Abstract
Signal regulatory protein α (SIRPα) is a glycoprotein receptor that recruits and signals via the tyrosine phosphatases SHP-1 and SHP-2. In macrophages SIRPα can negatively regulate the phagocytosis of host cells and the production of tumor necrosis factor alpha. Here we provide evidence that SIRPα can also stimulate macrophage activities, in particular the production of nitric oxide (NO) and reactive oxygen species. Ligation of SIRPα by antibodies or soluble CD47 triggers inducible nitric oxide synthase expression and production of NO. This was not caused by blocking negative-regulatory SIRPα-CD47 interactions. SIRPα-induced NO production was prevented by inhibition of the tyrosine kinase JAK2. JAK2 was found to associate with SIRPα in macrophages, particularly after SIRPα ligation, and SIRPα stimulation resulted in JAK2 and STAT1 tyrosine phosphorylation. Furthermore, SIRPα-induced NO production required the generation of hydrogen peroxide (H2O2) by a NADPH oxidase (NOX) and the phosphatidylinositol 3-kinase (PI3-K)-dependent activation of Rac1, an intrinsic NOX component. Finally, SIRPα ligation promoted SHP-1 and SHP-2 recruitment, which was both JAK2 and PI3-K dependent. These findings demonstrate that SIRPα ligation induces macrophage NO production through the cooperative action of JAK/STAT and PI3-K/Rac1/NOX/H2O2 signaling pathways. Therefore, we propose that SIRPα is able to function as an activating receptor.
Signal regulatory protein α (SIRPα) is a transmembrane glycoprotein receptor expressed predominantly by myeloid and neuronal cells (1, 10, 18, 53). The extracellular region of the molecule is composed of immunoglobulin (Ig)-like domains that mediate recognition of the broadly expressed cellular ligand CD47 (also called integrin-associated protein) (16, 39, 54). The cytoplasmic domain of SIRPα contains four immunoreceptor tyrosine-based inhibition motifs (ITIMs), which upon phosphorylation recruit and activate SH2-domain-containing phosphotyrosine phosphatases (PTPase) SHP-1 and SHP-2. This suggested that, in analogy to a variety of other ITIM-containing receptors (26, 34), SIRPα may function as an inhibitory receptor. There is now a considerable amount of evidence to support this idea. First, ectopic expression of SIRPα in fibroblasts has generally been shown to negatively regulate growth factor receptor signaling (15, 18). Unfortunately, apart from demonstrating a role for the SIRPα ITIMs, these studies have provided very little insight into the mechanism of regulation. For instance, the exact levels of cross talk and the physical associations required have not been established. Also, a major limitation of these studies is that fibroblasts, as opposed to the myeloid and neuronal cells that normally express SIRPα, lack detectable levels of SHP-1. Interestingly, experiments employing either macrophages from mice that lack the cytoplasmic domain of SIRPα or CD47-deficient target cells have demonstrated that SIRPα signaling upon interaction with CD47 negatively regulates the phagocytosis of host cells in vitro and in vivo, an effect that apparently involves SHP-1 (29, 30, 55). This indicates that SIRPα, upon coaggregation to the relevant activating receptors and PTPase recruitment and activation, negatively regulates immune effector responses and as such functions as some of the prototypic inhibitory receptors (e.g., KIR or FcγRIIB) do (26, 34). On the other hand, there is evidence that SIRPα is also involved in other macrophage functions, and this may require a different mode of action. For instance, we have shown that SIRPα expressed on monocytes is involved in transendothelial migration through interactions with CD47 on endothelial cells (5). Finally, antibodies against SIRPα have been reported to negatively regulate lipopolysaccharide (LPS)-induced tumor necrosis factor alpha (TNF-α) production in human monocytes (41). Unfortunately, the antibodies employed recognize not only SIRPα but also the highly related family member SIRPβ1 (3), which, based on its lack of cytoplasmic ITIMs and its capacity to signal via the immunoreceptor tyrosine-based activation motif-containing adaptor molecule DAP12, is more likely to function as an activating receptor (6, 47).
In the present study we have investigated the role of SIRPα in the production of proinflammatory mediators by macrophages. We have found that SIRPα ligation on macrophages results in the production of nitric oxide (NO). Our findings further suggest that this involves the cooperative action of JAK2/STAT and phosphatidylinositol 3-kinase (PI3-K)/Rac1/NADPH oxidase (NOX)/hydrogen peroxide (H2O2) signaling pathways. This supports the concept that SIRPα not only functions as a negative regulator but also provides positive signals.
MATERIALS AND METHODS
Antibodies.
Monoclonal antibodies (MAb) ED9, ED17 and MRC OX41 (all mouse IgG1) against rat SIRPα have been described previously (1) and are commercially available via Serotec (Oxford, United Kingdom). MAb OX101 (mouse IgG1) is directed against rat CD47 (54). Various mouse IgG1 antibodies, including OX45 (against rat CD48), OX34 (against rat CD2), OX27 (against rat MHCI), and BF5 (directed against human CD4; a kind gift of J. Wijdenes), were used as controls. MAb were purified from hybridoma supernatants cultured in RPMI 1640 containing 5% low-IgG fetal calf serum (FCS) (Life Technologies, Gaithersburg, MD). Fab and F(ab′)2 fragments were generated by standard papain and pepsin digestion, respectively. To prepare endotoxin-free preparations, MAb were run over a polymyxin B column (Pierce, Rockford, IL), and when tested by the Limulus assay they yielded final LPS concentrations in culture of <8 pg/ml. Rabbit anti-SHP-1, rabbit anti-SHP-2, and rabbit anti-SIRPα (used for Western blotting); rabbit anti-inducible nitric oxide synthase (anti-iNOS) (M-19); rabbit anti-Rac2; and rabbit anti-p65-NF-κB were obtained from Santa Cruz Biotechnology. Rabbit anti-SIRPα (used for immunoprecipitations), mouse anti-STAT3, and mouse anti-STAT1 (N terminus) were from BD Biosciences. Mouse anti-STAT1-PY701 was from Zymed. Antiphosphotyrosine (anti-PY) 4G10, rabbit anti-JAK2, and mouse anti-Rac1 (clone 23A8) were purchased from Upstate Biotechnology, Lake Placid, NY.
Cells, culture conditions, and measurement of NO, reactive oxygen species (ROS), and cytokines.
The rat NR8383 macrophage cell line and peritoneal macrophages, obtained by peritoneal lavage from 6- to 12-week-old WAG/Rij rats as described before (1), were cultured at 106 cells/ml in RPMI 1640 medium containing 10% FCS (Gibco BRL), 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Where indicated, cells were serum starved (0.5% FCS) for 24 h prior to stimulation. The stimuli anti-SIRPα or control IgG1 MAb (10 μg/ml) or F(ab)2 or F(ab′) fragments thereof (20 μg/ml), Escherichia coli strain 055:B5 LPS (100 ng/ml) (Difco, Detroit, MI), and rat recombinant gamma interferon (IFN-γ) (20 U/ml) (a generous gift from P. van der Meide) were added where indicated. The inhibitors LY294002 (40 μM) (Calbiochem), AG490 (0.4 to 100 μM) (Calbiochem), TAT-Rac1C-terminal peptide and TAT control peptide (200 μg/ml) (51) (kindly provided by P. Hordijk), bisindolylmaleimide I (10 nM) (Calbiochem), SN50 peptide (50 μg/ml) (Biomol Research Laboratories), and reactive oxygen radical scavengers superoxide dismutase (SOD) (104 U/ml) (Sigma), catalase (104 U/ml) (Sigma), and mannitol (10 mM) (BDH) were added as indicated, either simultaneously with the stimulus or in a preincubation. The NADPH oxidase inhibitor apocynin (Roth) was recrystallized and used at the indicated concentrations. After 18 to 24 h, NO production (using Griess reagent) (7) and TNF-α, interleukin-1β (IL-1β), and IL-6 production (using enzyme-linked immunosorbent assay) (22, 35) in culture supernatants were determined. All incubations were performed in triplicate, and results are expressed as means ± standard deviations (SD). The nitroblue tetrazolium (NBT) assay was performed as described previously (50). Briefly, peritoneal macrophages (0.5 × 106 in 0.5 ml culture medium) were allowed to adhere to coverslips in a 24-well tissue culture plate for 1.5 h. The cells were incubated with or without intact antibodies (40 μg/ml) simultaneously with NBT (0.5 mg/ml; Sigma). After 1 h cells were washed, fixed in methanol, and counterstained with nuclear fast red.
Fusion protein constructs and microsphere bead binding.
The rat SIRPα-CD4 domain 3 + 4 biotin (CD4d3 + 4) chimeric protein was constructed as previously described (4). Fusion proteins were expressed in 293T cells, concentrated from supernatants by using 10-kDa-cutoff Centricon filters (Amicon, Beverley, MA), and biotinylated using recombinant E. coli BirA enzyme (Avidity, Denver, CO) according to the manufacturer's instructions. Binding studies were carried out and analyzed by flow cytometry as previously described (54). Rat peritoneal macrophages or thymocytes (0.5 × 106) were plated in flat-bottomed microtiter plates and, where appropriate, incubated with OX101 or control (OX45) hybridoma supernatants for 1 h at 4°C. Fluorescent avidin-coated microspheres (catalog no. VFP-0552-5; Spherotech, Libertyville, Illinois) were coated with fusion proteins (2 μg/1.5 × 108 beads) for 1 h at 4°C. Washed beads were then incubated with antibody Fab fragments (20 μg/ml) where appropriate, washed again, sonicated, and incubated for 1 h at 4°C with the cells in 0.2% bovine serum albumin (BSA)-phosphate-buffered saline. Beads coated with CD4d3 + 4 were used as a negative control. CD47-Fc proteins were constructed, produced, and purified as described previously for the SIRPα-Fc and sialoadhesin(R97A)-Fc proteins (48, 49).
Immunoprecipitation and Western blotting.
Serum-starved cells were incubated in RPMI-HEPES plus 0.5% BSA at 37°C with the indicated inhibitors and then stimulated with 10 μg/ml of ED9 for the indicated periods, washed in ice-cold phosphate-buffered saline, and lysed in lysis buffer containing 0.5% NP-40, 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM MgCl2, 1 mM EGTA, protease inhibitors (10 μg/ml leupeptin, 1 mM pepstatin, 1 μg/ml aprotinin, 0.1 mg/ml Pefabloc), and phosphatase inhibitors (0.1 mM Na-orthovanadate, 10 mM NaF, 10 mM Na2P2O7). All subsequent steps were performed at 4°C. Centrifuged lysates were precleared for 1 h with protein A/G-Sepharose (Pharmacia), followed by incubation with antibody precoupled to protein A/G-Sepharose (0.2 to 1 μg/immunoprecipitation) for 1 h. After washing with lysis buffer, the precipitates were denatured in reducing sample buffer, electrophoresed (10% sodium dodecyl sulfate [SDS]-polyacrylamide gel electrophoresis), and blotted onto nitrocellulose membrane (Schleicher and Schuell). The blots were incubated with the indicated antibodies, followed by peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG (Pierce), and staining was visualized using Supersignal West Dura (Pierce) and a Gelimager (Epi Chemi II Darkroom combined with a 12 bit SensiCam charge-coupled device camera driven by LabWorks 4.0; from UVP). Stripping was done with Restore Western blot stripping buffer from Pierce.
For staining of total cellular protein, cells were incubated at 37°C as described above with or without inhibitors and stimuli and directly lysed in reducing SDS sample buffer. Western blotting of these samples was done as above. Equal loading was confirmed by Ponceau-S staining.
Rac1 pull-down.
Pull-down of GTP-bound Rac1 was done essentially as described before (32). In short, 2 × 107 NR8383 cells were incubated in 1 ml RPMI-HEPES plus 0.5% BSA at 37°C with the indicated inhibitors and/or 10 μg/ml anti-SIRPα (ED9). Cells were lysed on ice by addition of 0.5 ml ice-cold, 3× concentrated lysis buffer (final concentrations, 0.5% NP-40, 50 mM Tris pH 7.4, 150 mM NaCl, 5 mM MgCl2, 10 μg/ml leupeptin, 1 μg/ml aprotinin, and 0.1 mg/ml Pefabloc) supplemented with biotinylated PAK-CRIB peptide (2 μg/assay; kindly provided by J. Collard, Netherlands Cancer Institute, Amsterdam, The Netherlands). After 5 min, lysates were cleared by centrifugation at 4°C, transferred to fresh tubes containing streptavidin-agarose beads (20 μl packed; Sigma), and rotated for 30 min at 4°C. The beads were washed three times with lysis buffer, resuspended in reducing SDS sample buffer, and boiled. Western blots of the samples were stained with anti-Rac1 (Upstate) as described above.
Translocation of p65 NF-κB.
Peritoneal macrophages were cultured at 106 cells/ml in chamber slides for 48 h. Cells were stimulated with E. coli strain 055:B5 LPS (100 ng/ml) (Difco, Detroit, MI), intact MAb ED9 (10 μg/ml), or anti-human CD4 MAb (10 μg/ml) for 30 min. After stimulation, cells were fixed in methanol for 10 min and incubated for 1 h with anti-p65 NF-κB and donkey anti-rabbit Ig-fluorescein isothiocyanate. Nuclei were stained with Hoechst stain. Subsequently, slides were embedded in Fluorestab (ICN Biomedicals, Costa Mesa, CA) and viewed on an Eclipse E800 fluorescence microscope (Nikon, Badhoevedorp, The Netherlands).
RESULTS
Ligation of SIRPα induces macrophage NO production.
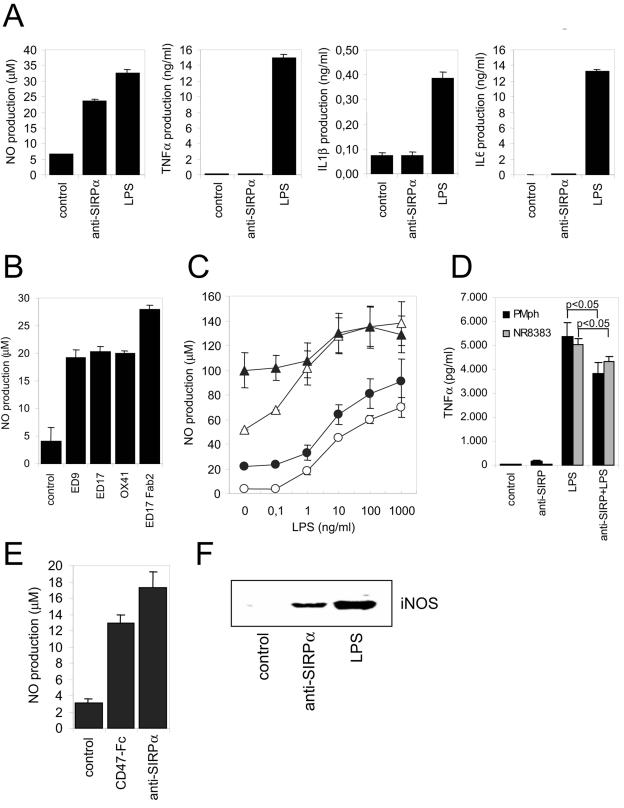
It has been shown that stimulation by ligands of SIRPα and/or SIRPβ1 leads to inhibition of LPS-induced cytokine/chemokine production in monocytes or macrophages (12, 41). To investigate whether (selective) SIRPα ligation can regulate the production of inflammatory mediators in macrophages, rat NR8383 alveolar macrophages were cultured in the presence of the rat SIRPα-specific MAb (1) and evaluated for NO, TNF-α, IL-1, and IL-6 production. As shown in Fig. 1A, anti-SIRPα triggered the production of NO to levels almost comparable to those obtained with 100 ng/ml LPS. SIRPα ligation did not result in the production of TNF-α, IL-1β, or IL-6. The effect on NO production was dependent on the concentration of antibody added, with an optimal response at ∼1 μg/ml (see Fig. S1A in the supplemental material). It was observed with several rat SIRPα-specific antibodies (Fig. 1B), of which ED9 and ED17 recognize an overlapping epitope and MRC OX41 recognizes a distinct epitope (1). No detectable effects on NO production were seen with a number of isotype-matched control antibodies directed against either human CD4 (BF5), CD2 (OX34), or MHCI (OX27). Furthermore, F(ab′)2 fragments were at least as efficient, thereby excluding the involvement (e.g., by co-cross-linking) of Fcγ receptors. When NO production in freshly isolated rat peritoneal macrophages was studied and SIRPα cross-linking was combined with other stimuli, such as LPS and IFN-γ, the effects were essentially additive and no evidence of synergy was obtained (Fig. 1C). On the other hand, SIRPα ligation moderately but significantly inhibited TNF-α production triggered by LPS (Fig. 1D), a result that corresponds with reported findings for human monocytes (41). To investigate whether ligation of SIRPα with MAb mimics the effect of its natural cellular ligand CD47, a recombinant fusion protein composed of the extracellular Ig domain of mouse CD47 and the Fc portion of human IgG1 (CD47-Fc) was used. As can be seen in Fig. 1E, CD47-Fc also induced NO production in NR8383 macrophages. This was also observed when immobilized (i.e., plastic-coated) CD47-Fc was employed (data not shown). No effects were detected with SIRPα-Fc or mutant (R97A) sialoadhesin-Fc proteins. The triggering of NO production by SIRPα ligation was accompanied by enhanced expression of iNOS protein (Fig. 1F).
FIG. 1.
Ligation of SIRPα induces macrophage NO production. (A) Rat NR8383 macrophages were cultured for 18 h. in the presence of control IgG1 (MAb BF5, 20 μg/ml), anti-SIRPα MAb ED9 (20 μg/ml), or LPS (100 ng/ml), and amounts of secreted NO, TNF-α, IL-1β, or IL-6 were determined. (B) NO production in NR8383 macrophages was measured after addition of different intact MAb or corresponding F(ab)2 fragments (all at 20 μg/ml; 18 h of incubation). (C) Effects of control IgG1 (20 μg/ml, ○), anti-SIRPα MAb ED9 (20 μg/ml, •), IFN-γ (20 U/ml, ▵), or ED9 plus IFN-γ (▴) either alone, or in combination with various LPS concentrations, on NO production in rat peritoneal macrophages. (D) TNF-α secretion by peritoneal macrophages (PMph) or NR8383 cells cultured in the presence or absence of ED9 (20 μg/ml) and/or LPS (100 ng/ml) for 18 h (P values were obtained by Student's t test). (E) NO production in NR8383 macrophages triggered by murine CD47-Fc protein (25 μg/ml; 18 h of incubation) or anti-SIRPα MAb ED9 (10 μg/ml). (F) Anti-SIRPα MAb ED9 (10 μg/ml; 18 h of incubation) induces iNOS protein expression in NR8383 macrophages as shown by Western blotting. Experiments were performed at least in triplicate, and results are shown as means ± SD.
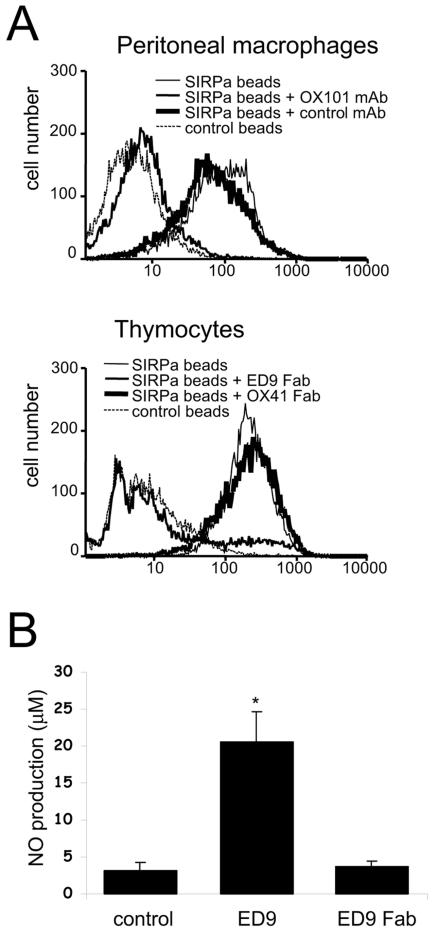
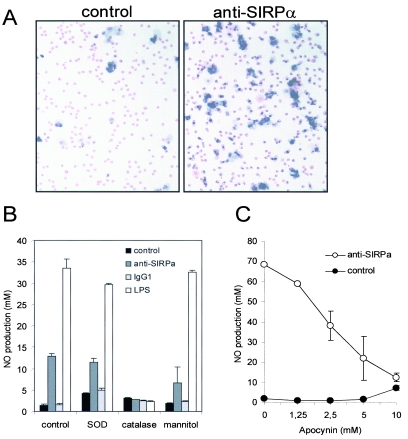
Macrophages, including NR8383 cells and rat peritoneal macrophages, express both SIRPα and its cell surface ligand CD47 (5) (results not shown). We investigated whether the observed effects on NO production either were due to blocking of putative SIRPα-CD47 interactions (i.e., antagonism) (5) or were the direct result of cross-linking of SIRPα by using the bivalent antibodies (i.e., agonism). We first tested whether fluorescent microspheres coated with a recombinant fusion protein composed of the three extracellular Ig domains of rat SIRPα and domains 3 and 4 of rat CD4 could bind in a CD47-dependent fashion to macrophages. Figure 2A shows that the SIRPα beads showed strong binding to rat peritoneal macrophages and that this was abolished by preincubation of the cells with anti-rat CD47 MAb OX101. This demonstrates that SIRPα can bind to macrophages and that essentially all binding is mediated by CD47. Next, we investigated whether monovalent ED9 Fab fragments could block the binding of SIRPα beads to rat thymocytes, which do not express SIRPα themselves but have previously been shown to interact with SIRPα beads in a CD47-dependent fashion (54). As can be seen in Fig. 2A, ED9 Fab fragments almost completely blocked binding, whereas Fab fragments of MAb OX41, which recognize a different SIRPα epitope (1), were ineffective. The availability of a monovalent (i.e., purely antagonistic) blocking reagent allowed us to test whether putative SIRPα-CD47 interactions control NO production by macrophages. As shown in Fig. 2B, ED9 Fab fragments did not induce NO production in NR8383 macrophages, while under the same conditions intact agonistic ED9 antibodies did, thus demonstrating that the effect requires surface oligomerization of SIRPα. Further cross-linking using secondary reagents (even at suboptimal concentrations of ED9) gave only limited enhancement of anti-SIRPα-induced NO production, perhaps suggesting that the formation of relatively small SIRPα aggregates on the cell surface is already sufficient to generate an optimal response (results not shown).
FIG. 2.
SIRPα-induced NO production by macrophages does not involve blocking of SIRPα-CD47 interactions. (A) Flow cytometric analysis of the binding of beads coated with rat SIRPα-CD4d3 + 4 chimeric protein (or control CD4d3 + 4 beads) to rat peritoneal macrophages or rat thymocytes. Where indicated, cells were preincubated with anti-CD47 MAb OX101 or control IgG1 (MAb OX45) or with anti-SIRPα ED9 Fab fragments or OX41 Fab fragments. (B) NO production by NR8383 cells stimulated with intact ED9 MAb (10 μg/ml) or ED9 Fab fragments (20 μg/ml). Data are expressed as the means ± SD from three independent experiments (*, P < 0.01 by Student's t test).
Taken together, these results show that activation of SIRPα with agonistic antibodies or CD47-Fc protein generates a signal that triggers expression of iNOS and the production of NO in macrophages.
SIRPα-mediated NO production involves JAK2-STAT activation but is NF-κB independent.
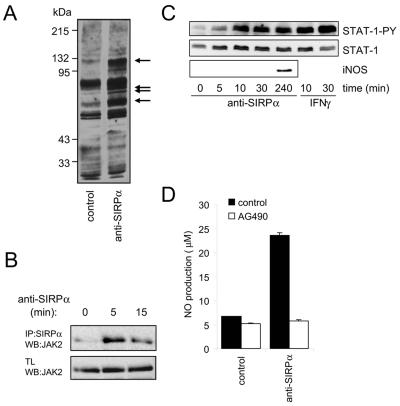
The above results demonstrated that SIRPα is able to provide negative as well as positive signals to regulate cellular macrophage functions. Subsequently, we focused on the signaling pathway(s) mediating SIRPα-induced NO production. The JAK-STAT and NF-κB pathways are known to play a critical role in the induction of iNOS gene expression in macrophages (11), and it was therefore of interest to investigate their possible involvement. Notably, the rat iNOS promoter (like its human and mouse counterparts) is known to contain functional STAT- and NF-κB-responsive elements (44). SIRPα is able to directly associate with the tyrosine kinase JAK2, albeit when both components are overexpressed in, e.g., COS-1 cells (42). Total protein tyrosine phosphorylation was increased within minutes following SIRPα ligation using intact or F(ab′)2 fragments of anti-SIRPα MAb in NR8383 cells (Fig. 3A). We investigated whether endogenous JAK2 associates with SIRPα in macrophages. As can be observed in Fig. 3B, immunoprecipitates of SIRPα from unstimulated NR8383 cells contain a low but detectable amount of JAK2, which is significantly increased after SIRPα stimulation. Parallel stainings of the total lysates with anti-PY antibody suggested an increase in JAK2 tyrosine phosphorylation after SIRPα triggering (results not shown; see, for example, Fig. 7C). To investigate whether STAT activation occurs after SIRPα ligation, we monitored phosphorylation of the essential STAT1 tyrosine Y701, which is known to be required for transcriptional activity (24). As shown in Fig. 3C, SIRPα triggering resulted in a rapid (within 5 to 10 min) STAT1 phosphorylation (STAT1-PY). The level of STAT1-PY was similar to that obtained with IFN-γ (20 U/ml), which constitutes a potent stimulus for JAK-STAT activation. The appearance of tyrosine-phosphorylated STAT1 preceded the induction of iNOS protein (Fig. 3C, lower panel), consistent with a role in iNOS gene expression. Finally, to address the involvement of JAK2 in SIRPα-mediated NO production, we tested the effect of the JAK2 inhibitor AG490 (tyrphostin B42) (23). As can be seen in Fig. 3D, AG490 completely blocked NO production induced via SIRPα. Dose-response experiments demonstrated complete inhibition at concentrations of 10 to 25 μM and higher and an 50% inhibitory concentration of ∼3 μM (see Fig. S1B in the supplemental material), which is consistent with reported data on JAK2 inhibition by AG490 in macrophages (2). Taken together, these data strongly suggest that SIRPα ligation, most likely via activation of associated JAK2, results in STAT1 activation and as a consequence in the induction of iNOS expression and the production of NO by macrophages.
FIG. 3.
Role of JAK2/STAT signaling in SIRPα-induced NO production in NR8383 macrophages. (A) Tyrosine phosphorylation in macrophages stimulated for 5 min with control IgG1 (MAb BF5, 20 μg/ml) or anti-SIRPα MAb ED9 (20 μg/ml). Total lysates of NR8383 cells subjected to Western blotting and staining with anti-PY MAb 4G10. Note that SIRPα ligation promotes tyrosine phosphorylation of several cellular proteins (arrows). (B) Immunoprecipitates (IP) of SIRPα in NR8383 cells after stimulation by anti-SIRPα MAb ED9 (10 μg/ml) or total lysates (TL) were Western blotted (WB) and probed with anti-JAK2. Note that JAK2 associates with SIRPα in particular after SIRPα ligation by anti-SIRPα MAb ED9 (10 μg/ml). (C) Anti-SIRPα MAb ED9 (10 μg/ml)- or IFN-γ (20 U/ml)-stimulated NR8383 cells were lysed directly in SDS sample buffer. The panels show the same Western blot probed sequentially with anti-STAT1-PY, total anti-STAT1, and anti-iNOS antibodies. (D) Anti-SIRPα (MAb ED9, 20 μg/ml, 18 h of incubation)-induced NO production with or without 30 min of preincubation with AG490 (10 μM). Results from representative experiment that was performed in triplicate are shown (results are expressed as means ± SD).
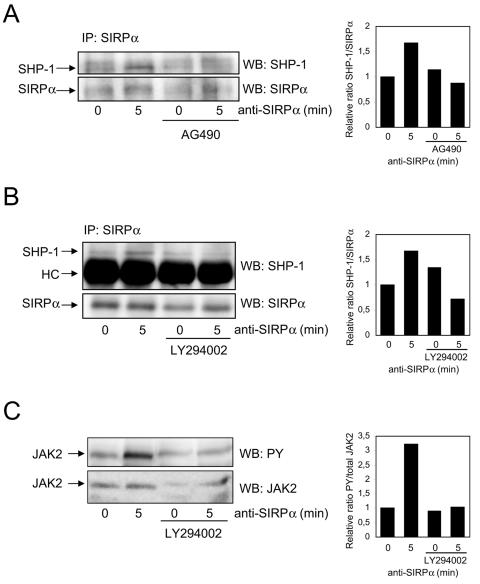
FIG. 7.
Ligation of SIRPα promotes the JAK- and PI3-K-dependent recruitment of SHP-1 in macrophages. NR8383 cells were preincubated with AG490 (10 μM; 30 min) or LY294002 (40 μM; 30 min), control cells were incubated with vehicle, and then cells were stimulated with anti-SIRPα (10 μg/ml ED9) for 5 min, lysed, and immunoprecipitated (IP) with anti-SIRPα Abs (rabbit anti-SIRPα from PharMingen in panels A and B) or directly lysed in reducing SDS sample buffer (C). Western blots (WB) of these samples were stained with the indicated antibodies recognizing SHP-1, SHP-2, SIRPα (Santa Cruz Ab), PY, or JAK2 as described in Materials and Methods. HC, heavy chain.
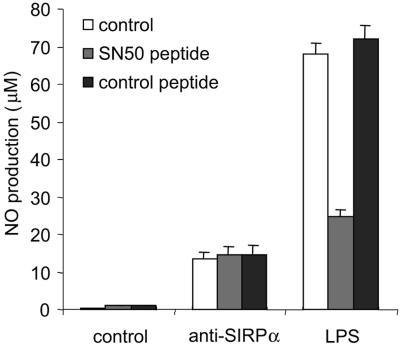
As indicated above, NF-κB could also be involved in SIRPα-induced NO production. Translocation of p65 NF-κB to the nucleus after SIRPα ligation was examined. Indeed, 30 min of SIRPα triggering on peritoneal macrophages resulted in a clear nuclear translocation of p65 NF-κB as shown by double staining with Hoechst dye (see Fig. S2 in the supplemental material). The extend of p65 NF-κB translocation observed was roughly similar to that observed with LPS. A cell-permeative peptide (SN50) containing the nuclear localization sequence of p50 NF-κB and capable of blocking transport of NF-κB across the nuclear membrane (25) was employed to address the role of NF-κB in SIRPα-induced NO production. As can be seen in Fig. 4, SN50 peptide at concentrations able to strongly inhibit LPS-induced NO production had no detectable effect on SIRPα-mediated NO production. This demonstrates that NF-κB, although activated by SIRPα, does not contribute to SIRPα-dependent NO production.
FIG. 4.
Effect of NF-κB inhibitor on SIRPα-induced NO production. Anti-SIRPα (MAb ED9, 20 μg/ml)- or LPS (100 ng/ml)-induced NO production (18 h incubation) in the absence or presence of cell permeative, NF-κB-inhibitory SN50 peptide or inactive control peptide (50 μg/ml, added 30 min before ED9/LPS) is shown. The experiment was performed in triplicate, and results are shown as means ± SD.
SIRPα-induced NO production requires H2O2.
Not only does the oxidative burst constitute an important antimicrobial activity in macrophages, but the generated ROS also act as second messengers/autocrine regulators in various cells, including macrophages (8, 21, 36). Therefore, we investigated the effect of SIRPα ligation on the oxidative burst and the role of ROS in SIRPα signaling. Indeed, SIRPα antibodies triggered the macrophage oxidative burst as measured with the NBT assay (Fig. 5A). The increased ROS production was independent of the antibody Fc portion, since it was also observed with F(ab′)2 fragments (data not shown). The possible involvement of individual extracellular ROS in the production of SIRPα-induced NO was investigated by using selective scavengers for superoxide (SOD), H2O2 (catalase), and hydroxyl radicals (mannitol) (50). H2O2 in particular was found to be essential for the SIRPα-induced production of NO (Fig. 5B). Apocynin, an inhibitor of NOX assembly and activation (43, 50), also inhibited SIRPα-mediated NO production (Fig. 5C). Furthermore, NO production was also completely inhibited with the antioxidants pyrrolidine dithiocarbamate (5 mM) and diphenylene iodonium (an inhibitor of flavoprotein-dependent oxidases) (50 μM) (data not shown). Addition of H2O2 or the ROS donor menadione to NR8383 cells did not result in detectable levels of NO (data not shown), suggesting that H2O2 alone is not sufficient for SIRPα-induced NO production.
FIG. 5.
SIRPα-induced triggering of the oxidative burst and the role of H2O2 in SIRPα-mediated NO production. (A) Superoxide production in rat peritoneal macrophages analyzed by NBT assay in the presence or absence of control IgG1 (MAb BF5, 20 μg/ml) or anti-SIRPα MAb (ED9, 20 μg/ml) for 90 min. (B) Effect of the ROS scavengers SOD (104 U/ml), catalase (104 U/ml), and mannitol (10 mM) on SIRPα-induced (ED9, 20 μg/ml, 18 h) NO production in NR8383 cells. (C) SIRPα MAb ED9-induced production of NO in NR8383 cells in the presence of the NADPH oxidase inhibitor apocynin. All experiments were performed in triplicate, and results are shown as means ± SD.
Taken together, these results demonstrate that SIRPα ligation activates NOX and that H2O2 is essential for SIRPα-induced NO production.
SIRPα signaling involves PI3-K-dependent activation of Rac1.
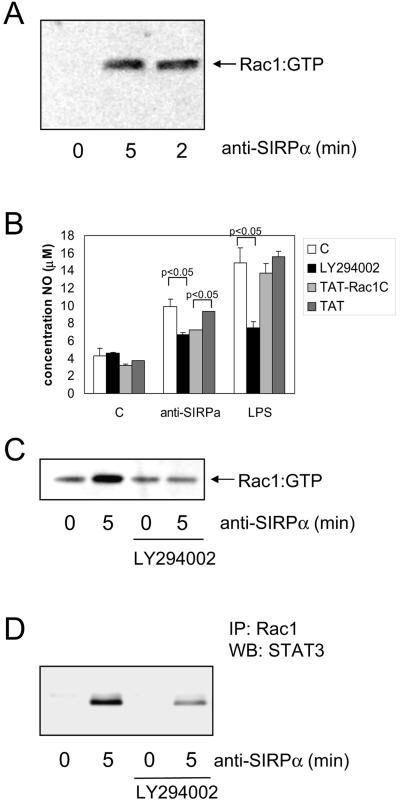
One way in which SIRPα could trigger assembly and activation of NOX in macrophages is through activation of the small GTPase Rac1, an intrinsic component of the NOX complex (33). Consistent with previous reports (57), we found that NR8383 macrophages express Rac1 but do not express detectable levels of Rac2 (data not shown). The amount of active (i.e., GTP-bound) Rac1 in macrophages was measured by employing a pull-down assay using biotinylated p21-activated kinase peptide (32). SIRPα ligation resulted in a rapid and potent Rac1 activation, as can be seen in Fig. 6A. The involvement of Rac1 in SIRPα-triggered NO production in NR8383 macrophages was evaluated by adding cell-permeative (i.e., HIV-TAT) peptides based on the C terminus (residues 178 to 188) of Rac1 (TAT-Rac1C) (51). This C-terminal domain has previously been shown to be required for activation of NOX (20). As can be seen in Fig. 6B, TAT-Rac1C inhibited NO production by ∼50%, while it had little effect on LPS-induced NO production. We observed no effect of the actin-depolarizing agent cytochalasin B (5 μM) on SIRPα-induced NO production. This and other observations essentially dissociate NO production through SIRPα signaling from the concomitant cytochalasin B-sensitive homotypic aggregation that was also observed after SIRPα ligation (J. Alblas et al., unpublished data). These findings suggest that, with respect to NO production via SIRPα, Rac1 acts primarily by activating NOX and not via modulation of the actin cytoskeleton.
FIG. 6.
Role of Rac1 and PI3-K in SIRPα-induced NO production in macrophages. (A) Rac1 activation was determined (see Materials and Methods for details) in NR8383 cells following SIRPα ligation using MAb ED9 (10 μg/ml). (B) NR8383 cells in the presence of LY294002 (40 μM; 30-min preincubation), cell permeable TAT-Rac1 C-terminal peptides (200 μM; 5-min preincubation), or control TAT peptide (200 μM) were subsequently stimulated by anti-SIRPα (10 μg/ml) or LPS (100 ng/ml). After 20 h, NO production in the supernatants was measured. Shown are results from a representative experiment performed in triplicate (means ± SD). The differences between control and LY294002 in the anti-SIRPα- and LPS-treated samples and between Tat and Tat-Rac1 in the anti-SIRPα-treated samples are statistically significant (P < 0.05 by Student's t test). (C and D) NR8383 cells were pretreated for 30 min with LY294002 (40 μM) and then stimulated with anti-SIRPα (10 μg/ml) for 5 min and lysed. From these lysates, active Rac1 was pulled down as described in Materials and Methods (C), and subsequently, total Rac1 was immunoprecipitated (IP) and the associated STAT3 shown on a Western blot (WB) (D). Shown are representative results from three experiments.
A candidate upstream regulator of Rac1 is PI3-K (31). In particular, SHP-2 has been shown to regulate the activity of the p85 subunit of PI3-K (56). To explore this possibility, we tested the effect of the PI3-K inhibitor LY294002 on SIRPα-induced Rac1 activation. As can be seen in Fig. 6C, LY294002 completely blocks Rac1 activation. Furthermore, it inhibits (by ∼60%) SIRPα-mediated NO production (Fig. 6B). Another documented pathway that can lead to Rac1 activation in macrophages involves protein kinase C (33). We found no effect of protein kinase C inhibition on SIRPα-induced Rac1 activation by using the broad-spectrum inhibitor bisindolylmaleimide I.
Considering the observed association of JAK2 with SIRPα and the role of JAK2 in SIRPα-induced NO production, we evaluated the effect of JAK2 inhibition by AG490 on Rac activity. We observed a nonspecific increase in Rac1 activation after treatment of the cells with AG490, indicative of a negative feedback route from JAK2 to Rac1 (results not shown). One possible level of integration of the Rac1 and JAK2-STAT signaling pathways is through direct interaction of Rac1 with STAT3 (40). Upon SIRPα activation, we observed the presence of STAT3 in immunoprecipitates of Rac1. This Rac1-STAT3 interaction could be partly inhibited by LY294002 (Fig. 6D), consistent with the PI3-K dependence of Rac1 activation.
Taken together, these findings demonstrate that triggering of SIRPα in macrophages involves the PI3-K-dependent activation of Rac1, which contributes to NOX activation, leading to H2O2 production and the subsequent generation of NO.
SIRPα ligation enhances SHP-1 and SHP-2 recruitment via JAK2 and PI3-K.
Clearly, it was of interest to investigate whether SIRPα ligation affected its association with SHP-1 and SHP-2. As shown by SIRPα immunoprecipitation and Western blotting with SHP-1- and SHP-2-specific antibodies (Fig. 7; see Fig. S1C in the supplemental material), SHP-1, and perhaps also some SHP-2, was found to be constitutively associated with SIRPα in untreated macrophages. After cross-linking (for 5 min) with either intact antibody or F(ab′)2 fragments, a clear increase in SIRPα-associated SHP-1 and SHP-2 was observed. In order to investigate whether the SIRPα ITIM tyrosines constitute substrates for JAK2 and as a result lead to enhanced PTPase docking, we studied the effect of AG490. The enhanced association of SHP-1 with SIRPα upon ligation for 5 min was essentially prevented by AG490 (Fig. 7A). Another factor that may have contributed to the enhanced association of PTPases with SIRPα upon ligation could be H2O2. H2O2 can act as a potent inhibitor of PTPases by reversibly modifying their active-site cysteine residues (27, 36, 37) and was anticipated to reduce the level of SIRPα dephosphorylation by SHP-1 and/or SHP-2, thereby increasing their own association with SIRPα (see also Fig. 8). Consistent with this, we observed that inhibition of PI3-K (by LY294002) apparently prevented the increased SHP-1 association with SIRPα (Fig. 7B). Also, the overall cellular increase in JAK2 tyrosine phosphorylation observed upon SIRPα ligation was prevented by LY294002 (Fig. 7C), indicating that SIRPα-triggered PI3-K activity contributes to JAK-STAT activation. Similar results were obtained when JAK2 was immunoprecipitated (data not shown).
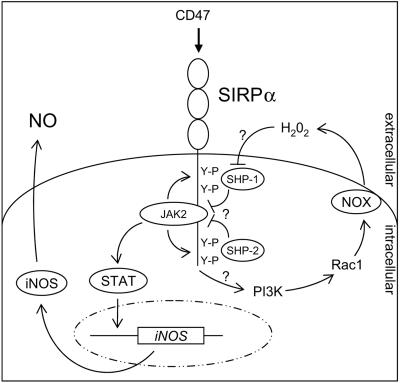
FIG. 8.
Model for the signaling events that mediate SIRPα-induced NO production in macrophages. SIRPα oligomerization by ligand triggers cross-phosphorylation of SIRPα-associated JAK2 and promotes SIRPα tyrosine phosphorylation, which then allows STAT docking and tyrosine phosphorylation. Activated STAT dimers induce iNOS gene expression and subsequent NO production. Simultaneously, SIRPα ligation triggers Rac1 activation, in a PI3-K-dependent fashion, followed by NOX assembly and oxidative burst generation. The resultant H2O2 inhibits the catalytic activity of SIRPα-associated SHP-1 and SHP-2, thereby preventing JAK2 and SIRPα dephosphorylation resulting in sustained JAK-STAT signaling and enhanced SHP-1 and SHP-2 retention by SIRPα.
DISCUSSION
In the present study we have investigated the role of SIRPα in the production of inflammatory mediators by macrophages. Our findings demonstrate that ligation of SIRPα on macrophages, induced by antibody or CD47-Fc, but without deliberate co-cross-linking to an activating receptor/pathway, results in the production of NO and ROS (Fig. 1 and 5). Furthermore, we have shown that this effect was due to agonistic ligation and oligomerization of surface SIRPα, rather than to the blocking of SIRPα-CD47 interactions (Fig. 2). The results provide evidence that the SIRPα-induced production of NO involves the tyrosine kinase JAK2, which is presumed to act by activation of one or more STAT proteins (Fig. 3). In addition, our results demonstrate an essential role for extracellular H2O2 produced by the macrophage NOX, which is probably assembled and activated by sequential PI3-K and Rac1 activation (Fig. 5 and 6). These findings suggest that SIRPα, upon ligation in macrophages, is able to provide positive signals that trigger NO production and perhaps other effects as well. In addition, they provide the first evidence that SIRPα signaling involves Rac1 and H2O2 and that SIRPα ligation in macrophages triggers JAK2-STAT activation.
It could be argued that the stimulatory action we observed is perhaps due to aggregation of a SIRPα-related “activating” SIRPβ1 molecule described for humans and mice (6, 13), which is associated with the ITAM-containing DAP12 molecule. However, in our experiments cross-reaction with putative SIRPβ1 on rat macrophages is very unlikely, because rats do not encode a SIRPβ1 orthologue or a closely related SIRPβ homologue (E. van Beek and T. K. van den Berg, unpublished data). In addition, immunoprecipitations with our SIRPα monoclonal antibodies from NR8383 cells detect only a single protein at 110 kDa, consistent with only the SIRPα protein being recognized (42). Also, we found that NO production can be induced by soluble CD47 protein, which binds SIRPα but not SIRPβ1 (3, 38), supporting the idea that SIRPα alone mediates all of the observed effects.
Based on the available evidence, we propose a working model in which ligation of SIRPα leads to JAK2/STAT activation and as a consequence iNOS gene expression and NO production (Fig. 8). SIRPα-induced JAK2/STAT activation likewise involves a sequence of events analogous to that proposed for cytokine receptors, including JAK2 cross-phosphorylation (probably driven by ligand-mediated oligomerization), SIRPα tyrosine phosphorylation by JAK2, STAT docking, and JAK2-mediated STAT tyrosine phosphorylation and activation. There is evidence that the C-terminal tyrosine residue of the SIRPα cytoplasmic tail functions as a substrate for JAK2-dependent phosphorylation (42). In addition to STAT1 tyrosine phosphorylation, we have also observed STAT1 serine phosphorylation in response to SIRPα ligation (unpublished observation). This is probably also relevant, since optimal STAT activation requires both tyrosine and serine phosphorylation (52).
Apart from JAK2/STAT activation, SIRPα ligation triggers activation of NOX, likewise via sequential PI3-K and Rac1 activation. The resultant ROS, in particular H2O2, apparently play a critical role in iNOS induction, possibly through the inactivation of SIRPα-associated SHP-1 and SHP-2, which are proposed to deliver negative feedback to JAK2/STAT activation by dephosphorylating the critical tyrosines in JAK2, SIRPα, and/or STAT. In general, there is evidence that ROS can act upstream from JAK2 in smooth muscle cells (9). Our view is supported by published evidence that JAK2 can indeed act as a substrate for SHP-1 (17) and, perhaps more importantly, by the observation that SIRPα tyrosine mutation enhances JAK2 and STAT phosphorylation (42). In a similar fashion, erythropoietin receptor-associated SHP-1 mediates dephosphorylation of JAK2 (19). While our present data do not provide direct evidence for the involvement of SHP-1 and SHP-2 inactivation in SIRPα-induced NO production, there is a growing body of evidence showing that hydrogen peroxide acts as a potent inhibitor of tyrosine phosphatases, including SHP-2 (27, 36, 37). We propose that the enhanced level of SIRPα-associated SHP-1 and SHP-2 after ligation (Fig. 7A) is not only caused by JAK2-dependent tyrosine phosphorylation of SIRPα (Fig. 7B) but is also the consequence of H2O2-dependent PTPase inactivation (Fig. 7C), which would reduce SIRPα dephosphorylation and as a result enhance PTP binding. This idea is also supported by genetic evidence, since macrophages from viable motheaten mutant mice, which have a catalytically inactive SHP-1, show SIRPα hyperphosphorylation and enhanced SHP-1 binding (45). Perhaps one of the most interesting aspects of our model is that high levels of NO production triggered via SIRPα require both JAK2/STAT activation but also SHP-1 and SHP-2 inactivation. Such a dual mechanism allows tight control over NO levels. This is probably important because NO, while being a potent bactericidal substance, may also be very harmful to the host.
An association between SIRPα and JAK2 upon overexpression of both components in COS cells has previously been reported (42). Here we provide evidence for a JAK2-SIRPα interaction in macrophages at endogenous levels of both components. The molecular basis of JAK2 binding to SIRPα remains to be established, but available evidence suggests that it is independent of the SIRPα tyrosines (42). Interestingly, mammalian SIRPα contains two proline-rich regions in the cytoplasmic domain, interspersed between the first and second tyrosines and the third and fourth tyrosines, respectively (see Fig. S3 in the supplemental material). Such “box 1” regions are typical of cytokine receptors and mediate their interaction with JAK2, suggesting that they may also be responsible for the binding of JAK2 by SIRPα. Mutation analysis suggests that the C-terminal box 1-like region of at least human SIRPα is dispensable for JAK2 binding (42). Whether JAK2 binding involves the other proline-rich region in SIRPα or interacts with SIRPα in a different way remains to be established. While the exact basis for JAK2 binding by SIRPα has not been clarified, it also remains difficult to understand how this association can be promoted by SIRPα ligation (Fig. 3B). The interaction of JAKs with various receptors is generally considered to be ligand independent, although the growth hormone receptor shows ligand-promoted JAK2 binding (14).
While we have demonstrated that SIRPα ligation triggers NO production in NR8383 cells and peritoneal macrophages, our results show that in the same cells these SIRPα agonists can inhibit LPS-induced TNF-α production (Fig. 1D). The latter is in agreement with the results of Smith et al. (41), demonstrating that antibodies against SIRPα (and SIRPβ1) partly inhibit LPS-induced TNF-α secretion in human monocytes, and with those of Gardai et al. (12) using murine RAW-264.7 macrophages. Clearly, our present results suggest that SIRPα can provide a signal that can differentially (i.e., either negatively or positively) regulate proinflammatory mediator production. An interesting aspect is that both signals are (albeit partly) PI3-K dependent. In fact, Smith et al. (41) demonstrated increased activity of PI3-K upon SIRPα ligation. Moreover, they showed evidence for a direct interaction between SHP-2 and PI3-K that is promoted by SIRPα ligation, which provides a possible mechanistic explanation for the link between SIRPα and PI3-K.
An important remaining question is whether ligation of SIRPα by cell surface CD47, which is broadly expressed by hematopoietic and nonhematopoietic cells and is likely to be abundantly available to macrophages in vivo, can also trigger NO production through SIRPα. At least some of the activities of CD47-Fc protein can indeed be mimicked by cell-bound CD47 (28). Our own findings with myeloid cells indicate that SIRPα-induced elevation of tyrosine phosphorylation (Fig. 3A) can be triggered by cell-bound CD47 (Alblas et al., unpublished data). However, the encounter with cell surface CD47 per se may not be sufficient to trigger NO production in macrophages (H. Honing et al., unpublished observations). This is very understandable, since in the absence of further restrictions CD47-SIRPα interactions could lead to excessive, undesirable NO production and cause damage to the host. Perhaps the configuration of CD47 in the membrane of the opposing cell is also a critical factor. An alternative possibility is that SIRPα-induced NO production could be triggered primarily by soluble ligands, such as the oligomeric surfactant protein A or D, which have recently been identified as SIRPα ligands (12).
Taken together, the present findings indicate for the first time that SIRPα, in addition to the reported negative signals, is able to positively regulate macrophage activity. This suggests that SIRPα cannot be simply regarded as an inhibitory receptor but rather should be regarded as a receptor that can provide either positive or negative signals depending on the nature of the effector function. We anticipate that the picture will become perhaps even more complicated when some of the other SIRPα-associated signaling components (46) are taken into consideration. The challenge will be to understand whether and how these pathways integrate and translate into the various cellular functions that are regulated by SIRPα in macrophages and other cells.
Supplementary Material
Acknowledgments
We thank Neil Barclay, Dirk Roos, and Jeroen den Hertog for valuable discussions. Peter van der Meide, John Wijdenes, Edwin Kanters, Allard Kaptein, John Collard, and Peter Hordijk are acknowledged for generously providing reagents, and Priscilla Heijnen, Ed Döpp, and Karel Holen are acknowledged for technical assistance.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Adams, S., L. J. van der Laan, E. Vernon-Wilson, C. Renardel de Lavalette, E. A. Dopp, C. D. Dijkstra, D. L. Simmons, and T. K. van den Berg. 1998. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J. Immunol. 161:1853-1859. [PubMed] [Google Scholar]
- 2.Bright, J. J., C. Natarajan, S. Sriram, and G. Muthian. 2004. Signaling through JAK2-STAT5 pathway is essential for IL-3-induced activation of microglia. Glia 45:188-196. [DOI] [PubMed] [Google Scholar]
- 3.Brooke, G., J. D. Holbrook, M. H. Brown, and A. N. Barclay. 2004. Human lymphocytes interact directly with CD47 through a novel member of the signal regulatory protein (SIRP) family. J. Immunol. 173:2562-2570. [DOI] [PubMed] [Google Scholar]
- 4.Brown, M. H., K. Boles, P. A. van der Merwe, V. Kumar, P. A. Mathew, and A. N. Barclay. 1998. 2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J. Exp. Med. 188:2083-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vries, H. E., J. J. Hendriks, H. Honing, C. R. De Lavalette, S. M. van der Pol, E. Hooijberg, C. D. Dijkstra, and T. K. van den Berg. 2002. Signal-regulatory protein alpha-CD47 interactions are required for the transmigration of monocytes across cerebral endothelium. J. Immunol. 168:5832-5839. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich, J., M. Cella, M. Seiffert, H. J. Buhring, and M. Colonna. 2000. Cutting edge: signal-regulatory protein beta 1 is a DAP12-associated activating receptor expressed in myeloid cells. J. Immunol. 164:9-12. [DOI] [PubMed] [Google Scholar]
- 7.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 8.Forman, H. J., and M. Torres. 2001. Redox signaling in macrophages. Mol. Aspects Med. 22:189-216. [DOI] [PubMed] [Google Scholar]
- 9.Frank, G. D., M. Mifune, T. Inagami, M. Ohba, T. Sasaki, S. Higashiyama, P. J. Dempsey, and S. Eguchi. 2003. Distinct mechanisms of receptor and nonreceptor tyrosine kinase activation by reactive oxygen species in vascular smooth muscle cells: role of metalloprotease and protein kinase C-delta. Mol. Cell. Biol. 23:1581-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujioka, Y., T. Matozaki, T. Noguchi, A. Iwamatsu, T. Yamao, N. Takahashi, M. Tsuda, T. Takada, and M. Kasuga. 1996. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol. Cell. Biol. 16:6887-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganster, R. W., B. S. Taylor, L. F. Shao, and D. A. Geller. 2001. Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-kappa B. Proc. Natl. Acad. Sci. USA 98:8638-8643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardai, S. J., Y. Q. Xiao, M. Dickinson, J. A. Nick, D. R. Voelker, K. E. Greene, and P. M. Henson. 2003. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115:13-23. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi, A., H. Ohnishi, H. Okazawa, S. Nakazawa, H. Ikeda, S. Motegi, N. Aoki, S. Kimura, M. Mikuni, and T. Matozaki. 2004. Positive regulation of phagocytosis by SIRPbeta and its signaling mechanism in macrophages. J. Biol. Chem. 279:29450-29460. [DOI] [PubMed] [Google Scholar]
- 14.He, K., X. Wang, J. Jiang, R. Guan, K. E. Bernstein, P. P. Sayeski, and S. J. Frank. 2003. Janus kinase 2 determinants for growth hormone receptor association, surface assembly, and signaling. Mol. Endocrinol. 17:2211-2227. [DOI] [PubMed] [Google Scholar]
- 15.Inagaki, K., T. Yamao, T. Noguchi, T. Matozaki, K. Fukunaga, T. Takada, T. Hosooka, S. Akira, and M. Kasuga. 2000. SHPS-1 regulates integrin-mediated cytoskeletal reorganization and cell motility. EMBO J. 19:6721-6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang, P., C. F. Lagenaur, and V. Narayanan. 1999. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J. Biol. Chem. 274:559-562. [DOI] [PubMed] [Google Scholar]
- 17.Jiao, H., K. Berrada, W. Yang, M. Tabrizi, L. C. Platanias, and T. Yi. 1996. Direct association with and dephosphorylation of Jak2 kinase by the SH2-domain-containing protein tyrosine phosphatase SHP-1. Mol. Cell. Biol. 16:6985-6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kharitonenkov, A., Z. Chen, I. Sures, H. Wang, J. Schilling, and A. Ullrich. 1997. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 386:181-186. [DOI] [PubMed] [Google Scholar]
- 19.Klingmuller, U., U. Lorenz, L. C. Cantley, B. G. Neel, and H. F. Lodish. 1995. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell 80:729-738. [DOI] [PubMed] [Google Scholar]
- 20.Kreck, M. L., J. L. Freeman, A. Abo, and J. D. Lambeth. 1996. Membrane association of Rac is required for high activity of the respiratory burst oxidase. Biochemistry 35:15683-15692. [DOI] [PubMed] [Google Scholar]
- 21.Lander, H. M. 1997. An essential role for free radicals and derived species in signal transduction. FASEB J. 11:118-124. [PubMed] [Google Scholar]
- 22.Lenczowski, M. J., A. M. Van Dam, S. Poole, J. W. Larrick, and F. J. Tilders. 1997. Role of circulating endotoxin and interleukin-6 in the ACTH and corticosterone response to intraperitoneal LPS. Am. J. Physiol. 273:R1870-R1877. [DOI] [PubMed] [Google Scholar]
- 23.Levitzki, A. 2002. Tyrosine kinases as targets for cancer therapy. Eur. J. Cancer 38(Suppl. 5):S11-S18. [DOI] [PubMed] [Google Scholar]
- 24.Levy, D. E. and J. E. Darnell, Jr. 2002. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell. Biol. 3:651-662. [DOI] [PubMed] [Google Scholar]
- 25.Lin, Y. Z., S. Y. Yao, R. A. Veach, T. R. Torgerson, and J. Hawiger. 1995. Inhibition of nuclear translocation of transcription factor NF-kappa-B by a synthetic peptide containing a cell membrane-permeable motif and nuclear-localization sequence. J. Biol. Chem. 270:14255-14258. [DOI] [PubMed] [Google Scholar]
- 26.Long, E. O. 1999. Regulation of immune responses through inhibitory receptors. Annu. Rev. Immunol. 17:875-904. [DOI] [PubMed] [Google Scholar]
- 27.Meng, T. C., T. Fukada, and N. K. Tonks. 2002. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell 9:387-399. [DOI] [PubMed] [Google Scholar]
- 28.Ogura, T., T. Noguchi, R. Murai-Takebe, T. Hosooka, N. Honma, and M. Kasuga. 2004. Resistance of B16 melanoma cells to CD47-induced negative regulation of motility as a result of aberrant N-glycosylation of SHPS-1. J. Biol. Chem. 279:13711-13720. [DOI] [PubMed] [Google Scholar]
- 29.Oldenborg, P. A., H. D. Gresham, and F. P. Lindberg. 2001. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J. Exp. Med. 193:855-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oldenborg, P. A., A. Zheleznyak, Y. F. Fang, C. F. Lagenaur, H. D. Gresham, and F. P. Lindberg. 2000. Role of CD47 as a marker of self on red blood cells. Science 288:2051-2054. [DOI] [PubMed] [Google Scholar]
- 31.Park, H. S., S. H. Lee, D. Park, J. S. Lee, S. H. Ryu, W. J. Lee, S. G. Rhee, and Y. S. Bae. 2004. Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol. Cell. Biol. 24:4384-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price, L. S., M. Langeslag, J. P. ten Klooster, P. L. Hordijk, K. Jalink, and J. G. Collard. 2004. Calcium signalling regulates translocation and activation of Rac. J. Biol. Chem. 278:39413-39421. [DOI] [PubMed] [Google Scholar]
- 33.Price, M. O., L. C. McPhail, J. D. Lambeth, C. H. Han, U. G. Knaus, and M. C. Dinauer. 2002. Creation of a genetic system for analysis of the phagocyte respiratory burst: high-level reconstitution of the NADPH oxidase in a nonhematopoietic system. Blood 99:2653-2661. [DOI] [PubMed] [Google Scholar]
- 34.Ravetch, J. V., and L. L. Lanier. 2000. Immune inhibitory receptors. Science 290:84-89. [DOI] [PubMed] [Google Scholar]
- 35.Rees, G. S., C. K. Gee, H. L. Ward, C. Ball, G. M. Tarrant, S. Poole, and A. F. Bristow. 1999. Rat tumour necrosis factor-alpha: expression in recombinant Pichia pastoris, purification, characterization and development of a novel ELISA. Eur. Cytokine Netw. 10:383-392. [PubMed] [Google Scholar]
- 36.Reth, M. 2002. Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immunol. 3:1129-1134. [DOI] [PubMed] [Google Scholar]
- 37.Salmeen, A., J. N. Andersen, M. P. Myers, T. C. Meng, J. A. Hinks, N. K. Tonks, and D. Barford. 2003. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 423:769-773. [DOI] [PubMed] [Google Scholar]
- 38.Seiffert, M., P. Brossart, C. Cant, M. Cella, M. Colonna, W. Brugger, L. Kanz, A. Ullrich, and H. J. Buhring. 2001. Signal-regulatory protein alpha (SIRPalpha) but not SIRPbeta is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34(+)CD38(−) hematopoietic cells. Blood 97:2741-2749. [DOI] [PubMed] [Google Scholar]
- 39.Seiffert, M., C. Cant, Z. Chen, I. Rappold, W. Brugger, L. Kanz, E. J. Brown, A. Ullrich, and H. J. Buhring. 1999. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood 94:3633-3643. [PubMed] [Google Scholar]
- 40.Simon, A. R., H. G. Vikis, S. Stewart, B. L. Fanburg, B. H. Cochran, and K. L. Guan. 2000. Regulation of STAT3 by direct binding to the Rac1 GTPase. Science 290:144-147. [DOI] [PubMed] [Google Scholar]
- 41.Smith, R. E., V. Patel, S. D. Seatter, M. R. Deehan, M. H. Brown, G. P. Brooke, H. S. Goodridge, C. J. Howard, K. P. Rigley, W. Harnett, and M. M. Harnett. 2003. A novel MyD-1 (SIRP-1alpha) signaling pathway that inhibits LPS-induced TNFalpha production by monocytes. Blood 102:2532-2540. [DOI] [PubMed] [Google Scholar]
- 42.Stofega, M. R., L. S. Argetsinger, H. Wang, A. Ullrich, and C. Carter-Su. 2000. Negative regulation of growth hormone receptor/JAK2 signaling by signal regulatory protein alpha. J. Biol. Chem. 275:28222-28229. [DOI] [PubMed] [Google Scholar]
- 43.Stolk, J., T. J. Hiltermann, J. H. Dijkman, and A. J. Verhoeven. 1994. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell Mol. Biol. 11:95-102. [DOI] [PubMed] [Google Scholar]
- 44.Teng, X., H. Zhang, C. Snead, and J. D. Catravas. 2002. Molecular mechanisms of iNOS induction by IL-1 beta and IFN-gamma in rat aortic smooth muscle cells. Am. J. Physiol. Cell Physiol. 282:C144-C152. [DOI] [PubMed] [Google Scholar]
- 45.Timms, J. F., K. Carlberg, H. Gu, H. Chen, S. Kamatkar, M. J. Nadler, L. R. Rohrschneider, and B. G. Neel. 1998. Identification of major binding proteins and substrates for the SH2-containing protein tyrosine phosphatase SHP-1 in macrophages. Mol. Cell. Biol. 18:3838-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Timms, J. F., K. D. Swanson, A. Marie-Cardine, M. Raab, C. E. Rudd, B. Schraven, and B. G. Neel. 1999. SHPS-1 is a scaffold for assembling distinct adhesion-regulated multi-protein complexes in macrophages. Curr. Biol. 9:927-930. [DOI] [PubMed] [Google Scholar]
- 47.Tomasello, E., C. Cant, H. J. Buhring, F. Vely, P. Andre, M. Seiffert, A. Ullrich, and E. Vivier. 2000. Association of signal-regulatory proteins beta with KARAP/DAP-12. Eur. J. Immunol. 30:2147-2156. [DOI] [PubMed] [Google Scholar]
- 48.van den Berg, T. K., D. Nath, H. J. Ziltener, D. Vestweber, M. Fukuda, I. van Die, and P. R. Crocker. 2001. Cutting edge: CD43 functions as a T cell counterreceptor for the macrophage adhesion receptor sialoadhesin (Siglec-1). J. Immunol. 166:3637-3640. [DOI] [PubMed] [Google Scholar]
- 49.van den Nieuwenhof, I., C. Renardel de Lavalette, N. Diaz, I. van Die, and T. K. van den Berg. 2001. Differential galactosylation of neuronal and haematopoietic signal regulatory protein-alpha determines its cellular binding-specificity. J. Cell Sci. 114:1321-1329. [DOI] [PubMed] [Google Scholar]
- 50.van der Goes, A., J. Brouwer, K. Hoekstra, D. Roos, T. K. van den Berg, and C. D. Dijkstra. 1998. Reactive oxygen species are required for the phagocytosis of myelin by macrophages. J. Neuroimmunol. 92:67-75. [DOI] [PubMed] [Google Scholar]
- 51.van Hennik, P. B., J. P. ten Klooster, J. R. Halstead, C. Voermans, E. C. Anthony, N. Divecha, and P. L. Hordijk. 2003. The C-terminal domain of Rac1 contains two motifs that control targeting and signaling specificity. J. Biol. Chem. 278:39166-39175. [DOI] [PubMed] [Google Scholar]
- 52.Varinou, L., K. Ramsauer, M. Karaghiosoff, T. Kolbe, K. Pfeffer, M. Muller, and T. Decker. 2003. Phosphorylation of the Stat1 transactivation domain is required for full-fledged IFN-gamma-dependent innate immunity. Immunity 19:793-802. [DOI] [PubMed] [Google Scholar]
- 53.Veillette, A., E. Thibaudeau, and S. Latour. 1998. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J. Biol. Chem. 273:22719-22728. [DOI] [PubMed] [Google Scholar]
- 54.Vernon-Wilson, E. F., W. J. Kee, A. C. Willis, A. N. Barclay, D. L. Simmons, and M. H. Brown. 2000. CD47 is a ligand for rat macrophage membrane signal regulatory protein SIRP (OX41) and human SIRPalpha 1. Eur. J. Immunol. 30:2130-2137. [DOI] [PubMed] [Google Scholar]
- 55.Yamao, T., T. Noguchi, O. Takeuchi, U. Nishiyama, H. Morita, T. Hagiwara, H. Akahori, T. Kato, K. Inagaki, H. Okazawa, Y. Hayashi, T. Matozaki, K. Takeda, S. Akira, and M. Kasuga. 2002. Negative regulation of platelet clearance and of the macrophage phagocytic response by the transmembrane glycoprotein SHPS-1. J. Biol. Chem. 277:39833-39839. [DOI] [PubMed] [Google Scholar]
- 56.Zhang, S. Q., W. G. Tsiaras, T. Araki, G. Wen, L. Minichiello, R. Klein, and B. G. Neel. 2002. Receptor-specific regulation of phosphatidylinositol 3′-kinase activation by the protein tyrosine phosphatase Shp2. Mol. Cell. Biol. 22:4062-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao, X., K. A. Carnevale, and M. K. Cathcart. 2003. Human monocytes use Rac1, not Rac2, in the NADPH oxidase complex. J. Biol. Chem. 278:40788-40792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.