Abstract
The corepressor mSin3A is the core component of a chromatin-modifying complex that is recruited by multiple gene-specific transcriptional repressors. In order to understand the role of mSin3A during development, we generated constitutive germ line as well as conditional msin3A deletions. msin3A deletion in the developing mouse embryo results in lethality at the postimplantation stage, demonstrating that it is an essential gene. Blastocysts derived from preimplantation msin3A null embryos and mouse embryo fibroblasts (MEFs) lacking msin3A display a significant reduction in cell division. msin3A null MEFs also show mislocalization of the heterochromatin protein, HP1α, without alterations in global histone acetylation. Heterozygous msin3A+/− mice with a systemic twofold decrease in mSin3A protein develop splenomegaly as well as kidney disease indicative of a disruption of lymphocyte homeostasis. Conditional deletion of msin3A from developing T cells results in reduced thymic cellularity and a fivefold decrease in the number of cytotoxic (CD8) T cells, while helper (CD4) T cells are unaffected. We show that CD8 development is dependent on mSin3A at a step downstream of T-cell receptor signaling and that loss of mSin3A specifically decreases survival of double-positive and CD8 T cells. Thus, msin3A is a pleiotropic gene which, in addition to its role in cell cycle progression, is required for the development and homeostasis of cells in the lymphoid lineage.
mSin3A functions as a platform that mediates the association of histone deacetylase (HDAC) enzymes and other chromatin-modifying activities with a large number of DNA-binding transcriptional repressors (for reviews, see references 4 and 29). In order to interact with a wide range of repressors, Sin3 proteins from yeast to mammals have evolved and maintained four imperfect repeats known as paired amphipathic helix (PAH) domains (6, 49, 63), each of which appear to possess innate specificity for recruiting a defined repertoire of transcriptional repressors or corepressors. Recent structural studies have begun to define the basis for specific interactions between the second PAH domain of mSin3A (PAH2) and the transcriptional repressors Mad1 and HBP1 (8, 11, 19, 57, 58). The association of Sin3 with sequence-specific transcription factors serves to bring the complex in the vicinity of the transcription factor target genes, thereby permitting the complex to modulate transcriptional output by altering chromatin structure.
Sin3 is thought to predominantly influence chromatin structure by mediating histone deacetylation through its recruitment of class I HDACs (HDAC1 and HDAC2) (3, 21, 22, 34, 41). Another Sin3 complex protein, SDS3, acts as a bridging protein between the HDACs and the Sin3 HDAC interaction domain (HID), a highly conserved region lying between PAH3 and PAH4 (2, 13, 15, 16, 36). The RbAp46 and RbAp48 proteins also associate with HDACs in the Sin3 complex and appear to target HDAC activity to histone tails. Other components of the complex may serve to couple Sin3 to specific repression systems. For example, SAP30 is involved in repression by antagonist-bound nuclear hormone receptors (33) and mediates binding to RbAp-HDAC1 (73), the Rb docking protein RBP1 (35), and the negative growth regulator p33ING1b (31).
While HDAC activity is clearly central to Sin3 repressive function, other proteins with the potential to modify chromatin have also been reported to be present in Sin3 complexes. These include histone methyltransferases (42, 68) as well as an O-linked N-acetylglucosamine transferase (69), both of which could in principle contribute additional repressive modifications to deacetylated histone tails. Furthermore, components of the Swi/Snf nucleosome remodeling complex have been detected in a subset of mSin3 complexes (31, 44, 54). Because a number of Sin3 complex components are apparently present at substoichiometric levels (54, 69, 72, 73), the composition of intracellular Sin3 complexes is likely to be heterogeneous, suggesting that specialized subsets of complexes may be directed towards specific repression functions.
In mice two distinct Sin3 genes, msin3A and msin3B, encode the mSin3A and mSin3B paralogs, respectively (6). Mammalian Sin3A and Sin3B proteins are approximately 57% identical throughout the length of their polypeptide chains, with the highest degree of homology localized in the PAH and HID regions (6). Both proteins have been found to be widely expressed (frequently within the same cells and tissues) and to bind many of the same transcriptional repressors and complex components (references 6, 8, and 57 and unpublished data). Therefore Sin3A and Sin3B have been generally assumed to perform redundant functions.
Biological roles of Sin3 complexes.
Given that Sin3 complexes are recruited by a wide range of transcription factors, it is not surprising that Sin3 functions have been linked to a large number of cellular processes. For example, in Saccharomyces cerevisiae Sin3 has been shown to play roles in the unfolded protein response (51), rRNA processing (39), and the response to DNA damage (26, 52). In Drosophila melanogaster, small interfering RNA knockdown of Sin3 compromises G2 progression without causing global changes in chromatin acetylation (46). Expression array analysis of Drosophila Sin3 knockdown cells indicated that dSin3 regulates cell cycle genes as well as many genes involved in mitochondrial genesis and function (47). Sin3 in mammalian cells has been implicated in the maintenance of pericentric heterochromatin and binding by HP1α (67). Furthermore, specific cellular functions related to proliferation arrest and differentiation mediated by regulators such as Rb and p130 (35, 48), p53 (31, 40, 74), and the Mad protein family (5, 27, 49, 50) have been linked to their ability to interact with Sin3 complexes.
Reports of new Sin3-interacting transcription factors and Sin3/HDAC complex components appear regularly (e.g., references 16, 58, 70, and 71), suggesting that the range of cellular functions involving Sin3 is likely to be even larger than those mentioned above. Nonetheless, it is apparent that many of the functions of Sin3 appear to converge on regulation of gene expression coupled to proliferation arrest and differentiation. In order to understand in more detail the role of Sin3 in vivo, we have generated mice with a constitutive or conditional disruption of the msin3A gene. We have employed the constitutive deletion to analyze the general effects of msin3A loss of function on murine development. We have found that msin3A is an essential gene and that heterozygous mice develop splenomegaly and glomerulopathy due to alterations in lymphoid homeostasis. Furthermore, because T-lymphoid cell development represents a compartment in which chromatin modification and remodeling (18, 30) play crucial roles, we have generated a conditional msin3A deletion in T cells and have found specific effects on T-cell proliferation, differentiation, and homeostasis.
MATERIALS AND METHODS
Targeting construct, colony generation, and genotyping.
A genomic DNA fragment containing the msin3A gene was isolated from a murine 129/Sv genomic library (a kind gift from P. Soriano, Fred Hutchinson Cancer Research Center [FHCRC]) using the msin3A cDNA (6) as a probe. The resulting clones were confirmed by sequencing. The mSin3ALoxP targeting vector was constructed as follows: a 2.76-kb EcoRI fragment, containing exon 3 of the msin3A gene, was used to form the long arm of the targeting vector. An upstream LoxP site and additional EcoRI and HindIII restriction sites were inserted as a double-stranded oligonucleotide into a unique XbaI site. The short arm (3′ end) of the vector was generated by PCR and cloned into the HindIII site of the plasmid. The final targeting vector, mSin3ALoxP, was sequenced in full to ensure the absence of point mutations. We screened clones, initially by PCR and then by Southern blotting analysis, using EcoRI and the region outlined in Fig. 1C as a probe to identify correctly targeted recombinants. The Neor cassette was removed by transient expression of Cre followed by PCR and Southern analysis. Correctly targeted embryonic stem (ES) cell clones were injected into blastocysts to generate chimeric mice, which were subsequently bred onto a C57BL/6J strain to produce germ line-targeted (msin3AL/+) animals. msin3AL/+ mice were initially intercrossed with a Mox2-Cre germ line deleter strain (61) and then backcrossed to produce msin3A+/− heterozygotes. Mice were routinely genotyped by multiplex PCR. Primer sequences used were as follows: SinFor, GATGTTTGCACATAAGTGTGG; SinRev, TTCAGCTTAACAATAGGAGAC; SinMut, ACAATTATGTTAATTTGAGAC. A PCR with all three primers was used to distinguish wild-type alleles (497-bp) and alleles with exon 3 deleted (583-bp). Wild-type (493-bp) and LoxP-targeted (545-bp) alleles can be distinguished using only SinFor and SinRev primers. PCR conditions were 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 90 s.
FIG. 1.
(A) The msin3A gene present on mouse chromosome 9 consists of 22 exons, with exon 2 containing the initiator ATG and first 63 amino acids. The msin3A locus is flanked by the mannohydrolase gene, man2c1, and the tyrosine phosphatase gene, ptpn9. (B) Targeting strategy and mutant alleles of msin3A. The Neor cassette and exon 2, the region to be deleted, were flanked by LoxP sites (triangles). The endogenous EcoRI sites plus one synthetic site, engineered into the 5′ LoxP element, used for Southern analysis are also depicted. The probe used for Southern blot analysis is shown. (C) Southern analysis of ES cell clones (digested with EcoRI; left panel) showing targeting of the msin3A locus (msin3ANeo), removal of the Neor cassette by transient expression of Cre (msin3AL), and the allele with exon 2 deleted (msin3A−). (Right panel) Genomic PCR analysis was also used to confirm the presence of the wild-type (1.6-kb) or deleted (0.6-kb) alleles.
Cellular analysis.
Mouse embryo fibroblasts (MEFs) were prepared from wild-type, msin3AL/+, and msin3AL/L embryonic day 14.5 (E14.5) embryos essentially as described previously (75), genotyped by PCR, and routinely cultured in Dulbecco's modified Eagle's medium plus 10% fetal calf serum, 50 mM β-mercaptoethanol, penicillin, and streptomycin. The deleted allele was reconstituted by infecting early-passage MEFs with an murine stem cell virus retrovirus expressing both Cre and green fluorescent protein (GFP) using standard protocols. Infected cells were isolated by sorting for GFP-positive populations using a Vantage cell sorter (Becton Dickinson). To perform growth curve analysis of Cre/GFP MEFs, 15,000 cells were plated into a 24-well plate, 3 days after viral infection, and were harvested and counted using a hemocytometer on the indicated days. Immortalized MEFs were derived as follows: supernatants containing a large T antigen retrovirus were prepared from Psi 2 packaging cells infected with pZIPNeo SV(X)1 vector (7). The supernatant was then used to infect wild-type and msin3AL/L MEFs by standard protocols followed by G418 selection. Cell cycle analysis of both primary and immortalized MEFs was performed by harvesting cells, 7 days after Cre/GFP infection and staining with Hoechst 33342 (Molecular Probes), followed by analysis using a FACSCalibur (Becton Dickinson) and CellQuest software.
Protein analysis.
Lysates for Western blotting were prepared from equivalent cell numbers as follows: cells were washed twice with phosphate-buffered saline (PBS) before addition of hot 2× protein loading buffer (4% sodium dodecyl sulfate [SDS], 10% [vol/vol] glycerol, 1% [vol/vol] β-mercaptoethanol) and boiling for 5 min, followed by sonication. Cell extracts were resolved using SDS-polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and then probed using standard protocols. All antibody incubations were performed in PBS containing 0.05% Tween 20 (vol/vol) and 2% bovine serum albumin (wt/vol). Standard horseradish peroxidase-conjugated secondary antibodies were used in conjunction with enhanced chemiluminescence reagents for Western analysis unless stated otherwise. Quantitative Western analysis was performed as above, but with Alexa Fluor 680-conjugated anti-rabbit (Molecular Probes) and IRDye 800-conjugated anti-mouse secondary (Rockland) antibodies. Blots probed in this fashion were analyzed using the LI-COR Odyssey linear detection system. Immunofluorescence was performed on MEFs grown in eight-well chamber slides, fixed in 2% paraformaldehyde, permeabilized with 0.5% Triton X-100, and then probed with the indicated antibodies in PBS, 5% normal goat serum, and 0.05% Tween 20. The following antibodies were used in this study: the P1E7 monoclonal was generated using the purified PAH2 domain of mSin3A (S. M. Cowley and R. N. Eisenman, FHCRC); anti-mSin3A (sc-994), anti-HDAC2 (sc-7899), anti-slp76 (sc-9062), anti-LAT (sc-7948), and anti-LCK (sc-13) rabbit polyclonals (Santa Cruz Biotechnology); anti-mSin3B monoclonal (sc-13145; Santa Cruz Biotechnology); antitubulin and antiactin monoclonal (Sigma); anti-mSDS (a gift from Don Ayer, Huntsman Institute, Salt Lake City, UT); anti-Runx3 (a gift from Yoram Groner, Weizmann Institute, Rehovot, Israel); and anti-HP1α (Upstate Biotechnology).
Isolation and cytometric flow analysis of lymphoid cells.
Cells were isolated from bone marrow, spleen, and thymus as described previously (25). Briefly, harvested cells were resuspended in Hanks' balanced salt solution (Gibco) plus 3% fetal calf serum (HyClone), and erythrocytes were then depleted by ammonium chloride lysis. Staining for surface markers using flow cytometry was performed as described previously (25), and 10,000 gated events were collected utilizing a FACSCalibur machine (Becton Dickinson). Data were then analyzed using CellQuest software (Becton Dickinson). Cells were stained with the following antibodies: fluorescein isothiocyanate (FITC)-conjugated anti-CD25 (Pharmingen), anti-CD8 (Caltag), phycoerythrin (PE)-conjugated CD4 (Caltag), anti-CD44 (Pharmingen), biotinylated anti-CD3ɛ (Pharmingen), and anti-heat-stable antigen.
T-cell proliferation assays.
T cells were cultured in RPMI 1640 medium (Gibco-BRL) supplemented with 10% fetal calf serum (HyClone), 2 mM l-glutamine (Gibco), 100 mM nonessential amino acids (Gibco), 100 U/ml penicillin, 100 U/ml streptomycin (Gibco). Cells were stimulated to divide by addition of anti-CD3 and anti-CD28 antibodies. For [3H]thymidine incorporation assays, cells were grown for 48 h following stimulation and then pulsed with [3H]thymidine for 24 h prior to harvesting onto printed glass fiber filtermats using an LKB-Wallac Cell Harvester. Incorporation of [3H]thymidine was measured using an LKBWallac 1205 Betaplate liquid scintillation counter. For cell division experiments, cells were labeled with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) according to the manufacturer's instructions. Cells were harvested after 48 and 96 h poststimulation and then analyzed using a FACSCalibur machine (Becton Dickinson).
RESULTS
Given the widespread expression pattern and putative transcriptional roles of mSin3A, it seemed possible that constitutive msin3A gene disruption would result in early lethality. Therefore, we generated a conditional msin3A allele that can be inactivated using standard Cre recombinase technology. The msin3A gene, present on mouse chromosome 9, consists of 22 exons spanning a region of 52 kb (Fig. 1A). We constructed a vector for targeting ES cells in which loxP recombination sites were introduced 5′ and 3′ to the first coding exon (Fig. 1B, exon 2). Correctly targeted ES cells (msin3ANeo/+) were determined by both PCR and Southern blotting (Fig. 1C). Following the removal the Neor cassette by transient expression of Cre, the resulting ES cell clones, msin3AL/+ (L for loxP), were injected into blastocysts to generate chimeric animals that were crossed onto a C57BL/6 line to generate germ line msin3AL/+ mice. msin3AL/+ mice were further crossed with a germ line deleter strain, Mox2-Cre (MORE) (61) to generate MORE-Sin3AL/+ animals, which were subsequently backcrossed to create germ line heterozygous msin3A+/− animals with a constitutive deletion.
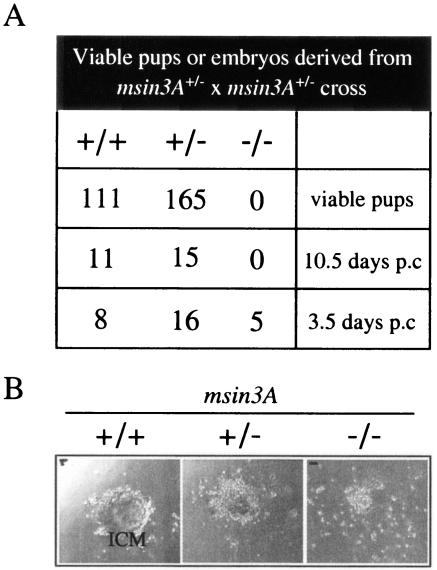
msin3A+/− pups appeared phenotypically normal and developed into fertile adults. Intercrosses between msin3A+/− mice generated both wild-type (msin3A+/+) and heterozygous (msin3A+/−) pups but failed to yield any homozygous null animals (msin3A−/−) (Fig. 2A). The number of msin3A+/− pups isolated was less than would be predicted from a normal Mendelian ratio (Fig. 2A), suggesting that there may be haploinsufficiency associated with the loss of one msin3A allele. Because the lack of viable msin3A−/− pups suggested an embryonic lethal phenotype, we examined embryos generated from the identical cross at different time points during development. None of the embryos genotyped at E10.5 were msin3A−/−. However, we did observe a number of empty decidua which were much reduced in size, suggesting that the absent msin3A−/− embryos had been reabsorbed at a point following implantation. A second, independently generated, msin3A null mouse line also results in the lethality of homozygous null embryos at the peri-implantation stage (J. H. Dannenberg et al., submitted for publication). Consistent with hypothesis that msin3A null embryos are reabsorbed at implantation, we were able to isolate E3.5 embryos with all three genotypes (Fig. 2A and B), which were cultured ex vivo by plating them onto gelatinized tissue culture plates. Although msin3A−/− embryos appeared phenotypically normal in appearance at the point of isolation, cells derived from the inner cell mass of nullizygous blastocysts produced much smaller colonies, suggesting that they fail to proliferate significantly in comparison to heterozygous and wild-type littermate controls (Fig. 2B). Taken together, our data suggest that mSin3A function is essential for embryonic development beyond the implantation stage.
FIG. 2.
Loss of msin3A causes early embryonic lethality. (A) The number and genotypes of pups and embryos generated from msin3A+/− heterozygous intercrosses are shown. (B) Embryos harvested at E3.5 from msin3A+/− heterozygous intercrosses were cultured in vitro on gelatinized tissue culture plates for 7 days. Representative examples of outgrowth cultures from wild-type, heterozygous, and nullizygous embryos are shown. ICM, inner cell mass.
Reduced proliferative potential in MEFs with msin3A deleted.
msin3A−/− embryos die too early in their development to isolate MEFs. However, we were able to generate MEFs from both wild-type and msin3AL/L targeted embryos, in which a deleted allele can be generated by infection with a Cre-expressing retrovirus, resulting in a reduction in expression of full-length mSin3A protein (Fig. 3A). Residual mSin3A observed in L/L cells is likely due to a subpopulation of cells that escaped infection. A comparison of Cre-infected cells revealed that loss of mSin3A causes a reduction in proliferative potential (Fig. 3B). The population doubling time of cultured Cre-infected msin3AL/L cells, beginning 3 days after infection, was increased by approximately twofold relative to Cre-infected msin3A+/+ cells. However, despite a reduction in the number of divisions, the cell cycle profile of CreGFP-msin3AL/L cells remained similar to wild type, with only a ∼7% increase in G2/M-phase cells (Fig. 3C). To circumvent the growth limitations of primary cells, we attempted to derive an msin3AL/L cell line by immortalizing targeted and wild-type MEFs, prior to Cre infection, with large T antigen. However, Cre-mediated deletion of mSin3A in immortalized MEFs resulted in profound apoptosis, as measured by the occurrence of a sub-G1 peak (Fig. 3D). The occurrence of the sub-G1 peak is concurrent with a decrease in the G2/M population, suggesting that post-S-phase cells are undergoing apoptosis, presumably in a p53-independent manner.
FIG. 3.
MEFs lacking mSin3A have a reduced proliferative potential. (A) Western blot analysis of mSin3A levels in GFP-positive MEF extracts 3 days after infection with a Cre/GFP-expressing virus. HDAC2 and actin levels were used to normalize protein extracts. (B) Growth curves of GFP-positive MEFs, 3 days after Cre/GFP infection, were determined by counting cells with a hemocytometer at the time points indicated. (C) Flow cytometric analysis of DNA content was used to determine the cell cycle profiles of wild-type, msin3AL/+, and msin3AL/L MEFs 7 days following Cre/GFP infection. The percentages of cells in each phase of the cell cycle are shown. (D) Wild-type, msin3AL/+, and msin3AL/L cells, prior to Cre infection, were immortalized by infection with large T antigen. These immortalized cells were then analyzed as for panel C, 7 days after Cre/GFP infection. (E) Cre/GFP-infected wild-type and msin3AL/L MEFs were analyzed by immunofluorescence. Specific antiserum was used to determine the localization of HP1α in GFP-positive cells; DAPI was used to stain DNA.
As a potential mechanism for these defects in cell-cycle progression, we analyzed the level and patterns of acetylated histones for changes caused by the loss of mSin3A-associated HDAC activity. The relative amounts of euchromatic markers, such as acetylated Lys9 on histone 3 (H3K9Ac) and Lys12 on histone 4 (H4K12Ac), did not change significantly in bulk histone extracts from primary MEFs with reduced mSin3A (data not shown). Although bulk histone acetylation was unaltered, it was possible that the distribution pattern of acetylated histones in the interphase nuclei of CreGFP-msin3AL/L cells might be affected. mSDS3, a core component of the mSin3A complex, was previously demonstrated to be required for the maintenance of pericentric heterochromatin in its hypoacetylated state (13). However, the distribution and amount of pericentric heterochromatin, identified by areas of intense 4′,6′-diamidino-2-phenylindole (DAPI) staining, did not appear to be altered and were found to lack H3K9Ac and H4K12Ac in both wild-type and msin3AL/L cells infected with Cre (see Fig. S1 in the supplemental material). In contrast, the localization of HP1α, which in wild-type MEFs displays punctate staining predominantly colocalized in pericentric heterochromatin, was frequently dispersed throughout the entire nucleus in CreGFP-msin3AL/L cells (Fig. 3E). No significant change in HP1α protein level was detected (Fig. S1 in the supplemental material). In conclusion, loss of mSin3A in MEFs, while causing little evident change in histone acetylation, leads to a reduction in proliferative potential, increased apoptosis in immortalized cells, and HP1α mislocalization.
A subset of msin3A heterozygous mice develop splenomegaly and membranous glomerulopathy.
Pathological analysis of mature msin3A+/− animals (over 5 months of age) revealed that a quarter of msin3A+/− mice (7 of 28) had spleens of greater than three times the normal size (i.e., >0.3 g), compared to 0 out of 10 wild-type littermates. Indeed, two heterozygous msin3A+/− animals had spleens with a mass of over 0.7 g, equivalent to seven times the average wild-type mass (Fig. 4A). Hematoxylin and eosin (H&E)-stained sections of normal and enlarged spleens revealed that the areas of white pulp, which contain the majority of splenic lymphoid cells, were increased in size in msin3A heterozygotes (Fig. 4B), indicating an increased lymphoid population. Consistent with this increased size, heterozygous mice with splenomegaly (represented by Het2) had four times the number of splenocytes compared to unaffected heterozygotes (represented by Het1) and wild-type controls (Fig. 4C). In contrast, the cellularity of thymus and bone marrow was largely unchanged. To account for this increase in splenic cellularity, we examined the lymphoid cell populations in the spleen. The percentages of B (B220 positive) and T cells (CD3ɛ positive) within the spleens of Het2 mice were comparable to wild-type controls (Fig. 4E). However, the total number of B cells and CD4 T cells were increased fourfold compared to controls, consistent with the overall increase in splenic cellularity (Fig. 4F). In contrast, CD8-positive T cells were present at wild-type levels in the enlarged spleens, indicating that the relative proportion of CD8 cells was sharply reduced. The CD4/CD8 ratio for the Het2 example was about eight- and sixfold greater than for wild-type and Het1 animals, respectively (Fig. 4G). On average, we observed an approximately fourfold increase in the ratio of CD4 versus CD8 cells in Het2 animals compared to Het1 and wild-type mice (Fig. 4H). Younger msin3A+/− mice (less than 12 weeks of age) showed no indication of splenic hypertrophy or altered CD4/CD8 ratio, suggesting a delayed or gradual onset. Quantitative Western blot analysis of T cells from wild-type, Het1, and Het2 animals revealed only a twofold reduction in mSin3A levels in Het2 mice compared to controls (Fig. 4D), perhaps explaining the gradual occurrence. Alternatively, the partial penetrant phenotype could be related to stochastic differences in the differentiation of cytokine-secreting T cells in msin3A+/− mice (see Discussion).
FIG. 4.
A subset of msin3A+/− heterozygous mice develop splenomegaly. (A) Comparison of spleens from wild-type (WT) and heterozygous msin3A+/− mice with splenomegaly (Het2). (B) Transverse tissue sections from wild-type and enlarged heterozygous spleens were stained with hematoxylin and eosin. Areas of white pulp (WP) and red pulp (RP) are indicated. Bar, 2 mm, approximately. (C) Representative examples of the numbers of thymocytes, splenocytes, and bone marrow cells from a wild-type (WT), unaffected msin3A+/− heterozygote (Het1), and msin3A+/− heterozygous mouse with splenomegaly (Het2) are shown. The cellularity was determined by counting with a hemocytometer. The masses of the spleens used for cell counts are also shown. (D) mSin3A levels were determined in thymocytes from WT, Het1, and Het2 animals by quantitative Western blotting using the LI-COR Odyssey system. The amount of mSin3A was normalized to tubulin and then expressed as a percentage relative to wild type. (E) Flow cytometric analysis was used to analyze the proportion of B (B220-positive) and T (CD3ɛ-positive) cells in spleens from wild-type, Het1, and Het2 mice. A representative flow cytometric histogram is shown. (F) Representative histogram showing the cellularity of CD4 single-positive, CD8 single-positive, and B cells was calculated from the proportion of each cell type present in the spleens of WT and Het2 animals. (G) Flow cytometric analysis was used to analyze the expression of CD4 and CD8 coreceptors on splenocytes harvested from WT, Het1, and Het2 animals. Splenocytes were stained with FITC-conjugated anti-CD8, PE-conjugated anti-CD4, and biotinylated anti-CD3ɛ, followed by streptavidin tricolor. A representative flow cytometric histogram is shown. (H) The average ratio of CD4 versus CD8 splenocytes was determined from the number of WT, Het1, and Het2 animals indicated. (I) Representative photo of the kidney glomeruli from an 11-month-old msin3A heterozygous mouse (Het2) and sex-matched normal littermate control mouse (WT), fixed in paraffin and stained with H&E. GL, renal glomeruli; T, renal tubule. The amorphous pink material in the renal tubule of the msin3A heterozygous mouse is protein, indicative of proteinuria. Extensive destruction of renal glomeruli is evident in the msin3A heterozygous mice. Two out of three 11-month-old msin3A heterozygous mice had membranous glomerulopathy, while 0 out of 2 wild-type littermate control mice exhibited disease.
Further histological analysis indicated increased infiltrates of lymphocytes in many tissues, including lung, liver, and kidney of Het2 animals versus normal littermate controls (data not shown). Moreover, examination of two out of three aged Het2 mice revealed severe membranous glomerulopathy (Fig. 4I), a kidney disease in humans and other species characterized by proteinuria and formation of immune deposits in the lamina rara of the glomerular basement membrane (for review, see reference 28). The Het2 mice examined had protein in their renal tubules (pink substance) and extensive protein deposits in their glomeruli basement membrane. Together, these studies suggest that mSin3A has a role in modulating lymphocyte homeostasis and that constitutive loss of one allele is sufficient to result in the expansion of CD4 and B lymphocytes and severe immune complex-mediated kidney disease.
Conditional deletion of msin3A in T cells results in reduced thymic cellularity and proliferative potential of mature T cells.
To further explore the possibility that loss of mSin3A might influence T-cell development, we generated a T-cell-specific msin3A deletion. msin3A-targeted mice (mSin3AL/L) were bred to animals expressing Cre under the control of the lck proximal promoter, which expresses Cre specifically in T cells throughout thymopoiesis (37). A comparison of thymocyte DNA derived from lckCre-msin3AL/+ and lckCre-msin3AL/L animals with DNA from targeted mice lacking Cre demonstrated a robust deletion of exon 2 in the presence, but not absence, of the recombinase (Fig. 5A). Characterization of thymocyte protein extracts from these same cells revealed a reduction in mSin3A levels compared to littermates lacking lckCre (Fig. 5B and see below). Other components of the mSin3 complex, including mSDS3 and HDAC2, were found to be expressed at comparable levels in all four genotypes (Fig. 5B). Thymocytes from a number of lckCre-mSin3AL/L mice expressed a truncated form of mSin3A as detected by an antibody to PAH2 (amino acids 304 to 386) (Fig. 5B, third row). Because this smaller protein is not recognized by an N-terminal antibody, we conclude that it lacks the N-terminal region and probably arises as a result of selection for an internal translational initiation event, since it is not detected in heterozygous deleted (lckCre-mSin3AL/+) T cells. In general, we observed a dose-dependent correlation between loss of all forms of mSin3A protein and increasing severity of the phenotypes which, taken together with the apparent selection, suggests that the truncated form of the protein does not function as a dominant negative. In addition, Western blot analysis of thymocyte protein extracts with an antibody raised to the C terminus of mSin3A failed to detect any other truncated forms of the protein (data not shown).
FIG. 5.
Characterization of thymocytes lacking mSin3A. (A) Genomic PCR analysis of thymocyte DNA derived from msin3AL/+ and msin3AL/L thymocytes with and without lckCre. The position of wild-type (WT), targeted (LoxP), and deleted (Δexon 2) alleles are shown (upper panel). (B) Western blot analysis of protein extracts derived from thymocytes with the indicated genotype. Antiserum which recognizes the PAH2 domain (amino acids 304 to 380) was used to detect mSin3A protein. The white arrowhead indicates the position of the truncated mSin3A protein. (C) The number of thymocytes harvested from individual animals with the indicated genotype was determined using a hemocytometer. The average cellularity was calculated using four mice of each genotype from three independent experiments. (D) Single cell populations of thymocytes harvested from mice with the indicated genotypes were stained with FITC-conjugated anti-CD25, anti-CD8, anti-gamma delta TCR, PE-conjugated anti-CD44, anti-CD4, and biotinylated-anti-CD3ɛ, followed by streptavidin tricolor. A representative flow cytometric histogram from 8-week-old mice is shown. (E) The average cellularity of each thymocyte subset was determined, using flow cytometric analysis and total thymic cellularity, from three independent experiments. (F) Splenocytes derived from the genotypes indicated were stimulated to proliferate ex vivo by addition of anti-CD3 and anti-CD28 antibodies. The cells were allowed to divide for 48 h and then pulsed with 3[H]thymidine for 24 h prior to harvesting. A representative histogram of the average 3[H]thymidine incorporated, normalized for the number of starting T cells, is shown. (G) Splenocytes were labeled with CFSE for 5 min at room temperature. The cells were then stimulated to proliferate as for panel B and then analyzed at 48 and 96 h by flow cytometry to assess division.
Phenotypic analysis of lckCre-mSin3AL/L T cells revealed a threefold reduction in total thymocyte cellularity in comparison to lckCre-mSin3AL/+ and control cells lacking lckCre, indicative of an impairment in thymopoiesis (Fig. 5C). The progression of T cells during development can be monitored through the expression of the cell surface markers CD4, CD8, and the T cell receptor (TCR), which help delineate the four major subsets of thymocytes. Immature TCR− CD4− CD8− (double-negative [DN]) cells proliferate and differentiate to become TCR+ CD4+ CD8+ (double-positive [DP]) cells before finally maturing into CD4+ or CD8+ single-positive (SP) naïve T cells. A comparison of CD4 and CD8 expression revealed that lckCre-mSin3AL/L mice had a threefold increase in the percentage of DN cells compared to controls, indicative of a partial block or delay in thymopoiesis and thus explaining the reduced thymic cellularity (Fig. 5D, top row; an increase from 4% to 12%). A delay in thymopoiesis at the DN stage, prior to proliferative expansion, results in a nearly normal number of DN T cells, while DP, CD4SP, and CD8SP T-cell populations, in contrast, are all greatly reduced (Fig. 5E, compare lckCre-msin3AL/L cells to lckCre-msin3AL/+ and the no-lckCre controls).
We next examined more specifically where loss of mSin3A function affected development of DN cells. Immature DN T cells can be further subdivided into four populations (DN1 to -4) based on their expression of CD25 (interleukin-2 [IL-2] receptor) and CD44 (Fig. 5C, second row). Maturation from the DN3 (CD25+, CD44−) to DN4 (CD25−, CD44−) stage of development, subsequent expansion, and allelic exclusion of the TCRβ chain are dependent upon successful in-frame rearrangement of the TCR β-chain and formation of the pre-TCR complex, collectively referred to as the “β-checkpoint.” Homozygous deleted msin3A T cells showed increased numbers of DN3 cells, suggesting that a reduction in mSin3A levels results in a dose-dependent delay or partial block at the β-checkpoint. However, the formation of gamma delta T cells, which express a variant TCR, was unaffected by loss of mSin3A (Fig. 5C, third row).
The reduction of thymic cellularity in lckCre-mSin3AL/L mice might be explained by an impairment in maturation (e.g., the β-checkpoint) or by a more generalized cell cycle defect, as suggested by the reduction in proliferative potential observed in cultured embryos and MEFs (Fig. 2B and 3B). To distinguish between these two possibilities, we assessed the ability of T cells from lckCre-msin3AL/L and control mice to proliferate ex vivo. T cells derived from the spleens of lckCre-msin3AL/+, lckCre-msin3AL/L, and non-Cre-expressing mice were stimulated to proliferate in culture by the addition of anti-CD3 and anti-CD28 antibodies, which mimic TCR activation. Proliferation was measured by the incorporation [3H]thymidine over a 48-hour period following antibody stimulation (Fig. 5F). Incorporation of [3H]thymidine was markedly reduced in lckCre-mSin3AL/L mice compared to lckCre-mSin3AL/+ and no-Cre controls. No proliferation of T cells derived from any of the mice occurred in the absence of stimulation (Fig. 5F, first four columns). Cell division was also assessed from the same splenocytes by labeling with the fluorescent dye CFSE prior to stimulation. Analysis of stimulated CD4SP cells revealed significant division of both mSin3AL/L and lckCre-mSin3AL/+ cells after 2 days, which increased over a 4-day period (Fig. 5G). The cells from lckCre-mSin3AL/L mice, in contrast, which had a reduced thymic cellularity and mSin3A levels compared to control cells, did not divide at all, even 4 days poststimulation.
Thus, we conclude that lckCre-mSin3AL/L mice have a reduced number of T cells, due to a partial block at the β-checkpoint stage of thymopoiesis and a reduction in proliferative potential of mature T cells, suggesting that loss of mSin3A may cause a generalized defect in cell cycle progression similar to that observed in cultured embryos and MEFs (Fig. 2 and 3).
Loss of mSin3A impairs the development of CD8SP T cells.
Closer examination of mature CD3hi thymocytes (CD3hi, upper row) revealed an additional effect on T-cell development whereby loss of msin3A resulted in a marked decrease in the percentage and total number of CD8SP (cytotoxic) T cells in lckCre-mSin3AL/L mice compared to controls (Fig. 5E and Fig. 6A, upper row). Consequently, the ratio of CD4SP to CD8SP cells is increased from 2.2 and 1.9 in LckCre-negative control animals to 7.2 in lckCre-mSin3AL/L mice lacking msin3A. Analysis of spleens from the same animals revealed a similar reduction in the percentage of CD8SP cells (Fig. 6A, bottom row). On average, we observed an increase in the ratio of CD4SP to CD8SP cells in the spleen from 1.4 in non-Cre-expressing animals to 2 and 5.3 in lckCre-mSin3AL/+ and lckCre-mSin3AL/L mice, respectively. This loss of CD8SP cells is reminiscent of the phenotype we observed in msin3A+/− mice with splenomegaly (Fig. 4E). However, the size and cellularity of spleens from lckCre-mSin3AL/L mice was not significantly different from control mice, in contrast to msin3A+/− Het2 mice (see Discussion).
FIG. 6.
Loss of msin3A inhibits the development of CD8SP T cells. (A) Thymocytes and splenocytes derived from mice with the indicated genotype were stained with anti-CD4-FITC, anti-CD4-PE, and anti-CD3ɛ antibodies. Α representative flow cytometric histogram is shown. (Β) The average ratio of CD4SP versus CD8SP cells in the spleen was determined from mice with the indicated genotypes. (C) Splenocytes expressing a transgenic T-cell receptor (OT-1) were harvested from 6-week-old mice with the indicated genotypes and then stained with FITC-conjugated anti-CD8, PE-conjugated anti-CD4, and biotinylated anti-CD3ɛ, followed by streptavidin tricolor. A representative histogram, with two mice of each genotype, is shown. (D) The LI-COR Odyssey quantitative Western blotting system was used to analyze the relative protein levels of mSin3A, mSin3B, and tubulin in the thymocytes indicated. The amounts of mSin3A and mSin3B were normalized to tubulin and then expressed as a percentage relative to wild-type (++) cells.
The maturation of CD8SP cells from their DP precursors is dependent upon many competing factors, chief among these being the interaction of the TCR, on thymocytes, with complexes of peptides and the major histocompatibility complex (MHC) I (for CD8SP) or II (for CD4SP) molecules expressed on thymic epithelial cells, a process called positive selection (see reference 53 for a review). The level of CD3ɛ and TCRβ expression, which indicates the formation of a competent TCR complex, was comparable in control and thymocytes with msin3A deleted (Fig. 5D, bottom row, and data not shown). This suggests that mSin3A modulates lymphocyte development and proliferation by controlling signaling pathways that function downstream of the TCR. To test this hypothesis more comprehensively, we bred lckCre-mSin3AL/L mice to OT-1 transgenic animals, which constitutively express an MHC class I-restricted TCR (24) and hence are artificially skewed towards the development of CD8 single-positive thymocytes (CD8SP) during positive selection. We reasoned that if mSin3A functioned downstream of the TCR, its loss should readily inhibit the increased accumulation of CD8SP cells on the OT-1 background. As previously published, expression of the OT-1 transgene on the wild-type (msin3A+/+) lckCre+ background increases the proportion of peripheral CD8SP cells (Fig. 6C, lckCre-msin3A+/+; OT-1 mice A and B; 16% and 19% CD8SP, respectively). However, this increased population of CD8SP is significantly reduced in T cells lacking mSin3A (Fig. 6C, compare lckCre-msin3A+/+; OT-1 and lckCre-mSin3AL/L; OT-1 mice; a reduction from 16% and 19% to 2% and 3% CD8SP cells, respectively). Interestingly, of the two heterozygous deleted mice (lckCre-mSin3AL/+; OT-1) used in this instance, the phenotype of T cells from the first (A) more closely resembled cells derived from a homozygous deleted genotype (only 7% CD8SP), while the second (B) was indistinguishable from wild type (23% CD8SP) (Fig. 6C, compare lckCre-mSin3AL/+; OT-1 A and B). Quantitative Western blotting (Fig. 6D) revealed that thymocytes from heterozygote A, displaying the strong reduction in CD8SP cells, had only 28% the amount of mSin3A compared to wild-type cells. By contrast, heterozygote B, which had wild-type levels of CD8SP cells, had 88% of wild-type mSin3A. The two lckCre-mSin3AL/L; OT-1 mice used for this experiment (A and B) had 16% and 8% the amount of wild-type mSin3A, respectively (Fig. 6D) and, consequently, the lowest levels of CD8SP cells. Thus, it appears that successful development of CD8SP cells is sensitive to the levels of mSin3A protein. We also observed that mSin3B protein levels were increased, compared to wild-type controls, from 1.2- to 2.3-fold in mice with reduced levels of mSin3A (Fig. 6D). However, there was no correlation between increased mSin3B levels and the proportion of CD8SP cells.
Thus, we conclude that mSin3A is required for the normal development of CD8SP T cells, in a dose-dependent manner.
Thymocytes lacking mSin3A have an intact T-cell signaling pathway but increased levels of apoptosis.
To investigate potential mechanisms for the reduction of DP and CD8SP cells in T cells with msin3A deleted, we examined whether the expression of essential components of pre-TCR and TCR-dependent signaling pathways are altered by loss of mSin3A. To start, we analyzed expression of the adaptor proteins LAT and SLP76, the Syk-family PTK ZAP-70, and the Src-family protein tyrosine kinase p56LCK (Fig. 7A), all of which are essential for normal T-cell development. A decrease in ZAP-70 (3a) or an increase in p56LCK protein levels (23), for instance, might reduce the number of CD8SP. However, we found that the expression of these proteins was unaffected by the loss of msin3A, compared to controls, when msin3A was deleted (Fig. 7B).
FIG. 7.
Thymocytes lacking mSin3A have an intact T-cell signaling pathway but increased levels of apoptosis. (A) Schematic diagram showing relative position of signaling molecules and nuclear factors in T-cell receptor-dependent signaling pathway. (B) Western blot analysis was used to determine the relative expression levels of the indicated proteins from the thymocyte protein extracts specified. (C) Thymocytes derived from mice with the indicated genotype were analyzed for apoptosis by staining with annexin V-FITC and 7-aminoactinomycin (7-AAD) followed by flow cytometric analysis. A representative histogram is shown. (D) Thymocytes of the indicated genotype were isolated and then cultured ex vivo in Dulbecco's modified Eagle's medium for a 24-h period before being analyzed for apoptosis. Apoptosis of individual thymocyte populations was analyzed by costaining with anti-CD4 and anti-CD8 antisera followed by annexin V. Each assay was performed in triplicate. A representative histogram is shown.
We next examined the expression of several nuclear proteins that likely function downstream of these signaling molecules. The transcriptional repressors Runx-1 and Runx-3 are required for the silencing of the CD4 locus and the development of CD8SP cells (62, 66). But again, we found that Runx-1 and Runx-3 protein levels in cells with msin3A deleted were also largely unchanged compared to controls (Fig. 7B and data not shown). Finally, since mSin3A is the central component of an HDAC-containing complex, we also wanted to assess whether acetylation of core histones was affected in lckCre-mSin3AL/L cells. Western blot analysis showed that levels of H3K9Ac and H4K12Ac were unaltered in bulk histones by loss of mSin3A (Fig. 7B and data not shown). These results suggest that loss of mSin3A is not likely affecting T-cell development and proliferation by directly inhibiting the expression of membrane-proximal signaling molecules known to be important for T-cell development, or the nuclear proteins Runx1 and Runx3, which are important for the development of CD8 lineage cells.
We had previously shown that loss of mSin3A in immortalized MEFs results in an increase in sub-G1 apoptotic cells (Fig. 3). We therefore asked whether the impairment of DP and CD8SP cells in msin3A-deficient thymocytes may be due in part to increased selective apoptosis during development. Consistent with this hypothesis, we observed an almost twofold increase in apoptosis of thymocytes with msin3A deleted, compared to heterozygous deleted and no-Cre controls (Fig. 7C; 4% to 7% annexin V-positive cells). We next analyzed individual thymocyte populations for apoptosis following 24 h in culture. We found that loss of mSin3A caused an increase in apoptosis in DP and CD8SP thymocytes compared to controls (Fig. 7D) while, in contrast, DN and CD4SP cell populations lacking msin3A showed no significant change in the percentage of apoptotic cells. Taken together with our previous results in mSin3A-deficient MEFs, these results suggest that the decrease in CD8SP cells we observed in lckCre-mSin3AL/L mice is caused by a reduction in cell viability.
DISCUSSION
msin3A is an essential gene.
Sin3 orthologs, possessing highly conserved PAH and HID regions, are present in all eukaryotes from yeast to humans. Deletion of Sin3 in fission yeast (12) and fruit flies (43, 45) results in lethality. In this study, we demonstrate that msin3A is also an essential gene for mammalian development (Fig. 2). Because the highly related mSin3A paralog, mSin3B, is widely expressed (e.g., Fig. 6 and 7), our results suggest that these two genes are not functionally redundant. This is surprising, given that no clear differences between mSin3A and mSin3B in terms of binding and complex formation have been reported. The up-regulation of mSin3B in T cells with reduced mSin3A suggests that compensation does occur, albeit at a level insufficient to prevent phenotypic deficiencies (Fig. 6 and 7). It is likely that the presence of this close relative masks otherwise more prominent phenotypes, such as those observed when other components of the Sin3/HDAC complex have been deleted. Mouse knockout studies for mSDS3 (13) and HDAC1 (32) reveal similarities and a few discrepancies with our mSin3A knockout data. Loss of any one of the three genes results in early embryonic lethality at the postimplantation stage. Moreover, an increase in the ratio of viable wild-type to heterozygous pups in the knockouts suggests that haploinsufficiency can also be detrimental. The apparent cause of lethality in all three instances is a marked reduction in proliferative potential.
In three independent systems, cultured blastocysts (Fig. 2), MEFs (Fig. 3), and mature T cells (Fig. 5), we observed that a reduction in mSin3A correlated with decreased cell division. However, while the doubling time of these cells was increased, flow cytometric analysis revealed only a subtle change in cell cycle profile, suggesting that loss of mSin3A may affect changes in both G1 and G2/M phases (Fig. 3). Acute deletion of msin3A in MEFs (CreGFP-Sin3AL/L) produces a modest increase in the levels of CDK inhibitors p21 and p27 compared to control cells (see Fig. S1A in the supplemental material), possibly accounting for a delay in G1 phase similar to that observed in cells with HDAC1 deleted (32). On the other hand, cells lacking mSDS3 develop a G2/M arrest due to defective chromosome segregation caused by aberrant acetylation of pericentric heterochromatin (13). Similar findings were also obtained in Schizosaccharomyces pombe cells lacking the Sin3 ortholog, Pst1p (55). However, our examination, using immunofluorescence, of the pericentric heterochromatin in cells with msin3A deleted indicated that it was still present in a hypoacetylated state (see Fig. S1B in the supplemental material). There was also no evidence of aneuploidy, equivalent to that observed in mSDS3-disrupted cells, in CreGFP-Sin3AL/L MEFs (Fig. 3C and D). A strong chromatin phenotype such as this might only be expected to only occur upon deletion of both msin3 genes, or by inactivation of a protein which mediates function of both mSin3 proteins, such as mSDS3. We did, however, detect a reduced number of distinct HP1α foci in CreGFP-Sin3AL/L MEFs compared to control cells (Fig. 3), consistent with previous reports (67). HP1α is thought to be targeted to heterochromatin via numerous transient interactions (for review, see reference 38). Perhaps the best characterized of these involves binding to trimethylated lysine 9 on histone 3 (H3-K9). In this regard, it is interesting that that mice null for the Sin3A-associated H3-K9 methyltransferase ESET (68) die at a peri-implantation stage (14), similar to msin3A−/− animals. HP1α localization can also be modified by prolonged exposure to the HDAC inhibitor trichostatin A, which causes histone hyperacetylation without a global change in histone methylation (59). These findings suggest that one of the targets of trichostatin A might be the mSin3/HDAC complex. However, loss of mSin3A—unlike HDAC1, but similar to mSDS3—does not cause a change in bulk histone acetylation (Fig. 7 and data not shown) (12, 55). It therefore seems unlikely that loss of mSin3A would cause a global change in gene expression. Indeed, only 3% of genes (399/13,137) were found to have altered expression, the majority with less than a twofold change, when microarray studies were performed in Drosophila cells with reduced dSin3 (47).
We propose that loss of msin3A causes multiple effects, including mislocalization of the heterochromatin-associated protein HP1α and induction of cyclin-dependent kinase inhibitors, which result in a reduction of cellular proliferation. While we did not observe a global increase in histone acetylation, loss of mSin3A may lead to a more subtle disruption of localized regions of heterochromatin that are perhaps reflected in the observed alterations of HP1α binding. Such changes may ultimately have a deleterious effect on regulated gene expression during proliferation and development. The embryonic lethality we observe peri-implantation is thus likely due to a failure in proliferation once maternal stores of mSin3A have been depleted. Our conclusions are in general agreement with a second, independently generated msin3A null mouse which dies in early development and whose MEFs exhibit reduced proliferation and altered HP1α localization but not hypoacetylation of pericentric heterochromatin or altered H3K9 trimethylation (Dannenberg et al., submitted).
Systemic reduction in mSin3A alters peripheral lymphoid homeostasis and causes membranous glomerulopathy.
Constitutive msin3A+/− mice with splenomegaly have an expanded total number of CD4 T cells and B cells. Thymocytes derived from these mice have a twofold reduction in mSin3A protein compared to wild-type and unaffected heterozygote animals (Fig. 4D). Thus, the affected msin3A+/− mice appear unable to compensate for the loss of one msin3A allele. However, we cannot be certain whether this is sufficient to generate the disruption in lymphoid homeostasis. In all likelihood, the mixed 129/Sv and C57BL/6J genetic background of the msin3A+/− mice also plays a role in modifying the severity and penetrance of the phenotype. We are in the process of backcrossing the msin3A+/− mice to generate pure 129/Sv and C57BL/6J lines to further explore these phenomena. In contrast, T-cell-specific disruption of msin3A in lckCre-msin3AL/L animals does not result in splenomegaly, we presume this is either because the lymphoproliferative phenotype observed in the constitutive deletion is not T-cell autonomous or because the near-complete deletion of sin3A during T-cell development results in strong selection for mature T cells that can compensate for reduced mSin3A. Nonetheless, we observed a reduced population of CD8SP T cells in both systems (Fig. 4F and 6A), leading us to conclude that a reduction in mSin3A protein levels curtails cytotoxic T-cell development.
Flow cytometric and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling analyses of enlarged spleens from msin3A+/− mice reveal normal levels of FasL and significant levels of apoptotic cells (data not shown), suggesting that the splenomegaly phenotype we observe is not due to a defect in the Fas pathway, as occurs in lpr or gld mice, which lack Fas or FasL (1, 60, 64). Rather, the splenomegaly phenotype of msin3A+/− mice, combined with the impairment in both pre-TCR signaling and TCR-induced proliferation, is reminiscent of mice deficient in the LAT adaptor (56). Despite impaired development of DP cells and impaired T-cell proliferation in response to TCR stimulation, LAT-deficient mice later develop a lymphoproliferative disorder characterized by an expansion of CD4 cells with a T helper 2 IL-4-secreting cytokine profile. Since both LAT and Sin3A are expressed in B and T cells, the lymphoproliferative phenotypes in LAT or sin3A+/− mice may reflect defects in both B and T cells (as well as additional cell types), while T-cell-specific disruption of Sin3 in lckCre-sin3AL/L animals does not result in splenomegaly, presumably because the lymphoproliferative phenotype is not T-cell intrinsic. Mice heterozygous for sin3A also develop membranous glomerulopathy, which is also consistent with the expansion of Th2 cytokine-secreting CD4 T cells, which would stimulate B cells and plasma cells to produce excessive antibodies that deposit in the kidney glomeruli.
Regulation of T-cell differentiation by chromatin-modifying factors.
A growing number of transcriptional regulators and chromatin remodeling factors have been implicated in T-cell development. The Swi/Snf complex proteins Brg1 and BAF57 act in a reciprocal manner to silence and activate the CD4 and CD8 loci, respectively, in immature cells (9, 10, 17). The transcriptional regulator Ikaros, which associates with mSin3A (30), also plays a role in activating the CD8 locus (20; see reference 18 for review). Conversely, Mi-2β, a component of the NuRD complex, is required to initiate CD4 expression during the DN-to-DP transition (65), while the repressors Runx-1 and -3 are required to silence it in immature T cells and mature CD8SP cells, respectively (62, 66). Underscoring the important role played by transcriptional regulation, we have found that selective deletion of msin3A in developing thymocytes causes a reduction in the total number and proportion of cytotoxic T cells (Fig. 6A). This novel function of mSin3A is likely to occur downstream of the TCR-dependent signaling pathway, because deletion of msin3A was able to block the accumulation of CD8SP cells even in the presence of a transgenic MHC class I-restricted TCR (Fig. 6C). By taking advantage of the variability in mSin3A expression levels in lckCre-Sin3AL/+ thymocytes, we were able to demonstrate that small decreases in mSin3A protein did not effect CD8SP development, while larger reductions produced a concomitant decrease in the level of CD8SP cells (Fig. 6C).
Thymocytes from a number of lckCre-msin3AL/L mice expressed a truncated form of mSin3A which lacks a wild-type N terminus (Fig. 5B; determined by Western blotting using antisera raised to different portions of the mSin3A molecule). This protein is probably generated through an internal initiation event, since the first coding exon (exon 2) has been deleted. T cells undergo a proliferative expansion as they progress from the DN to DP stage and are therefore prone to clonal expansion. Thus, a small percentage of cells expressing a truncated form of mSin3A may be selected. Since mSin3A is required for cellular proliferation, it seems likely that this truncated form of mSin3A is more likely to be a hypomorphic form of the protein rather than dominant negative. In general, much less of the truncated mSin3A protein was detected than the wild type, and loss of all forms of the protein correlated with a stronger phenotype.
Our analysis of protein extracts from purified populations of wild-type DN, DP, CD4SP, and CD8SP cells revealed that they all express equivalent amounts of mSin3A (data not shown). Therefore, it is the selective recruitment of mSin3A by other finely regulated transcription factors, and not regulation of mSin3A protein levels per se, that is likely to be important. Only in the context of lckCre-msin3AL/L mice, where mSin3A levels become limiting, do we observe a reduction in the number of CD8SP cells. We believe that mSin3A function is necessary for the viability CD8SP cells, since a reduction in the level of mSin3A protein causes an increase in the percentage of apoptotic cells and thus a concomitant decrease in CD8SP cell numbers. Work is in progress to try and discover the mechanism by which CD8SP cells with msin3A deleted are more susceptible to apoptosis while CD4SP cells are not.
In summary, we have demonstrated an essential role for mSin3A in embryogenesis and the development of T cells. The impairment of thymic DP and CD8 T-cell development in lckCre-msin3AL/L mice, and also the alteration in splenic lymphoid homeostasis in msin3A+/− mice, suggests that msin3A is a pleiotropic gene required not only for cell cycle progression but also for the proper development and homeostasis of lymphoid cells.
ADDENDUM
Dannenberg et al. (12a) have also found that targeted deletion of msin3A produces early embryonic lethality and inhibits proliferation and survival of fibroblasts in culture.
Supplementary Material
Acknowledgments
We thank Philippe Soriano for advice and encouragement throughout the course of this work, Elizabeth Wayner for technical expertise in the generation of P1E7 monoclonal antibody, and Piper Treuting for assistance with photos of H&E-stained slides, Mark Tsang for analysis of apoptotic thymocytes, and the FHCRC Histology Core for the processing, embedding, and H&E staining of tissue samples. We also thank Ron DePinho for communicating results prior to publication. We are very grateful for the kind gifts of antibodies from Don Ayer (anti-mSDS3; Huntsman Institute, Salt Lake City, UT) and Yoram Groner (anti-Runx3; Weizmann Institute, Rehovot, Israel).
This work was supported by postdoctoral fellowships from the Rett Syndrome Research Foundation (to S.M.C.) and the American Cancer Society (to S.M.M.) and by grants to R.N.E. (NIH/NCI RO1CA57138), B.I. (R01AI0535468 from the NIAID and University of Washington Royalty Research Fund), and to H.D.L. (5RO1HD18184 and 5RO1JL65898). R.N.E. is an American Cancer Society Research Professor.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Adachi, M., S. Suematsu, T. Kondo, J. Ogasawara, T. Tanaka, N. Yoshida, and S. Nagata. 1995. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat. Genet. 11:294-300. [DOI] [PubMed] [Google Scholar]
- 2.Alland, L., G. David, H. Shen-Li, J. Potes, R. Muhle, H. C. Lee, H. J. Hou, K. Chen, and R. A. DePinho. 2002. Identification of mammalian Sds3 as an integral component of the Sin3/histone deacetylase corepressor complex. Mol. Cell. Biol. 22:2743-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alland, L., R. Muhle, H. Hou, Jr., J. Potes, L. Chin, N. Schreiber-Agus, and R. A. DePinho. 1997. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature 387:49-55. [DOI] [PubMed] [Google Scholar]
- 3a.Arpaia, E., M. Shahar, H. Dadi, A. Cohen, and C. M. Roifman. 1994. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking Zap-70 kinase. Cell 76:947-958. [DOI] [PubMed] [Google Scholar]
- 4.Ayer, D. E. 1999. Histone deacetylases: transcriptional repression with siners and nurds. Trends Cell Biol. 9:193-198. [DOI] [PubMed] [Google Scholar]
- 5.Ayer, D. E., C. D. Laherty, Q. A. Lawrence, A. P. Armstrong, and R. N. Eisenman. 1996. Mad proteins contain a dominant transcription repression domain. Mol. Cell. Biol. 16:5772-5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayer, D. E., Q. A. Lawrence, and R. N. Eisenman. 1995. Mad-Max transcriptional repression is mediated by ternary complex formation with mammalian homologs of yeast repressor Sin3. Cell 80:767-776. [DOI] [PubMed] [Google Scholar]
- 7.Brown, M., M. McCormack, K. G. Zinn, and M. P. Farrell. 1986. A recombinant murine retrovirus for simian virus large T cDNA transforms mouse fibroblasts to anchorage-independent growth. J. Virol. 60:290-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brubaker, K., S. M. Cowley, K. Huang, L. Loo, G. S. Yochum, D. E. Ayer, R. N. Eisenman, and I. Radhakrishnan. 2000. Solution structure of the interacting domains of the Mad-Sin3 complex: implications for recruitment of a chromatin-modifying complex. Cell 103:655-665. [DOI] [PubMed] [Google Scholar]
- 9.Chi, T. H., M. Wan, P. P. Lee, K. Akashi, D. Metzger, P. Chambon, C. B. Wilson, and G. R. Crabtree. 2003. Sequential roles of Brg, the ATPase subunit of BAF chromatin remodeling complexes, in thymocyte development. Immunity 19:169-182. [DOI] [PubMed] [Google Scholar]
- 10.Chi, T. H., M. Wan, K. Zhao, I. Taniuchi, L. Chen, D. R. Littman, and G. R. Crabtree. 2002. Reciprocal regulation of CD4/CD8 expression by SWI/SNF-like BAF complexes. Nature 418:195-199. [DOI] [PubMed] [Google Scholar]
- 11.Cowley, S. M., R. S. Kang, J. V. Frangioni, J. J. Yada, A. M. DeGrand, I. Radhakrishnan, and R. N. Eisenman. 2004. Functional analysis of the Mad1-mSin3A repressor-corepressor interaction reveals determinants of specificity, affinity, and transcriptional response. Mol. Cell. Biol. 24:2698-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dang, V. D., M. J. Benedik, K. Ekwall, J. Choi, R. C. Allshire, and H. L. Levin. 1999. A new member of the Sin3 family of corepressors is essential for cell viability and required for retroelement propagation in fission yeast. Mol. Cell. Biol. 19:2351-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Dannenberg, J.-H., G. David, S. Zhong, J. van der Torre, W. H. Wong, and R. A. DePinho. mSin3A co-repressor regulates diverse transcriptional networks governing normal and neoplastic growth and survival. Genes Dev., in press. [DOI] [PMC free article] [PubMed]
- 13.David, G., G. M. Turner, Y. Yao, A. Protopopov, and R. A. DePinho. 2003. mSin3-associated protein, mSds3, is essential for pericentric heterochromatin formation and chromosome segregation in mammalian cells. Genes Dev. 17:2396-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodge, J. E., Y. K. Kang, H. Beppu, H. Lei, and E. Li. 2004. Histone H3-K9 methyltransferase ESET is essential for early development. Mol. Cell. Biol. 24:2478-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorland, S., M. L. Deegenaars, and D. J. Stillman. 2000. Roles for the Saccharomyces cerevisiae SDS3, CBK1 and HYM1 genes in transcriptional repression by SIN3. Genetics 154:573-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleischer, T. C., U. J. Yun, and D. E. Ayer. 2003. Identification and characterization of three new components of the mSin3A corepressor complex. Mol. Cell. Biol. 23:3456-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gebuhr, T. C., G. I. Kovalev, S. Bultman, V. Godfrey, L. Su, and T. Magnuson. 2003. The role of Brg1, a catalytic subunit of mammalian chromatin-remodeling complexes, in T cell development. J. Exp. Med. 198:1937-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgopoulos, K. 2002. Haematopoietic cell-fate decisions, chromatin regulation and ikaros. Nat. Rev. Immunol. 2:162-174. [DOI] [PubMed] [Google Scholar]
- 19.Guezennec, X. L., G. Vriend, and H. G. Stunnenberg. 2004. Molecular determinants of the interaction of Mad with the PAH2 domain of mSin3. J. Biol. Chem. 279:25823-25829. [DOI] [PubMed] [Google Scholar]
- 20.Harker, N., T. Naito, M. Cortes, A. Hostert, S. Hirschberg, M. Tolaini, K. Roderick, K. Georgopoulos, and D. Kioussis. 2002. The CD8α gene locus is regulated by the Ikaros family of proteins. Mol. Cell 10:1403-1415. [DOI] [PubMed] [Google Scholar]
- 21.Hassig, C. A., T. C. Fleischer, A. N. Billin, S. L. Schreiber, and D. E. Ayer. 1997. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell 89:341-347. [DOI] [PubMed] [Google Scholar]
- 22.Heinzel, T., R. M. Lavinsky, T.-M. Mullen, M. Söderström, C. D. Laherty, J. Torchia, W.-M. Yang, G. Brard, S. G. Ngo, J. R. Davie, E. Seto, R. N. Eisenman, D. W. Rose, C. K. Glass, and M. G. Rosenfeld. 1997. N-CoR, mSin3, and histone deacetylase-containing complexes in nuclear receptor and Mad repression. Nature 387:43-48. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Hoyos, G., S. J. Sohn, E. V. Rothenberg, and J. Alberola-Ila. 2000. Lck activity controls CD4/CD8 T cell lineage commitment. Immunity 12:313-322. [DOI] [PubMed] [Google Scholar]
- 24.Hogquist, K. A., S. C. Jameson, W. R. Heath, J. L. Howard, M. J. Bevan, and F. R. Carbone. 1994. T cell receptor antagonist peptides induce positive selection. Cell 76:17-27. [DOI] [PubMed] [Google Scholar]
- 25.Iritani, B. M., K. A. Forbush, M. A. Farrar, and R. M. Perlmutter. 1997. Control of B cell development by Ras-mediated activation of Raf. EMBO J. 16:7019-7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jazayeri, A., A. D. McAinsh, and S. P. Jackson. 2004. Saccharomyces cerevisiae Sin3p facilitates DNA double-strand break repair. Proc. Natl. Acad. Sci. USA 101:1644-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasten, M. M., D. E. Ayer, and D. J. Stillman. 1996. SIN3-dependent transcriptional repression by interaction with the Mad1 DNA-binding protein. Mol. Cell. Biol. 16:4215-4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerjaschki, D. 2004. Pathomechanisms and molecular bias of membranous glomerulopathy. Lancet 364:1194-1196. [DOI] [PubMed] [Google Scholar]
- 29.Knoepfler, P. S., and R. N. Eisenman. 1999. Sin meets NuRD and other tails of repression. Cell 99:447-450. [DOI] [PubMed] [Google Scholar]
- 30.Koipally, J., A. Renold, J. Kim, and K. Georgopoulos. 1999. Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. EMBO J. 18:3090-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuzmichev, A., Y. Zhang, H. Erdjument-Bromage, P. Tempst, and D. Reinberg. 2002. Role of the Sin3-histone deacetylase complex in growth regulation by the candidate tumor suppressor p33ING1. Mol. Cell. Biol. 22:835-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagger, G., D. O'Carroll, M. Rembold, H. Khier, J. Tischler, G. Weitzer, B. Schuettengruber, C. Hauser, R. Brunmeir, T. Jenuwein, and C. Seiser. 2002. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J. 21:2672-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laherty, C. D., A. N. Billin, R. M. Lavinsky, G. S. Yochum, A. C. Bush, J.-M. Sun, J. R. Davie, D. W. Rose, M. G. Rosenfeld, D. E. Ayer, and R. N. Eisenman. 1998. SAP30, a component of the mSin3 corepressor complex involved in N-CoR-mediated repression by specific nuclear hormone receptor and homeodomain proteins. Mol. Cell 2:33-42. [DOI] [PubMed] [Google Scholar]
- 34.Laherty, C. D., W.-M. Yang, J.-M. Sun, J. R. Davie, E. Seto, and R. N. Eisenman. 1997. Histone deacetylases associated with the mSin3 corepressor mediate Mad transcriptional repression. Cell 89:349-356. [DOI] [PubMed] [Google Scholar]
- 35.Lai, A., B. K. Kennedy, D. A. Barbie, N. R. Bertos, X. J. Yang, M.-C. Theberge, S.-C. Tsai, E. Seto, Y. Zhang, A. Kuzmichev, W. S. Lane, D. Reinberg, E. Harlow, and P. E. Branton. 2001. RBP1 recruits the mSIN3-histone deacetylase complex to the pocket of retinoblastoma tumor suppressor family proteins found in limited discrete regions of the nucleus at growth arrest. Mol. Cell. Biol. 21:2918-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lechner, T., M. J. Carrozza, Y. Yu, P. A. Grant, A. Eberharter, D. Vannier, G. Brosch, D. J. Stillman, D. Shore, and J. L. Workman. 2000. Sds3 (suppressor of defective silencing 3) is an integral component of the yeast Sin3/Rpd3 HDAC complex and is required for histone deacetylase activity. J. Biol. Chem. 275:40961-40966. [DOI] [PubMed] [Google Scholar]
- 37.Lee, P. P., D. R. Fitzpatrick, C. Beard, H. K. Jessup, S. Lehar, K. W. Makar, M. Perez-Melgosa, M. T. Sweetser, M. S. Schlissel, S. Nguyen, S. R. Cherry, J. H. Tsai, S. M. Tucker, W. M. Weaver, A. Kelso, R. Jaenisch, and C. B. Wilson. 2001. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15:763-774. [DOI] [PubMed] [Google Scholar]
- 38.Maison, C., and G. Almouzni. 2004. HP1 and the dynamics of heterochromatin maintenance. Nat. Rev. Mol. Cell Biol. 5:296-304. [DOI] [PubMed] [Google Scholar]
- 39.Meskauskas, A., J. L. Baxter, E. A. Carr, J. Yasenchak, J. E. Gallagher, S. J. Baserga, and J. D. Dinman. 2003. Delayed rRNA processing results in significant ribosome biogenesis and functional defects. Mol. Cell. Biol. 23:1602-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy, M., J. Ahn, K. K. Walker, W. H. Hoffman, R. M. Evans, A. J. Levine, and D. L. George. 1999. Transcriptional repression by wild-type p53 utilizes histone deacetylases, mediated by interaction with mSin3a. Genes Dev. 13:2490-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagy, L., H.-Y. Kao, D. Chakravarti, R. J. Lin, C. A. Hassig, D. E. Ayer, S. L. Schreiber, and R. M. Evans. 1997. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell 89:373-380. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura, T., T. Mori, S. Tada, W. Krajewski, T. Rozovskaia, R. Wassell, G. Dubois, A. Mazo, C. M. Croce, and E. Canaani. 2002. ALL-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol. Cell 10:1119-1128. [DOI] [PubMed] [Google Scholar]
- 43.Neufeld, T. P., A. Tang, and G. M. Rubin. 1998. A genetic screen to identify components of the sina signaling pathway in Drosophila eye development. Genetics 148:277-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pal, S., R. Yun, A. Datta, L. Lacomis, H. Erdjument-Bromage, J. Kumar, P. Tempst, and S. Sif. 2003. mSin3A/histone deacetylase 2- and PRMT5-containing Brg1 complex is involved in transcriptional repression of the Myc target gene cad. Mol. Cell. Biol. 23:7475-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pennetta, G., and D. Pauli. 1998. The Drosophila Sin3 gene encodes a widely distributed transcription factor essential for embryonic viability. Dev. Genes Evol. 208:531-536. [DOI] [PubMed] [Google Scholar]
- 46.Pile, L. A., E. M. Schlag, and D. A. Wassarman. 2002. The SIN3/RPD3 deacetylase complex is essential for G2 phase cell cycle progression and regulation of SMRTER corepressor levels. Mol. Cell. Biol. 22:4965-4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pile, L. A., P. T. Spellman, R. J. Katzenberger, and D. A. Wassarman. 2003. The SIN3 deacetylase complex represses genes encoding mitochondrial proteins: implications for the regulation of energy metabolism. J. Biol. Chem. 278:37840-37848. [DOI] [PubMed] [Google Scholar]
- 48.Rayman, J. B., Y. Takahashi, V. B. Indjeian, J. H. Dannenberg, S. Catchpole, R. J. Watson, H. te Riele, and B. D. Dynlacht. 2002. E2F mediates cell cycle-dependent transcriptional repression in vivo by recruitment of an HDAC1/mSin3B corepressor complex. Genes Dev. 16:933-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schreiber-Agus, N., L. Chin, K. Chen, R. Torres, G. Rao, P. Guida, A. I. Skoultchi, and R. A. DePinho. 1995. An amino-terminal domain of Mxi1 mediates anti-Myc oncogenic activity and interacts with a homolog of the yeast repressor SIN3. Cell 80:777-786. [DOI] [PubMed] [Google Scholar]
- 50.Schreiber-Agus, N., and R. A. DePinho. 1998. Repression by the mad(mxi1)-sin3 complex. Bioessays 20:808-818. [DOI] [PubMed] [Google Scholar]
- 51.Schroder, M., R. Clark, C. Y. Liu, and R. J. Kaufman. 2004. The unfolded protein response represses differentiation through the RPD3-SIN3 histone deacetylase. EMBO J. 23:2281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott, K. L., and S. E. Plon. 2003. Loss of Sin3/Rpd3 histone deacetylase restores the DNA damage response in checkpoint-deficient strains of Saccharomyces cerevisiae. Mol. Cell. Biol. 23:4522-4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sebzda, E., S. Mariathasan, T. Ohteki, R. Jones, M. F. Bachmann, and P. S. Ohashi. 1999. Selection of the T cell repertoire. Annu. Rev. Immunol. 17:829-874. [DOI] [PubMed] [Google Scholar]
- 54.Sif, S., A. J. Saurin, A. N. Imbalzano, and R. E. Kingston. 2001. Purification and characterization of mSin3A-containing Brg1 and hBrm chromatin remodeling complexes. Genes Dev. 15:603-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silverstein, R. A., W. Richardson, H. Levin, R. Allshire, and K. Ekwall. 2003. A new role for the transcriptional corepressor SIN3; regulation of centromeres. Curr. Biol. 13:68-72. [DOI] [PubMed] [Google Scholar]
- 56.Sommers, C. L., C. S. Park, J. Lee, C. Feng, C. L. Fuller, A. Grinberg, J. A. Hildebrand, E. Lacana, R. K. Menon, E. W. Shores, L. E. Samelson, and P. E. Love. 2002. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science 296:2040-2043. [DOI] [PubMed] [Google Scholar]
- 57.Spronk, C. A. E. M., M. Tessari, A. M. Kaan, J. F. A. Jansen, M. Vermeulen, H. G. Stunnenberg, and G. W. Vuister. 2000. The Mad1-Sin3B interaction involves a novel helical fold. Nat. Struct. Biol. 7:1100-1104. [DOI] [PubMed] [Google Scholar]
- 58.Swanson, K. A., P. S. Knoepfler, K. Huang, R. S. Kang, S. M. Cowley, C. D. Laherty, R. N. Eisenman, and I. Radhakrishnan. 2004. HBP1 and Mad1 repressors bind the Sin3 corepressor PAH2 domain with opposite helical orientations. Nat. Struct. Mol. Biol. 11:738-746. [DOI] [PubMed] [Google Scholar]
- 59.Taddei, A., C. Maison, D. Roche, and G. Almouzni. 2001. Reversible disruption of pericentric heterochromatin and centromere function by inhibiting deacetylases. Nat. Cell Biol. 3:114-120. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi, T., M. Tanaka, C. I. Brannan, N. A. Jenkins, N. G. Copeland, T. Suda, and S. Nagata. 1994. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 76:969-976. [DOI] [PubMed] [Google Scholar]
- 61.Tallquist, M. D., and P. Soriano. 2000. Epiblast-restricted Cre expression in MORE mice: a tool to distinguish embryonic vs. extra-embryonic gene function. Genesis 26:113-115. [DOI] [PubMed] [Google Scholar]
- 62.Taniuchi, I., M. Osato, T. Egawa, M. J. Sunshine, S. C. Bae, T. Komori, Y. Ito, and D. R. Littman. 2002. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell 111:621-633. [DOI] [PubMed] [Google Scholar]
- 63.Wang, H., I. Clark, P. R. Nicholson, I. Herskowitz, and D. J. Stillman. 1990. The Saccharomyces cerevisiae SIN3 gene, a negative regulator of HO, contains four paired amphipathic helix motifs. Mol. Cell. Biol. 10:5927-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watanabe-Fukunaga, R., C. I. Brannan, N. G. Copeland, N. A. Jenkins, and S. Nagata. 1992. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356:314-317. [DOI] [PubMed] [Google Scholar]
- 65.Williams, C. J., T. Naito, P. G. Arco, J. R. Seavitt, S. M. Cashman, B. De Souza, X. Qi, P. Keables, U. H. Von Andrian, and K. Georgopoulos. 2004. The chromatin remodeler Mi-2β is required for CD4 expression and T cell development. Immunity 20:719-733. [DOI] [PubMed] [Google Scholar]
- 66.Woolf, E., C. Xiao, O. Fainaru, J. Lotem, D. Rosen, V. Negreanu, Y. Bernstein, D. Goldenberg, O. Brenner, G. Berke, D. Levanon, and Y. Groner. 2003. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc. Natl. Acad. Sci. USA 100:7731-7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xin, H., H. G. Yoon, P. B. Singh, J. Wong, and J. Qin. 2004. Components of a pathway maintaining histone modification and heterochromatin protein 1 binding at the pericentric heterochromatin in mammalian cells. J. Biol. Chem. 279:9539-9546. [DOI] [PubMed] [Google Scholar]
- 68.Yang, L., Q. Mei, A. Zielinska-Kwiatkowska, Y. Matsui, M. L. Blackburn, D. Benedetti, A. A. Krumm, G. J. J. Taborsky, and H. A. Chansky. 2003. An ERG (ets-related gene)-associated histone methyltransferase interacts with histone deacetylases 1/2 and transcription co-repressors mSin3A/B. Biochem. J. 369:651-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang, X., F. Zhang, and J. E. Kudlow. 2002. Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: coupling protein O-GlcNAcylation to transcriptional repression. Cell 110:69-80. [DOI] [PubMed] [Google Scholar]
- 70.Yochum, G. S., and D. E. Ayer. 2001. Pf1, a novel PHD zinc finger protein that links the TLE corepressor to the mSin3A-histone deacetylase complex. Mol. Cell. Biol. 21:4110-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yochum, G. S., and D. E. Ayer. 2002. Role for the mortality factors MORF4, MRGX, and MRG15 in transcriptional repression via associations with Pf1, mSin3A, and Transducin-Like Enhancer of Split. Mol. Cell. Biol. 22:7868-7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang, Y., R. Iratni, H. Erdjument-Bromage, P. Tempst, and D. Reinberg. 1997. Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell 89:357-364. [DOI] [PubMed] [Google Scholar]
- 73.Zhang, Y., Z.-W. Sun, R. Iratni, H. Erdjument-Bromage, P. Tempst, M. Hampsey, and D. Reinberg. 1998. SAP30, a novel protein conserved between human and yeast, is a component of a histone deacetylase complex. Mol. Cell 1:1021-1031. [DOI] [PubMed] [Google Scholar]
- 74.Zilfou, J. T., W. H. Hoffman, M. Sank, D. L. George, and M. Murphy. 2001. The corepressor mSin3a interacts with the proline-rich domain of p53 and protects p53 from proteasome-mediated degradation. Mol. Cell. Biol. 21:3974-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zindy, F., C. M. Eischen, D. H. Randle, T. Kamijo, J. L. Cleveland, C. J. Sherr, and M. F. Roussel. 1998. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 12:2424-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.