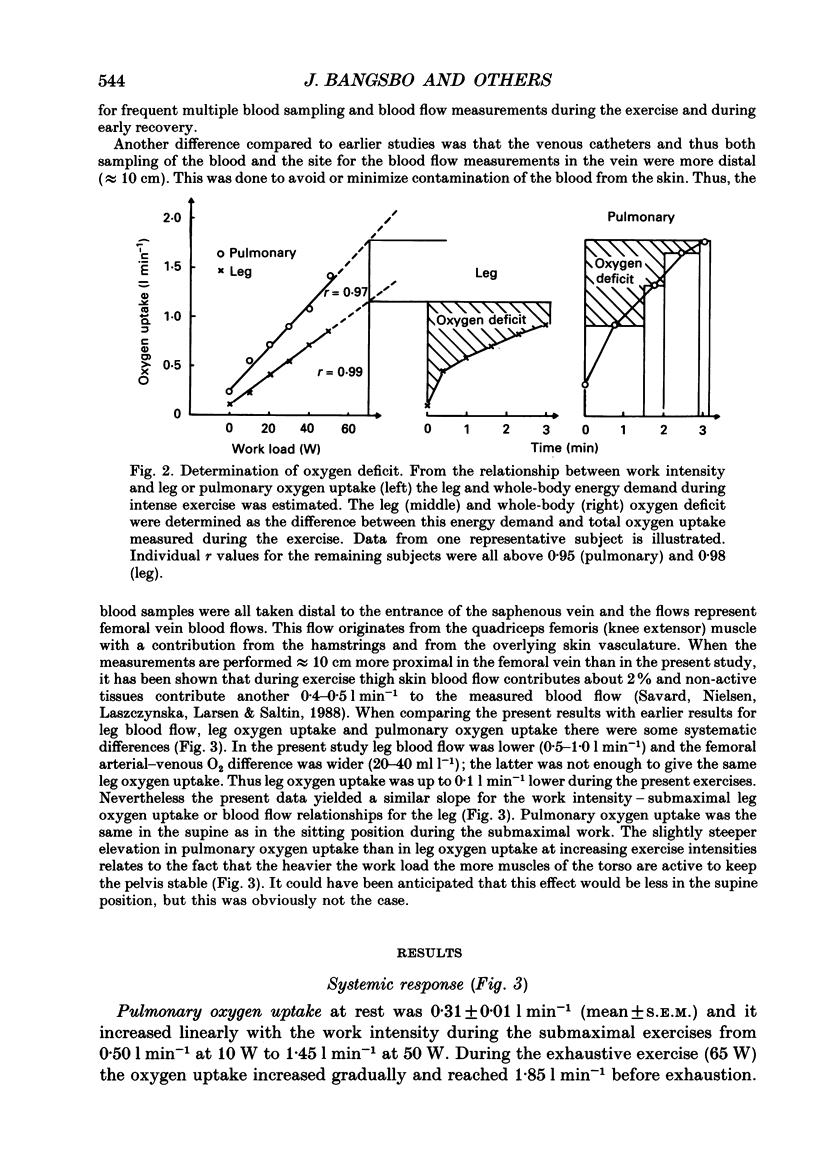
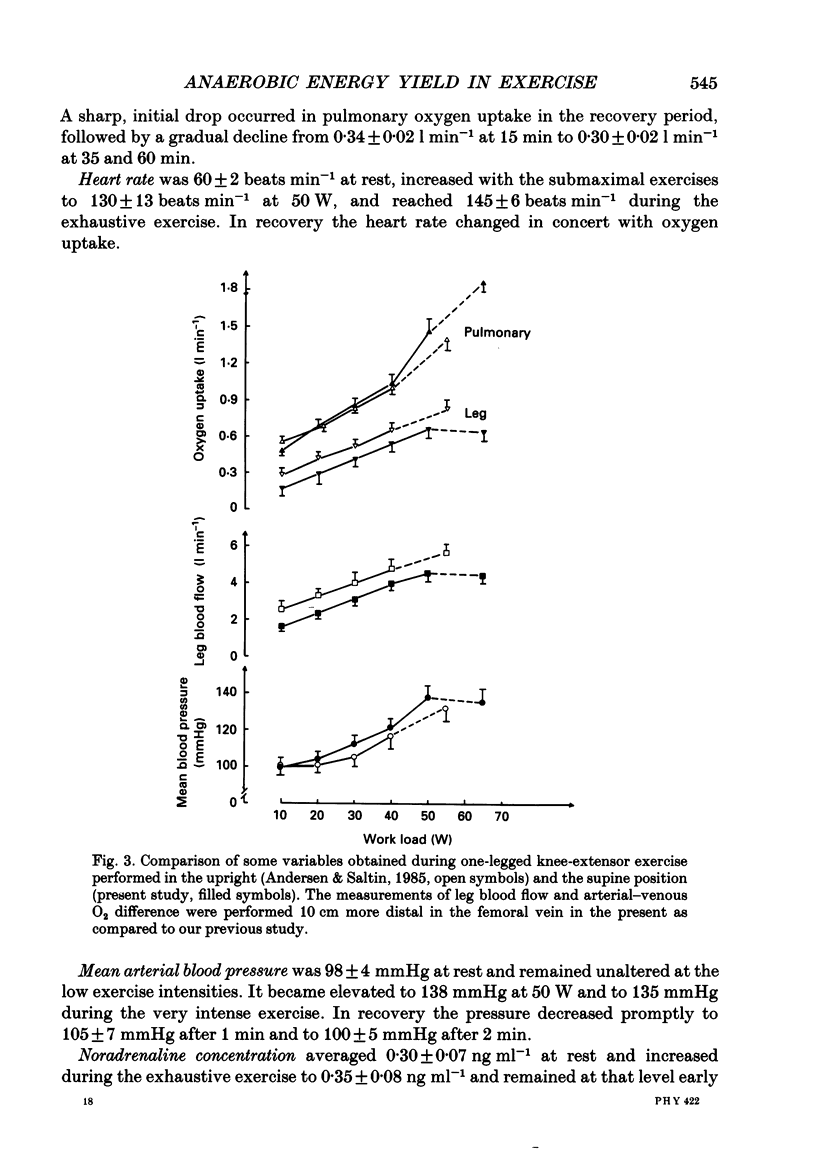
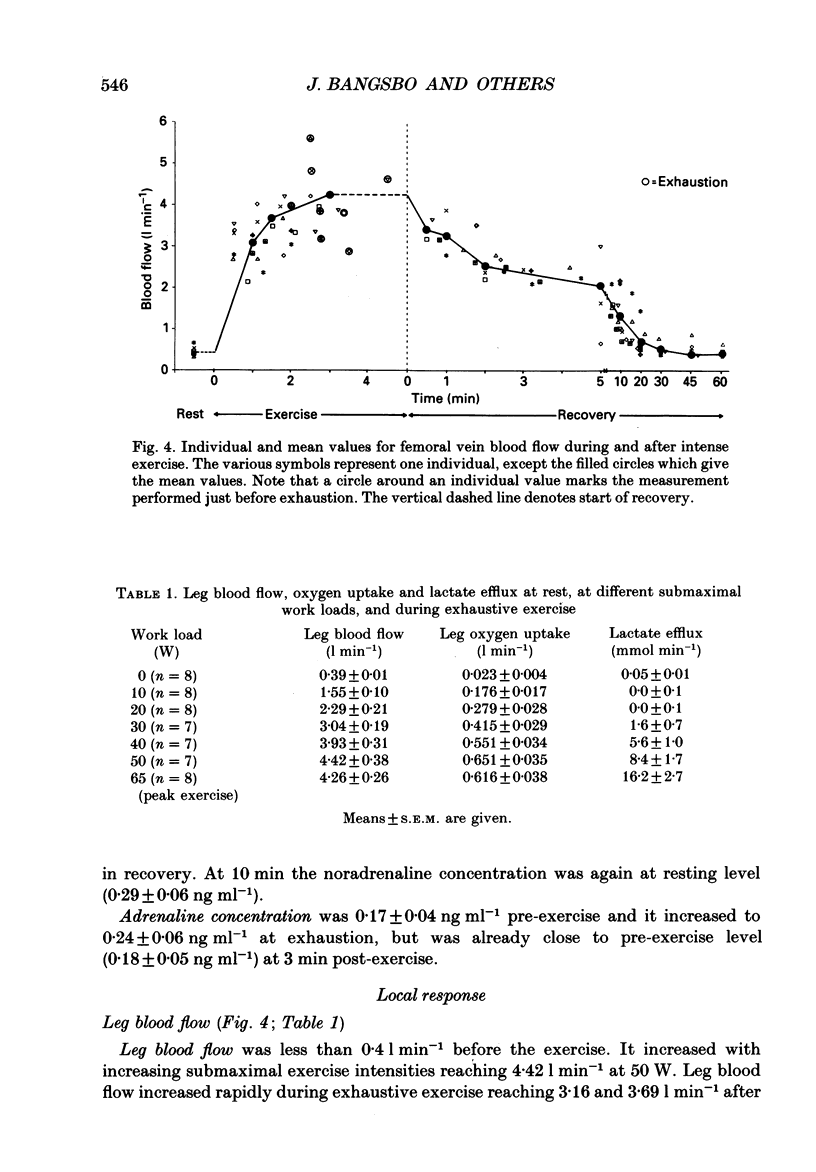
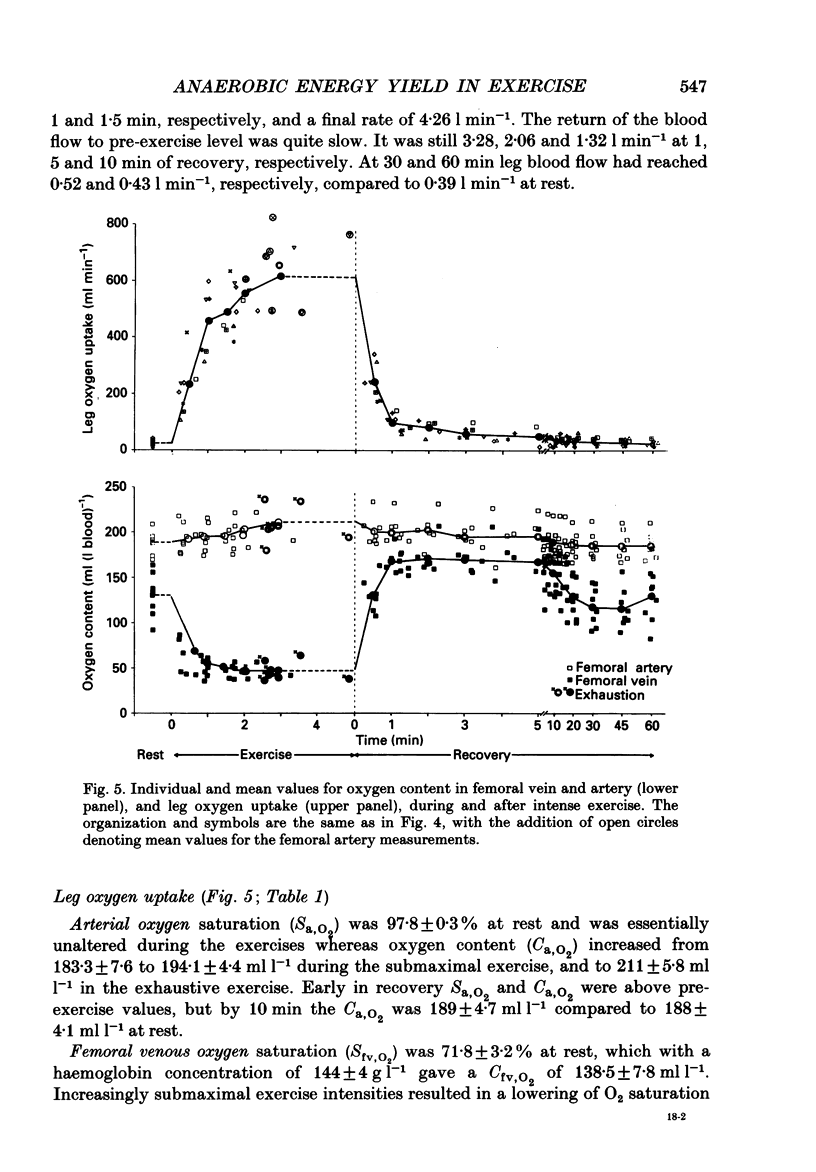
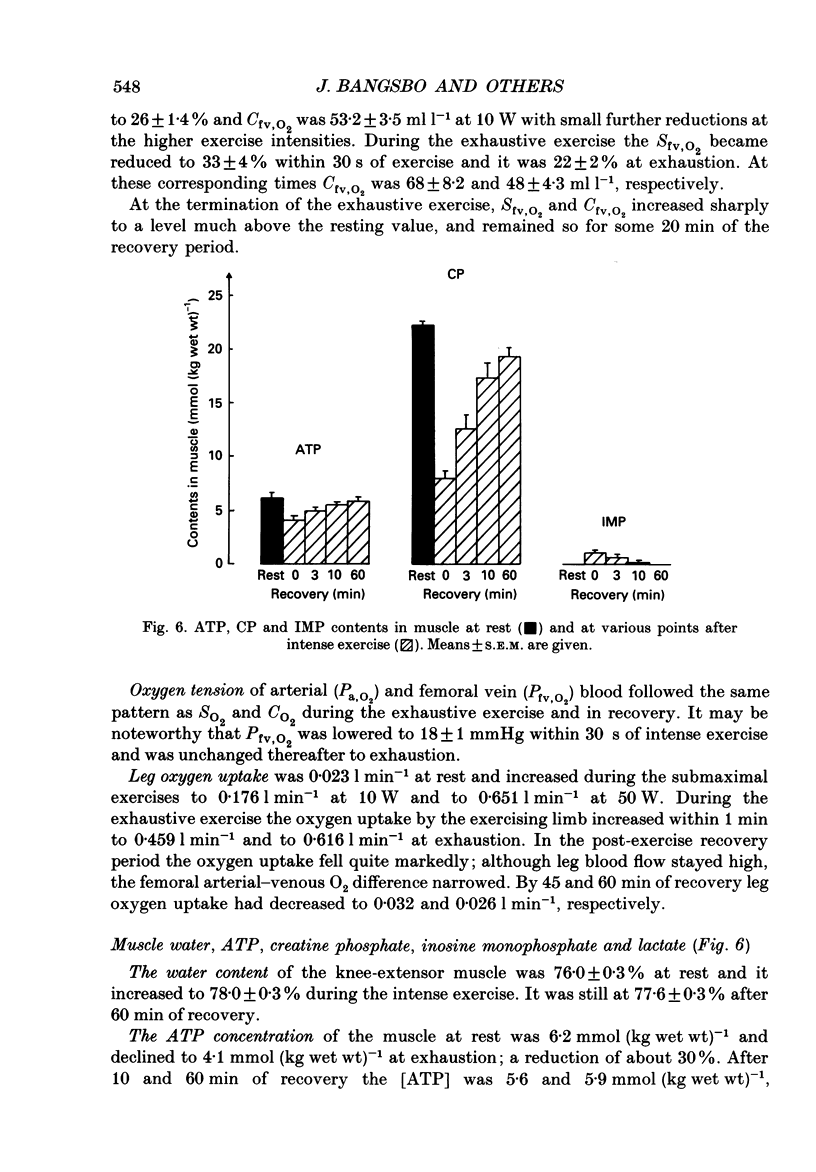
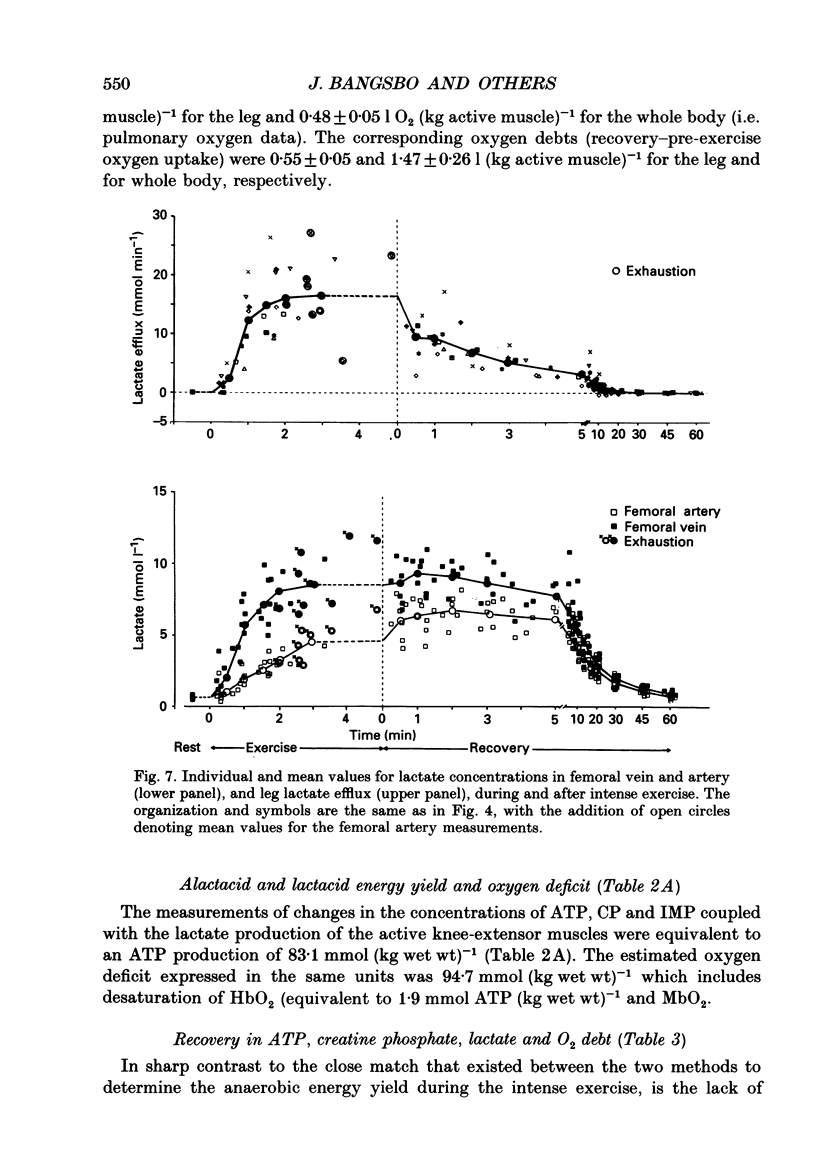
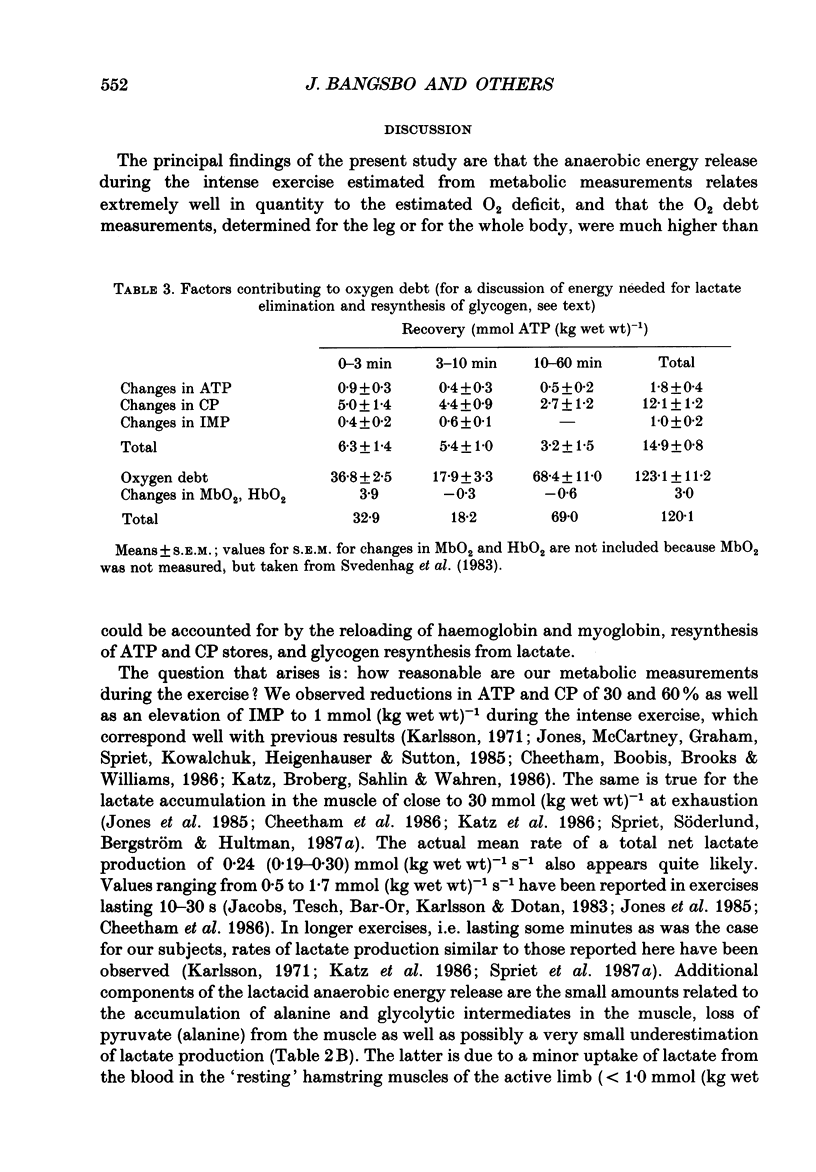
1. Eight subjects performed one-legged, dynamic, knee-extensor exercise, first at 10 W followed by 10 min rest, then at an intense, exhaustive exercise load (65 W) lasting 3.2 min. After 60 min recovery, exercise was performed for 8-10 min each at 20, 30, 40 and 50 W. Measurements of pulmonary oxygen uptake, heart rate, blood pressure, leg blood flow, and femoral arterial-venous differences of oxygen content and lactate were performed as well as determination of ATP, creatine phosphate (CP) inosine monophosphate (IMP) and lactate concentrations on biopsy material from the quadriceps muscle before and immediately after the intense exercise, and at 3, 10 and 60 min into recovery. 2. Individual linear relations (r = 0.95-1.00) between the power outputs for submaximal exercise and oxygen uptakes (leg and pulmonary) were used to estimate the energy demand during intense exercise. Pulmonary and leg oxygen deficits determined as the difference between energy demand and oxygen uptake were 0.46 and 0.48 l (kg active muscle)-1, respectively. Limb and pulmonary oxygen debts (oxygen uptake during 60 min of recovery - pre-exercise oxygen uptake) were 0.55 and 1.65 l (kg active muscle)-1, respectively. 3. During the intense exercise, muscle [ATP] decreased by 30% and [CP] by 60% from resting concentrations of 6.2 and 22.4 mmol (kg wet wt)-1, respectively, and [IMP] increased to 1.1 mmol (kg wet wt)-1. Muscle [lactate] increased from 2 to 28.1 mmol (kg wet wt)-1, and the concomitant net lactate release was 14.8 mmol (kg wet wt)-1 or about 1/3 of the total net lactate production. During recovery 70% of the accumulated lactate was released to the blood, and the nucleotides and CP returned to about 40 and 85% of pre-exercise values at 3 and 10 min of recovery, respectively. 4. Total reduction in ATP and CP (and elevation of IMP) during the intense exercise amounted to 16.4 mmol ATP (kg wet wt)-1, which together with the lactate production accounted for 83.1 mmol ATP (kg wet wt)-1. In addition 6-8 mmol ATP (kg wet wt)-1 are made available related to accumulation of glycolytic intermediates including pyruvate (and alanine). Estimated leg oxygen deficit corresponded to an ATP production of 94.7 mmol ATP kg-1; this value included 3.1 mmol kg-1 related to unloading of HbO2 and MbO2.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTRAND P. O., SALTIN B. Oxygen uptake during the first minutes of heavy muscular exercise. J Appl Physiol. 1961 Nov;16:971–976. doi: 10.1152/jappl.1961.16.6.971. [DOI] [PubMed] [Google Scholar]
- Andersen P., Adams R. P., Sjøgaard G., Thorboe A., Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol (1985) 1985 Nov;59(5):1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Andersen P., Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985 Sep;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr R., Ingnes I., Vaage O., Sejersted O. M., Newsholme E. A. Effect of duration of exercise on excess postexercise O2 consumption. J Appl Physiol (1985) 1987 Feb;62(2):485–490. doi: 10.1152/jappl.1987.62.2.485. [DOI] [PubMed] [Google Scholar]
- Brooks G. A. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc. 1985 Feb;17(1):22–34. [PubMed] [Google Scholar]
- Cheetham M. E., Boobis L. H., Brooks S., Williams C. Human muscle metabolism during sprint running. J Appl Physiol (1985) 1986 Jul;61(1):54–60. doi: 10.1152/jappl.1986.61.1.54. [DOI] [PubMed] [Google Scholar]
- Christensen N. J., Vestergaard P., Sørensen T., Rafaelsen O. J. Cerebrospinal fluid adrenaline and noradrenaline in depressed patients. Acta Psychiatr Scand. 1980 Feb;61(2):178–182. doi: 10.1111/j.1600-0447.1980.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Cooke R., Franks K., Luciani G. B., Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol. 1988 Jan;395:77–97. doi: 10.1113/jphysiol.1988.sp016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs I., Tesch P. A., Bar-Or O., Karlsson J., Dotan R. Lactate in human skeletal muscle after 10 and 30 s of supramaximal exercise. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):365–367. doi: 10.1152/jappl.1983.55.2.365. [DOI] [PubMed] [Google Scholar]
- Jones N. L., McCartney N., Graham T., Spriet L. L., Kowalchuk J. M., Heigenhauser G. J., Sutton J. R. Muscle performance and metabolism in maximal isokinetic cycling at slow and fast speeds. J Appl Physiol (1985) 1985 Jul;59(1):132–136. doi: 10.1152/jappl.1985.59.1.132. [DOI] [PubMed] [Google Scholar]
- Jorfeldt L., Juhlin-Dannfelt A., Karlsson J. Lactate release in relation to tissue lactate in human skeletal muscle during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1978 Mar;44(3):350–352. doi: 10.1152/jappl.1978.44.3.350. [DOI] [PubMed] [Google Scholar]
- KNUTTGEN H. G. Oxygen debt, lactate, pyruvate, and excess lactate after muscular work. J Appl Physiol. 1962 Jul;17:639–644. doi: 10.1152/jappl.1962.17.4.639. [DOI] [PubMed] [Google Scholar]
- Karlsson J. Lactate and phosphagen concentrations in working muscle of man with special reference to oxygen deficit at the onset of work. Acta Physiol Scand Suppl. 1971;358:1–72. [PubMed] [Google Scholar]
- Karlsson J., Saltin B. Lactate, ATP, and CP in working muscles during exhaustive exercise in man. J Appl Physiol. 1970 Nov;29(5):596–602. doi: 10.1152/jappl.1970.29.5.598. [DOI] [PubMed] [Google Scholar]
- Katz A., Broberg S., Sahlin K., Wahren J. Muscle ammonia and amino acid metabolism during dynamic exercise in man. Clin Physiol. 1986 Aug;6(4):365–379. doi: 10.1111/j.1475-097x.1986.tb00242.x. [DOI] [PubMed] [Google Scholar]
- Kawai M., Güth K., Winnikes K., Haist C., Rüegg J. C. The effect of inorganic phosphate on the ATP hydrolysis rate and the tension transients in chemically skinned rabbit psoas fibers. Pflugers Arch. 1987 Jan;408(1):1–9. doi: 10.1007/BF00581833. [DOI] [PubMed] [Google Scholar]
- Krogh A., Lindhard J. The changes in respiration at the transition from work to rest. J Physiol. 1920 May 18;53(6):431–439. doi: 10.1113/jphysiol.1920.sp001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J. Patterns in mammalian muscle energetics. J Exp Biol. 1985 Mar;115:165–177. doi: 10.1242/jeb.115.1.165. [DOI] [PubMed] [Google Scholar]
- Linnarsson D., Karlsson J., Fagraeus L., Saltin B. Muscle metabolites and oxygen deficit with exercise in hypoxia and hyperoxia. J Appl Physiol. 1974 Apr;36(4):399–402. doi: 10.1152/jappl.1974.36.4.399. [DOI] [PubMed] [Google Scholar]
- MARGARIA R., CERRETELLI P., DIPRAMPERO P. E., MASSARI C., TORELLI G. Kinetics and mechanism of oxygen debt contraction in man. J Appl Physiol. 1963 Mar;18:371–377. doi: 10.1152/jappl.1963.18.2.371. [DOI] [PubMed] [Google Scholar]
- Maehlum S., Grandmontagne M., Newsholme E. A., Sejersted O. M. Magnitude and duration of excess postexercise oxygen consumption in healthy young subjects. Metabolism. 1986 May;35(5):425–429. doi: 10.1016/0026-0495(86)90132-0. [DOI] [PubMed] [Google Scholar]
- Medbø J. I., Mohn A. C., Tabata I., Bahr R., Vaage O., Sejersted O. M. Anaerobic capacity determined by maximal accumulated O2 deficit. J Appl Physiol (1985) 1988 Jan;64(1):50–60. doi: 10.1152/jappl.1988.64.1.50. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Mikines K. J., Galbo H., Kiens B. Effect of exercise on insulin action in human skeletal muscle. J Appl Physiol (1985) 1989 Feb;66(2):876–885. doi: 10.1152/jappl.1989.66.2.876. [DOI] [PubMed] [Google Scholar]
- Rose R. J., Hodgson D. R., Kelso T. B., McCutcheon L. J., Reid T. A., Bayly W. M., Gollnick P. D. Maximum O2 uptake, O2 debt and deficit, and muscle metabolites in Thoroughbred horses. J Appl Physiol (1985) 1988 Feb;64(2):781–788. doi: 10.1152/jappl.1988.64.2.781. [DOI] [PubMed] [Google Scholar]
- Roth D. A., Stanley W. C., Brooks G. A. Induced lactacidemia does not affect postexercise O2 consumption. J Appl Physiol (1985) 1988 Sep;65(3):1045–1049. doi: 10.1152/jappl.1988.65.3.1045. [DOI] [PubMed] [Google Scholar]
- Saltin B., Gagge A. P., Bergh U., Stolwijk J. A. Body temperatures and sweating during exhaustive exercise. J Appl Physiol. 1972 May;32(5):635–643. doi: 10.1152/jappl.1972.32.5.635. [DOI] [PubMed] [Google Scholar]
- Savard G. K., Nielsen B., Laszczynska J., Larsen B. E., Saltin B. Muscle blood flow is not reduced in humans during moderate exercise and heat stress. J Appl Physiol (1985) 1988 Feb;64(2):649–657. doi: 10.1152/jappl.1988.64.2.649. [DOI] [PubMed] [Google Scholar]
- Spriet L. L., Söderlund K., Bergström M., Hultman E. Anaerobic energy release in skeletal muscle during electrical stimulation in men. J Appl Physiol (1985) 1987 Feb;62(2):611–615. doi: 10.1152/jappl.1987.62.2.611. [DOI] [PubMed] [Google Scholar]
- Spriet L. L., Söderlund K., Bergström M., Hultman E. Skeletal muscle glycogenolysis, glycolysis, and pH during electrical stimulation in men. J Appl Physiol (1985) 1987 Feb;62(2):616–621. doi: 10.1152/jappl.1987.62.2.616. [DOI] [PubMed] [Google Scholar]
- Svedenhag J., Henriksson J., Sylvén C. Dissociation of training effects on skeletal muscle mitochondrial enzymes and myoglobin in man. Acta Physiol Scand. 1983 Feb;117(2):213–218. doi: 10.1111/j.1748-1716.1983.tb07199.x. [DOI] [PubMed] [Google Scholar]
- Walløe L., Wesche J. Time course and magnitude of blood flow changes in the human quadriceps muscles during and following rhythmic exercise. J Physiol. 1988 Nov;405:257–273. doi: 10.1113/jphysiol.1988.sp017332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Prampero P. E., Boutellier U., Marguerat A. Efficiency of work performance and contraction velocity in isotonic tetani of frog sartorius. Pflugers Arch. 1988 Oct;412(5):455–461. doi: 10.1007/BF00582533. [DOI] [PubMed] [Google Scholar]
- di Prampero P. E. Energetics of muscular exercise. Rev Physiol Biochem Pharmacol. 1981;89:143–222. doi: 10.1007/BFb0035266. [DOI] [PubMed] [Google Scholar]


