Abstract
Semaphorins are cell surface and secreted proteins that provide axonal guidance in neuronal tissues and regulate cell motility in many cell types. They act by binding a family of transmembrane receptors known as plexins, which belong to the c-Met family of scatter factor receptors but lack an intrinsic tyrosine kinase domain. Interestingly, we have recently shown that Plexin-B1 is highly expressed in endothelial cells and that its activation by Semaphorin 4D elicits a potent proangiogenic response (J. R. Basile, A. Barac, T. Zhu, K. L. Guan, and J. S. Gutkind, Cancer Res. 64:5212-5224, 2004). In searches for the underlying molecular mechanism, we observed that Semaphorin 4D-stimulated endothelial cell migration requires the activation of the phosphatidylinositol 3-kinase (PI3K)-Akt pathway. Surprisingly, we found that Plexin-B1 stimulates PI3K-Akt through the activation of an intracellular tyrosine kinase cascade that involves the sequential activation of PYK2 and Src. This results in the tyrosine phosphorylation of Plexin-B1, the rapid recruitment of a multimeric signaling complex that includes PYK2, Src, and PI3K to Plexin-B1 and the activation of Akt. These findings suggest that Plexin-B1 may achieve its numerous physiological functions through the direct activation of intracellular tyrosine kinase cascades.
The semaphorins are a family of cell surface and soluble proteins that control neurite extension, guide axonal growth, maintain established neuronal pathways, and regulate the proliferation and activation of B cells (41, 57). More than 20 semaphorins have been identified to date, and they are grouped into eight classes based upon their phylogenetic characteristics: classes 1 and 2 are found in invertebrates, classes 3 to 7 in vertebrates, and an eighth class, class V, has been identified in some nonneurotropic DNA viruses (2, 28). Semaphorins exhibit homology to the family of scatter factor proteins, which participate in branching morphogenesis, axonal guidance in neuronal tissues (30), and normal and aberrant proliferation and enhanced cell motility in many other cell types, particularly those of epithelial origin (59). Semaphorins and the scatter factors share homology in an approximately 500-amino-acid-long segment called the Sema domain and a cysteine-rich motif of about 80 amino acids called the Met-related sequence (MRS), named after the prototypical scatter factor receptor c-Met (28, 64). The ligand for c-Met is hepatocyte growth factor (HGF), also known as scatter factor-1 (34, 54, 59). HGF acts through c-Met to maintain tissue homeostasis in the adult by regulating cell proliferation, motility, and cell survival and by controlling intercellular contacts and cell-matrix interactions (7, 32). Inappropriate activation of the HGF pathway in neoplasia leads to dysregulated invasive growth, a process where tumor cells weaken tissue integrity, migrate out of the tumor mass, and invade distant sites, giving rise to metastatic disease (8, 14).
The semaphorins bind a recently identified family of single pass transmembrane receptors, the plexins (53), which also share homology in their extracellular segments with semaphorins and scatter factor receptors, such as c-Met. However, the intracellular region of the plexins fail to exhibit homology with any other known protein but are highly conserved within and across species (34, 53). In humans, there are at least nine plexins grouped into four families, A through D, most of which have been shown to mediate neuronal cell adhesion and contact, fasciculation, and axon guidance (53, 54). The A family of plexins are the most thoroughly studied and have been shown to participate in vascular morphogenesis in addition to their best-understood role in axon path finding (6, 47, 48). These receptors associate in a complex with members of the neuropilin class of cell surface receptors, neuropilin-1 and neuropilin-2, when bound by their ligands, the class 3 semaphorins (27, 52). Also first identified as regulators of axonal growth (20, 26), the neuropilins have functions in angiogenesis (33). For example, neuropilin-1 can also bind the proangiogenic factor vascular endothelial growth factor, a potent mitogenic and chemotactic cytokine whose receptors are expressed on the surface of endothelial cells (33).
In contrast to the class 3 semaphorins, Semaphorin 4D appears to bind directly to its receptor, Plexin-B1 (53). Despite recent advances, however, the signal transduction mechanisms utilized by Plexin-B1 remain poorly understood. Evidence now points to the B plexins as regulators of small GTP-binding protein signaling by acting on guanine nucleotide exchange factors (GEFs) for Rho GTPases (3, 16, 17, 39, 50) or functioning as GTP-activating proteins (GAPs) for the R-Ras family of GTPases (35, 36). There is also evidence that Plexin-B1 competes with p21-activated kinase for Rac binding, thus inhibiting Rac-dependent processes by sequestering the active form of Rac (61, 62).
Recently, we have observed that Plexin-B1 is highly expressed in endothelial cells, and that its activation by Semaphorin 4D promotes endothelial cell migration and tubulogenesis through a signaling pathway that is highly dependent on the ability of Plexin-B1 to stimulate Rho (5). In an effort aimed at unraveling the molecular mechanisms underlying this novel biological function of Plexin-B1, we took advantage of the availability of potent phosphatidylinositol 3-kinase (PI3K) inhibitors to examine whether this signaling molecule, often associated with processes that involve directional cell migration (31), participates in the proangiogenic response initiated by Plexin-B1. Indeed, we observe here that PI3K inhibitors abolish the chemotactic response in endothelial cells to Semaphorin 4D. This was consistent with the ability of Semaphorin 4D to stimulate Akt potently in a PI3K-dependent manner. The molecular dissection of the mechanisms whereby Plexin-B1 stimulates PI3K and Akt revealed that binding of Semaphorin 4D to Plexin-B1 or the dimerization of the intracellular region of Plexin-B1 by a receptor-chimera approach promotes the sequential activation of an intracellular tyrosine kinase cascade initiated by the nonreceptor tyrosine kinases PYK2 and Src and the consequent tyrosine phosphorylation of Plexin-B1 and the p85 regulatory subunit of PI3K, independent of residues involved in the ability of Plexin-B1 to act as a Ras GAP. Interestingly, this results in the recruitment of a multimeric signaling complex that includes PYK2, Src, and PI3K to Plexin-B1 and the activation of Akt. Furthermore, we provide evidence that activation of PYK2 is necessary for the tyrosine phosphorylation of the intracellular portion of Plexin-B1 and that PYK2 and Src and their regulated pathways are required for endothelial cell migration in response to Plexin-B1 stimulation. Together, these findings support the emerging notion that plexins of the B family participate in a growing number of biological responses by initiating a complex array of signaling events through the direct association with Rho GEFs and the concomitant activation of cytoplasmic tyrosine kinase cascades which in turn promote the assembly and binding of multimeric signaling complexes to tyrosine-phosphorylated Plexin-B.
MATERIALS AND METHODS
Cell culture.
Porcine aorta endothelial cells were cultured in HAM F-12 media (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum and 100 U/ml penicillin/streptomycin/amphotericin B (Sigma). 293T embryonic kidney cells were grown and maintained in Dulbecco's modified Eagle medium (DMEM) (Sigma) supplemented with 10% fetal bovine serum and 100 U/ml penicillin/streptomycin/amphotericin B.
Production of soluble Semaphorin 4D.
Semaphorin 4D was produced and purified as described previously (5). Briefly, the extracellular portion of mouse Semaphorin 4D was subjected to PCR, and the resulting product was cloned into the plasmid pSecTag2B (Invitrogen, Carlsbad, CA). This construct was transfected into 293T cells growing in serum free media using the calcium chloride (Fluka Chemika; Sigma Aldrich, St. Louis, MO)-N,N′-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid-buffered saline (Fluka Chemika) method (12). Media containing soluble Semaphorin 4D were collected 1 and 2 days posttransfection and purified with TALON metal affinity resin (Clontech Laboratories, Palo Alto, CA) according to manufacturer's instructions. Concentration and purity of the TALON eluates were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis with silver stain (Amersham Life Science, Piscataway, NJ) and the Bio-Rad assay (Bio-Rad, Hercules, CA). In all cases, media collected from parallel transfectants using the empty pSecTag2B vector were used as controls.
Immunoblot analysis.
Cells were lysed in buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40) supplemented with protease inhibitors (0.5 mM phenylmethylsulfonyl fluoride, 1 μl/ml aprotinin, and 1 μl/ml leupeptin) and phosphatase inhibitors (2 mM NaF and 0.5 mM sodium orthovanadate) for 15 min at 4°C. After centrifugation, protein concentrations were measured using the Bio-Rad assay. One-hundred micrograms of protein from each sample was subjected to SDS-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane (Immobilon P; Millipore Corp., Billerica, MA). The membranes were then incubated with the appropriate antibodies. The antibodies used were as follows: p85 (Cell Signaling Technology, Beverly, MA); Akt (Cell Signaling Technology); phospho-Akt (Thr308 and Ser 473 Cell Signaling Technology); phospho-tyrosine (clone 4G10 [Upstate Biotechnology, Lake Placid, New York] and ICN and PY-20 [Valeant Pharmaceuticals, Costa Mesa, CA]); PYK2 (BD Biosciences, Palo Alto, CA); phospho-PYK2 (Biosource, Camarillo, CA); Src (s.c. N-16; Santa Cruz Biotechnology, Santa Cruz, CA); phospho-Src (Biosource); myc (BABCO, Richmond, CA); and tubulin (TU-02; Santa Cruz). Proteins were detected using the SuperSignal West Pico Chemiluminescence System (Pierce).
Immunoprecipitation.
Normal endothelial cells or cells stably expressing Trk-A/Plexin-B1 chimeras were washed in phosphate-buffered saline and treated for the indicated periods of time with 200 ng/ml Semaphorin 4D or 100 ng/ml nerve growth factor (NGF), respectively. Cells were washed twice with phosphate-buffered saline and lysed in EDTA-β-glycerophosphate-Tris · Cl lysis buffer (see above). Trk-A/Plexin-B1 receptors were immunoprecipitated from the cleared lysates by incubation for 2 h at 4°C with anti-myc antibody (9E11; Covance Research Products, Berkeley, CA) or anti-HA (HA11; Covance Research Products) as a control. Other proteins were immunoprecipitated with the indicated antibody and blotted for phosphorylated tyrosine using a combination 1:1 of PY-20 and 4G10 anti-phosphotyrosine antibodies (see above). Immunocomplexes were recovered with the aid of gamma-bind Sepharose beads (Pharmacia, Pfizer, New York, NY). Lysates and immunoprecipitates were analyzed by Western blotting after SDS-polyacrylamide gel electrophoresis and transfer to a polyvinylidene difluoride membrane (Immobilon P; Millipore). Immunocomplexes were visualized by a SuperSignal West Pico Chemiluminescence System (Pierce).
Migration assays.
Endothelial cells were serum starved for 24 h and placed in the top well of a Boyden chamber while serum-free HAM F-12 containing the indicated chemoattractant was placed in the bottom well. The two chambers were separated by a polyvinylpropylene-free membrane (Osmonics; 8 μm pore size; GE Water Technologies, Trevose, PA) coated with 10 μg/ml fibronectin (GIBCO, Carlsbad, CA). Ten percent fetal bovine serum was used as a positive control, and serum-free HAM F-12 containing 0.1% bovine serum albumin (BSA) was the negative control. After 7 h, the chamber was disassembled and the membrane was stained with Diff-Quick Stain (Dade Behring, Deerfield, Illinois), placed on a glass slide, and scanned. Densitometric quantitation was performed with National Institutes of Health image software, and cell migration was expressed as staining intensity relative to the negative control wells. Each experiment was performed in triplicate or sextuplicate, and averages and standard deviations were calculated. Where indicated, cells were transfected with pCEFL control or pCEFL DN-PYK2 using Lipofectamine Plus (Invitrogen) supplemented with CombiMag transfection agent (Oz Biosciences, Marseille, France) to achieve high transfection efficiency (determined to be ≥76% by fluorescence-activated cell sorter analysis of pCEFL enhanced green fluorescent protein [EGFP]-transfected endothelial cells). Cells were also treated with 10 μM PP2 (Calbiochem, La Jolla, CA), 50 μM LY294002 (Biosource), or vehicle control prior to migration. The chemoattractants used were 200 ng/ml soluble Semaphorin 4D or 100 ng/ml NGF (Upstate Biotechnology).
Trk-A/plexin fusion proteins.
Trk-A/Plexin-B1 fusion proteins were made as previously described (5). Briefly, the intracellular portion Plexin-B1 was cut out of the plasmid pCEFL EGFP Plexin-B1 with NheI/NotI and cloned in frame with the N-terminal, extracellular, and transmembrane portion of the rat NGF receptor Trk-A in the vector pCEFL-HA. The construct was then transfected into PAE cells using Superfect (QIAGEN), and stable cells were selected in 1 mM G418 (Calbiochem). A Trk-A/PlexinB1 mutant lacking key residues involved in Ras GAP activity were generated as previously described (35, 36) using the QuikChange II XL Site-Direcred Mutagenesis kit according to manufacturer's instructions (Stratagene, La Jolla, CA). Briefly, PCR amplifications were performed using primers designed with base pair substitutions in which arginine residues were mutated to alanine at amino acids 1677, 1678, and 1984, corresponding to the intracellular portion of human Plexin-B1 (45). The sequences for the PCR primers (Sigma) were as follows: R1677 and 1678A, 5′ AACCCCAAGCTGATGCTGGCCGCGACAGAGACTGTGGTGGAG 3′; R1984A, 5′ TGGAAGACCAACAGCTTGCCGCTAGCGTTCTGGATCAATATAATA 3′. Mutations were confirmed by sequencing.
RESULTS
Semaphorin 4D stimulates the PI3K-Akt pathway in endothelial cells: a role in Semaphorin 4D-induced chemotaxis.
As activation of the PI3K-Akt pathway represents a crucial event involved in many aspects of cell migration (31), we asked whether this biochemical route participates in Semaphorin 4D-mediated endothelial cell chemotaxis. As an experimental approach, migration assays were performed using a Boyden chamber with Semaphorin 4D as the chemoattractant for porcine aortic endothelial cells that were pretreated with vehicle control, dimethyl sulfoxide (DMSO), or 50 μM LY294002, a specific inhibitor of PI3K (63). As shown in Fig. 1A, cells pretreated with DMSO migrated towards Semaphorin 4D at levels similar to that seen in positive control wells containing 10% serum (S), while cells pretreated with LY294002 failed to exhibit a chemotactic response to Semaphorin 4D significantly above that of negative controls, implying that activation of PI3K is necessary for Semaphorin 4D-mediated endothelial cell chemotaxis. Similar results were obtained when using wortmannin as a specific PI3K inhibitor (data not shown).
FIG. 1.
A role for the PI3K-Akt pathway in endothelial cell migration in response to Semaphorin 4D. (A) PI3K inhibition prevents Semaphorin 4D-mediated endothelial cell migration. Cells were preincubated with either DMSO vehicle control (−) or 50 μM of the PI3K inhibitor LY294002 (+) and used in a chemotaxis assay with purified (200 ng/ml) Semaphorin 4D as the chemoattractant (S4D). Media containing 10% fetal bovine serum (S) were used as positive controls for migration. The bars represent the fold increase of migration as determined by densitometry relative to that seen in negative control wells containing 0.1% BSA (C). (B) Endothelial cells were treated with 200 ng/ml Semaphorin 4D (S4D) for the indicated periods of time, lysed, and immunoprecipitated for the PI3K subunit p85 (IP: p85) and then immunoblotted for the presence of phosphorylated tyrosine residues (WB: P-Y). Phosphotyrosine was seen in p85 immunoprecipitates from Semaphorin 4D-treated cells starting at 1 min after treatment (upper panel). Total p85 was used as a loading control (WB: p85; lower panel). (C) Semaphorin 4D activates Akt in endothelial cells. Cells were treated with 200 ng/ml Semaphorin 4D (S4D) for the indicated periods of time, lysed, and analyzed for the presence of phosphorylated Akt. Phospho-Akt (P-Akt, Thr308) was seen in cells starting at 1 min after treatment (upper panel). Total Akt was used as a loading control (WB: Akt; lower panel). (D) Semaphorin 4D-mediated activation of Akt can be inhibited by LY294002 in endothelial cells. Cells preincubated with either vehicle control (C) or 50 μM LY294002 (LY) were treated with 200 ng/ml Semaphorin 4D (S4D) for the indicated periods of time, lysed, and analyzed for the presence of phosphorylated Akt. Phospho-Akt (P-Akt, Thr308) was seen in cells starting at 1 min after treatment in DMSO-pretreated cells but not in those treated with LY294002 (upper panel). Total Akt was used as a loading control (WB: Akt; lower panel).
Tyrosine phosphorylation or binding of the Src homology 2 (SH2) domain of the PI3K p85 regulatory subunit to tyrosine-phosphorylated target proteins are the most frequent mechanisms resulting in the activation of class 1 PI3K catalytic subunits (10, 60). Therefore, to begin exploring how Semaphorin 4D stimulates PI3Ks, we examined p85 immunoprecipitates from Semaphorin 4D-treated endothelial cells for the presence of phosphorylated tyrosine. As shown in Fig. 1B, the presence of phosphotyrosine in p85-immunoprecipitated fractions was readily detectable starting at 1 min after Semaphorin 4D treatment (upper panel). Total p85 was used as the loading control (lower panel). This finding suggests that Semaphorin 4D promotes the activation of PI3Ks by the tyrosine phosphorylation of their p85 regulatory subunit.
Consistent with the requirement for PI3K activation for cell migration in response to Semaphorin 4D, treatment of endothelial cells with this semaphorin led to the rapid accumulation of Akt phosphorylated in threonine (Thr) 308 (Fig. 1C), which represents the activated form of this kinase (1). Indeed, limited levels of phospho-Thr 308 Akt were observed in serum starved cells, and an increase in Akt-phosphorylated species could be detected as early as 1 min after Semaphorin 4D treatment, which became even more prominent at 3 min and persisted up to 1 h following treatment (Fig. 1C). Semaphorin 4D-induced Akt phosphorylation and activation was abolished by pretreatment with LY294002 compared to vehicle-treated controls (Fig. 1D). These results indicate that Semaphorin 4D activates PI3K and Akt in endothelial cells and that this response is necessary for endothelial cell chemotaxis.
NGF treatment of endothelial cells stably expressing Trk-A/Plexin-B1 chimeric receptors results in Akt activation.
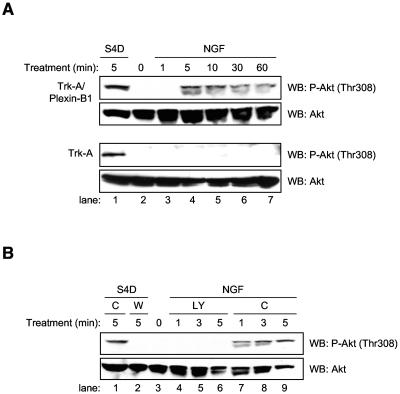
We have previously shown that chimeric receptor constructs containing the extracellular portion of the NGF receptor, Trk-A, fused to the intracellular portion of Plexin-B1 could elicit Plexin-B1 signaling in response to treatment with NGF (5). Therefore, we used endothelial cells stably expressing these constructs, or the extracellular portion of Trk-A alone without the cytoplasmic portion of Plexin-B1 as a control, to determine if the Akt activation we observed from Semaphorin 4D treatment was a specific result of Plexin-B1 stimulation. Figure 2A shows that NGF treatment of these cells results in phosphorylation and activation of Akt similar to that of Semaphorin 4D-treated control endothelial cells. This effect was not observed in endothelial cells stably expressing the extracellular portion of Trk-A alone (Fig. 2A), even though these cells still responded with Akt phosphorylation and activation in response to Semaphorin 4D treatment. The occasional appearance of Akt immunoreactivity as a doublet appears to be dependent on the running conditions of the SDS-PAGE gels rather than on the treatment. Next, endothelial cells stably expressing Trk-A/Plexin-B1 receptors were preincubated with DMSO as a control or the PI3K inhibitor LY294002 and analyzed for Akt phosphorylation in response to NGF. Figure 2B shows inhibition of Akt phosphorylation in cells pretreated with LY294002, similar to that seen in Semaphorin 4D-treated control cells pretreated with wortmannin, while Akt activation in cells preincubated with the DMSO vehicle control was similar to that of Semaphorin 4D-treated control endothelial cells. These results suggest that stimulation of Plexin-B1 signaling is sufficient for activation of PI3K and the subsequent phosphorylation and activation of Akt.
FIG. 2.
NGF treatment of endothelial cells stably expressing Trk-A/Plexin-B1 chimeric receptor constructs induces Akt phosphorylation. (A) Cells stably expressing extracellular Trk-A or Trk-A fused to the intracellular portion of Plexin-B1 (Trk-A/Plexin-B1) were treated with 100 ng/ml NGF for the indicated periods of time, lysed, and analyzed for the presence of phosphorylated Akt (P-Akt, Thr308; upper panels). Cells treated with 200 ng/ml Semaphorin 4D for 5 min were used as a positive control (S4D; lane 1). Total Akt was used as a loading control (WB: Akt; lower panels). (B) NGF-induced phosphorylation of Akt in endothelial cells stably expressing Trk-A/Plexin-B1 chimeric receptor constructs can be inhibited by LY294002. Cells stably expressing Trk-A/Plexin-B1 chimeric receptors were preincubated with either 50 μM LY294002 (LY) or DMSO vehicle control (C) and treated with 100 ng/ml NGF for the indicated periods of time, lysed, and analyzed for the presence of phosphorylated Akt. Phospho-Akt (P-Akt, Thr308) was seen after treatment in control pretreated cells (lanes 7 to 9, upper panel) but not in those growing in LY294002 (lanes 4 to 6, upper panel). Cells treated with 200 ng/ml Semaphorin 4D and DMSO (C) or 100 nM Wortmannin (W) for 5 min were used as a positive control (lane 1) and negative control (lane 2), respectively. Total Akt was used as a loading control (WB: Akt, lower panel).
Endothelial cell migration and Akt phosphorylation and activation in response to Semaphorin 4D does not require residues involved in Plexin-B1 Ras GAP activity.
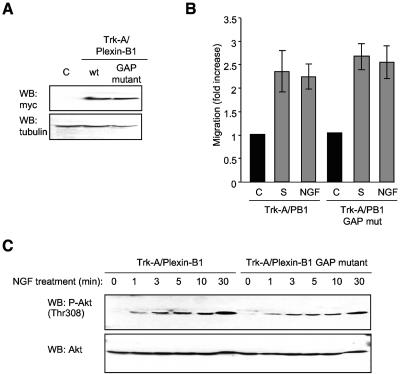
The intracellular segment of Plexin-B1 shares sequence similarity with Ras family-specific GTPase activating proteins, especially R-Ras GAP (24, 43, 44). Indeed, recent evidence points to Plexin-B1 as having Ras GAP activity (35, 36). In order to determine its significance in Semaphorin 4D-mediated Plexin-B1 signaling in endothelial cells, we generated endothelial cells stably expressing a Plexin-B1 Ras GAP mutant in which key arginine residues that are required for Ras GAP activity were mutated (35-37). Cells expressed the Trk-A/Plexin-B1 wild type and Trk-A/Plexin-B1 Ras GAP mutant to similar levels (Fig. 3A), and both cell populations migrated toward wells containing NGF to an extent similar to that seen in positive control wells containing 10% serum (Fig. 3B). Furthermore, NGF promoted a pattern of phosphorylation of Akt in cells expressing the Ras GAP mutants that was comparable to that elicited in cells expressing the wild-type Trk-A/Plexin-B1 chimeras (Fig. 3C), suggesting that the chemotactic signaling of Plexin-B1 in endothelial cells occurs independently of the ability of the receptor to act as a Ras GAP.
FIG. 3.
Endothelial cell migration and Akt phosphorylation and activation in response to Semaphorin 4D occur independently of residues involved in Plexin-B1 Ras GAP activity. (A) Transfected cells show immunoreactivity for the Ras GAP mutant form of the chimera Trk-A/Plexin-B1 (GAP mutant), at levels similar to that seen in cells expressing the wild-type intracellular portion of Plexin-B1 fused to Trk-A (wt, upper panel). Empty vector transfected cells were used as a negative control (C) and tubulin as the loading control (WB: tubulin, lower panel). (B) Endothelial cells expressing Trk-A/Plexin-B1 or Trk-A/Plexin-B1 Ras GAP mutant chimeras were used in a cell migration assay with 100 ng/ml NGF as the chemoattractant (NGF). Media containing 10% fetal bovine serum (S) was used as a positive control for migration. The bars represent the fold increase of migration as determined by densitometry relative to that seen in negative control wells containing 0.1% BSA (C). (C) Cells expressing Trk-A/Plexin-B1 or Trk-A/Plexin-B1 Ras GAP mutant chimeras were treated with 200 ng/ml Semaphorin 4D for the indicated periods of time, lysed, and analyzed for the presence of phosphorylated Akt. Phospho-Akt (WB: P-Akt [Thr308]) was seen in response to NGF treatment of Trk-A/Plexin-B1 expressing cells and in those expressing the Ras GAP mutant (upper panel). Total Akt was used as a loading control (WB: Akt, lower panel). mut, mutant.
Semaphorin 4D stimulates the phosphorylation and activation of PYK2 and Src: a role for Src in PI3K activation.
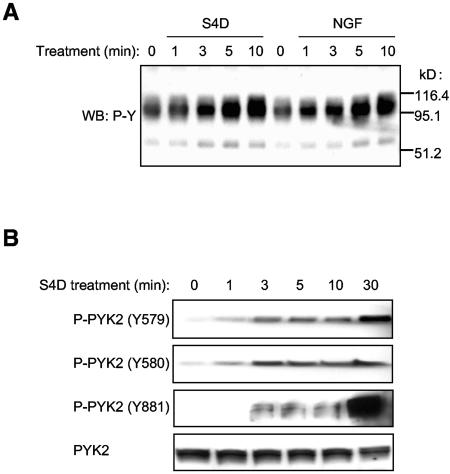
The finding that Semaphorin 4D promotes the activation of PI3Ks by the tyrosine phosphorylation of the p85 regulatory subunit suggests that Semaphorin 4D may promote the activation of intracellular tyrosine kinases and their subsequent signaling cascades. Thus, we analyzed global changes in the pattern of protein tyrosine phosphorylation in Semaphorin 4D-treated endothelial cells as well as in NGF-treated endothelial cells stably expressing Trk-A/Plexin-B1 chimeric receptors to identify proteins involved in the Plexin-B1-initiated tyrosine kinase signaling pathway. Figure 4A shows that Semaphorin 4D treatment results in the appearance of bands consistent with changes in tyrosine phosphorylation in a number of proteins of approximately 110 and 60 kDa in size. This prompted us to investigate the activation status of the cytoplasmic tyrosine kinases of the FAK and Src family, which exhibit similar molecular weights. Though we cannot specifically rule out FAK activation or the participation of this kinase in Plexin-B1 signaling in endothelial cells, we failed to detect any changes in the pattern of FAK phosphorylation in numerous experiments (data not shown). Instead, we did detect changes in the phosphorylation status of residues reflecting the activation of the FAK family member PYK2. Endothelial cells treated with Semaphorin 4D were analyzed for phosphorylation and activation of this kinase using antibodies specific for different sites of tyrosine phosphorylation on PYK2. Figure 4B demonstrates that tyrosine residues 579 and 580 are phosphorylated as early as 1 min after treatment with Semaphorin 4D. Of interest, these tyrosine residues are located in the activation loop of PYK2, and their phosphorylation lead to the relief of autoinhibition and the consequent activation of PYK2, thus reflecting its kinase activity (4). On the other hand, tyrosine residue 881, an autophosphorylation site that corresponds to a proposed Grb-2 binding site in the C-terminal domain of PYK2 (4), was phosphorylated starting at approximately 3 min after treatment with Semaphorin 4D and accumulated over time. Total PYK2 levels did not change, which also served as a loading control. Thus, these data suggest that Semaphorin 4D treatment leads to the rapid activation of PYK2 and the subsequent autophosphorylation of certain sites involved in signal transmission, such as tyrosine residue 881.
FIG. 4.
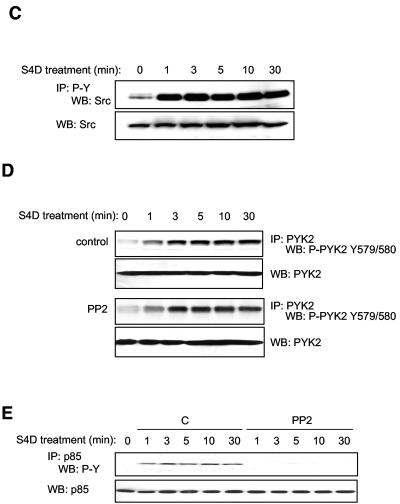
Semaphorin 4D induces the tyrosine phosphorylation of PYK2 and Src in endothelial cells. (A) Treatment of endothelial cells with Semaphorin 4D and cells stably expressing Trk-A/Plexin-B1 chimeric receptor constructs with NGF induces changes in tyrosine phosphorylation. Endothelial cells and cells stably expressing Trk-A/Plexin-B1 chimeric receptors were treated with 200 ng/ml Semaphorin 4D (S4D) or 100 ng/ml NGF (NGF), respectively, for the indicated periods of time, lysed, and analyzed for the presence of tyrosine phosphoproteins (WB: P-Y). Changes in tyrosine phosphorylation of proteins of approximately 110 and 60 kDa are detected. (B) PYK2 is activated in endothelial cells in response to S4D. Endothelial cells were treated with 200 ng/ml Semaphorin 4D for the indicated periods of time, lysed, and immunoblotted with antibodies specific for different sites of tyrosine phosphorylation on PYK2. The levels of phosphorylation on Y579 and 580, residues that reflect kinase activity, and Y881, corresponding to the proposed Grb-2 binding site in the C-terminal domain, are observed starting at 1 min and 3 min after treatment with Semaphorin 4D, respectively. (C) Src is found in tyrosine-phosphorylated molecular complexes in endothelial cells in response to S4D. Cells were treated with 200 ng/ml Semaphorin 4D for the indicated periods of time, lysed, and immunoprecipitated for tyrosine-phosphorylated proteins. The detection of Src in the tyrosine immunoprecipitated fraction dramatically increased at 1 min after treatment (IP: P-Y and WB: Src; upper panel). Total Src was used as a loading control (WB: Src; lower panel). (D) Blockade of Src has no effect on Semaphorin 4D-induced PYK2 activation. Endothelial cells were preincubated with either DMSO vehicle control (C) or 10 μM PP2 (PP2) and treated with 200 ng/ml Semaphorin 4D for the indicated periods of time. Cells were lysed, immunoprecipitated for PYK2 (IP: PYK2), and immunoblotted for the presence of the phosphorylated tyrosine residues Y579 and Y580, located in the activation loop (WB: P-PYK2 Y579/580). Phosphorylated tyrosine residues were detected in the immunoprecipitated fraction starting at approximately 1 min after treatment in both control and PP2 treated populations. Total PYK2 was used as a loading control (WB: PYK2). (E) Blockade of Src prevents Semaphorin 4D-induced phosphorylation of p85 PI3K subunit. Endothelial cells were preincubated with either DMSO vehicle control (C) or 10 μM PP2 (PP2) and treated with 200 ng/ml Semaphorin 4D for the indicated periods of time. Cells were lysed, immunoprecipitated for p85, and immunoblotted for the presence of the phosphorylated tyrosine residues (IP: p85 and WB: P-Y). Phosphorylated tyrosine residues were detected in the immunoprecipitated fraction starting at approximately 1 min after treatment in the DMSO populations but not in cells incubated with PP2 (upper panel). Total p85 was used as a loading control (WB: p85; lower panel). MW, molecular mass.
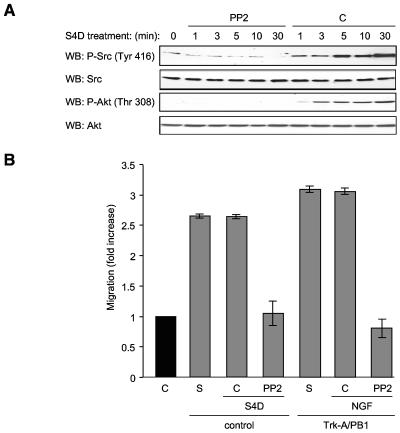
We then analyzed Plexin-B1-mediated phosphorylation and activation of Src, which is often found in functional association with FAK family cytoplasmic tyrosine kinases (4, 46, 49). Initially, we detected Src in an immunoblot of tyrosine-phosphorylated proteins immunoprecipitated from Semaphorin 4D-treated cells starting at 1 min after treatment and persisting out to 30 min (Fig. 4C). Total Src was used as a loading control. The activation of Src upon Semaphorin 4D treatment was further confirmed by monitoring the phosphorylation status of Src on tyrosine 416, which reflects its enzymatic activity (Fig. 5A).
FIG. 5.
Blockade of Src prevents Semaphorin 4D-induced Akt activation and cell migration. (A) PP2 blocks Src and Akt phosphorylation in Semaphorin 4D-treated endothelial cells. Endothelial cells were preincubated with either 10 μM PP2 (PP2) or DMSO (C) and treated with 200 ng/ml Semaphorin 4D for the indicated periods of time, lysed, and analyzed for the presence of phosphorylated Akt and Src. Phospho-Src (WB: P-Src) was seen after 1 min of treatment in cells preincubated with DMSO but not in cells grown in PP2 (upper panels). Total Src was used as a loading control (WB: Src). Phosphorylation of Akt is also seen in DMSO-treated control cells but not in PP2 treated populations [WB: P-Akt (Thr 308), lower panels]. Total Akt is used as a loading control (WB: Akt). (B) PP2 blocks Semaphorin 4D/Plexin-B1-mediated chemotaxis. Endothelial cells (control) or cells stably expressing Trk-A/Plexin-B1 chimeric receptors (Trk-A/PB1) were preincubated with either DMSO vehicle control (C) or 10 μM of the Src inhibitor PP2 (PP2) and then used in a cell migration assay with Semaphorin 4D (S4D) or 100 ng/ml NGF (NGF) as the chemoattractants. Media containing 10% fetal bovine serum (S) were used as positive controls for migration. The bars represent the fold increase of migration as determined by densitometry relative to that seen in negative control wells containing 0.1% BSA (C).
We next examined whether PYK2 activation was dependent on the stimulation of Src kinases by treating cells with Semaphorin 4D in the presence of the small-molecule Src inhibitor, PP2 (22). As shown in Fig. 4D, the presence of phosphotyrosines 579 and 580 was detected in PYK2-immunoprecipitated fractions starting at 1 min after Semaphorin 4D treatment in both control and PP2-treated groups, thus suggesting that Semaphorin 4D promotes the activation of PYK2 independent of Src activity. Surprisingly, however, inhibition of Src abolished the tyrosine phosphorylation of p85, as judged by the lack of accumulation of tyrosine-phosphorylated species of p85 upon Semaphorin 4D treatment when cells were stimulated in the presence of PP2 (Fig. 4E). This finding suggested that Semaphorin 4D promotes the activation of PI3Ks by the tyrosine phosphorylation of their p85 regulatory subunit in a Src-dependent manner.
Src kinases are necessary for Semaphorin 4D-induced activation of the PI3K-Akt pathway and endothelial cell migration.
To further study the role of Src kinases in Plexin-B1 signaling, we pretreated endothelial cells with PP2 and then treated them with Semaphorin 4D and immunoblotted for the phosphorylated and activate forms Src and Akt. As expected, whereas Semaphorin 4D led to the accumulation of Src phosphorylated on tyrosine 416, a reflection of its increased enzymatic activity (58), cells pretreated with PP2 failed to exhibit Src phosphorylation, demonstrating that PP2 could successfully inhibit Src activation in endothelial cells (Fig. 5A). Cells incubated with PP2 also failed to exhibit Akt phosphorylation in response to Semaphorin 4D (Fig. 5A), confirming that Src activity is necessary for PI3K activation by Semaphorin 4D. To determine if Src inhibition had an effect on Semaphorin 4D/Plexin-B1-mediated endothelial cell migration, chemotaxis assays were performed on endothelial cells and cells stably expressing Trk-A/Plexin-B1 chimeric receptors after pretreatment with vehicle control or PP2. Control cells migrated toward wells containing Semaphorin 4D or NGF (Fig. 5B) at levels similar to that seen in positive control wells containing 10% serum, while the PP2-treated populations failed to migrate to the appropriate chemoattractant when compared to negative controls. These results suggested that Src is necessary for Semaphorin 4D/Plexin-B1-mediated endothelial cell chemotaxis.
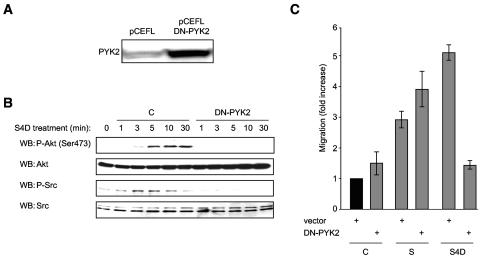
PYK2 activity is necessary for Semaphorin 4D/Plexin-B1-mediated activation of Akt and Src and endothelial cell chemotaxis.
To determine the role of PYK2 in activation of Akt and Src, we used a highly effective method to transfect endothelial cells with a control vector or a vector coding for a kinase-dead, dominant-negative form of PYK2 (PYK2-K475R, DN-PYK2) (15, 25). Cells were then treated with Semaphorin 4D and examined for the phosphorylated and hence activated forms of these proteins. Transfected cells showed enhanced expression of PYK2 (Fig. 6A), indicating successful overexpression of the kinase deficient form of PYK2, when compared to empty vector-transfected controls (pCEFL). While phosphorylation of Akt and Src were readily detected in control cells, cells expressing DN-PYK2 failed to demonstrate a response to Semaphorin 4D (Fig. 6B). These results show that Akt and Src activation observed in Semaphorin 4D-treated endothelial cells is dependent upon Plexin-B1-mediated activation of PYK2. It also suggests that PYK2 and Src may be activated sequentially, with PYK2 acting upstream of Src.
FIG. 6.
A dominant-negative form of PYK2 blocks Semaphorin 4D/Plexin-B1-mediated Akt and Src phosphorylation and chemotaxis. (A) Transfected cells show immunoreactivity for PYK2, indicating overexpression of the kinase-dead, dominant-negative version of PYK2 (DN-PYK2), compared to empty vector-transfected controls (pCEFL). (B) Cells transfected with empty vector control DNA (C) or kinase-dead dominant-negative PYK2 (DN-PYK2) were treated with 200 ng/ml Semaphorin 4D for the indicated periods of time, lysed, and analyzed for the presence of phosphorylated Akt and Src. Phospho-Akt [WB: P-Akt (Ser 473); upper panels] and phospho-Src (WB: P-Src; lower panels) were seen in response to Semaphorin 4D treatment of control cells but not in those expressing DN-PYK2. Total Akt and Src were used as loading controls. (C) Endothelial cells transfected with empty vector control DNA (vector) or kinase-dead dominant-negative PYK2 (DN-PYK2) were used in a cell migration assay with 200 ng/ml Semaphorin 4D as the chemoattractant (S4D). Media containing 10% fetal bovine serum (S) were used as positive controls for migration. The bars represent the fold increase of migration as determined by densitometry relative to that seen in negative control wells containing 0.1% BSA (C).
To determine the effects of PYK2 on Plexin-B1-mediated endothelial cell migration, we subjected vector-transfected control cells and cells transfected with DN-PYK2 to a migration assay with Semaphorin 4D as the chemoattractant. Vector-transfected control cells migrated toward wells containing Semaphorin 4D (Fig. 6C) at levels similar to that seen in positive control wells containing 10% serum, while the DN-PYK2-transfected populations failed to exhibit cell migration relative to negative controls (Fig. 6C). These results suggest that the activity of PYK2 is necessary for the initiation of the signaling events participating in Semaphorin 4D/Plexin-B1-mediated endothelial cell chemotaxis.
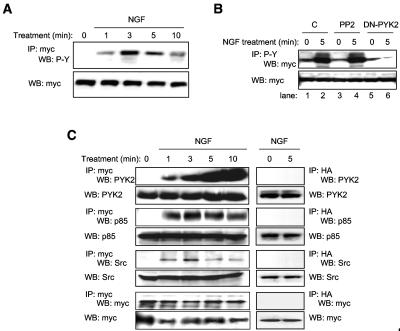
PYK2 is recruited to the Plexin-B1 receptor along with PI3K and Src and is required for Plexin-B1 phosphorylation.
Though the nature of the kinase involved is still unclear, there is evidence that the intracellular portion of Plexin-B1 is phosphorylated at a tyrosine residue upon binding to Semaphorin 4D (11). Indeed, tyrosine phosphorylation of Plexin-B1 could provide a docking site for the SH2 domains of the p85 regulatory subunit of PI3K, thus providing a possible mechanism for the activation of the PI3K-Akt pathway that we observed in Semaphorin 4D-treated endothelial cells. To establish the kinetics of Plexin-B1 phosphorylation in response to ligand, we treated endothelial cells stably expressing myc-tagged Trk-A/Plexin-B1 chimeric receptors with NGF, immunoprecipitated the receptor complex, and immunoblotted for phosphorylated tyrosine residues. Phosphorylated receptor already can be observed as early as 1 min after treatment, persisting for up to 10 min (Fig. 7A). To determine which of the kinases activated by Plexin-B1 signaling are required for phosphorylation of Plexin-B1, we transfected with myc-tagged Trk-A/Plexin-B1 chimeric receptor constructs and either DN-PYK2, EGFP, or EGFP preceded by incubation with the Src inhibitor PP2, followed by treatment with NGF. Immunoblotting for myc in phosphotyrosine-immunoprecipitated fractions revealed phosphorylation of the receptor after NGF treatment of controls and in PP2-pretreated cells in this cellular system that enables high levels of protein expression (Fig. 7B), suggesting that Src activity is not necessary for Plexin-B1 phosphorylation. However, in cells expressing DN-PYK2, no change in receptor phosphorylation was seen in response to NGF (Fig. 7B). These results suggest that while active Src is necessary for PI3K-mediated activation of Akt and endothelial cell chemotaxis, it is not necessary for Plexin-B1 phosphorylation. In contrast, PYK2 is necessary for both endothelial cell chemotaxis and Plexin-B1 phosphorylation.
FIG. 7.
Plexin-B1 activation promotes the assembly of multimeric signaling complexes. (A) Plexin-B1 is tyrosine phosphorylated upon activation. Lysates of endothelial cells stably expressing myc-tagged Trk-A/Plexin-B1 chimeric receptors treated with 100 ng/ml NGF for the indicated periods of time were immunoprecipitated for the receptor (IP: myc) and immunoblotted for the presence of phosphorylated tyrosine residues (WB: P-Y). A phosphotyrosine band of the appropriate size begins to appear in the immunoprecipitated fraction at about 1 min (upper panel). Total receptor (WB: myc) was used as a loading control (lower panel). (B) 293T cells were cotransfected with myc-tagged Trk-A/Plexin-B1 and either DN-PYK2 (DN-PYK2), control DNA (C), or control DNA preceded by incubation with the Src inhibitor PP2 (PP2), followed by treatment with 100 ng/ml NGF. An immunoblot for myc was performed on phosphotyrosine-immunoprecipitated fractions (IP: P-Y and WB: myc). An increase in receptor phosphorylation is seen in response to NGF in control cells (lane 2) and cells pretreated with PP2 (lane 4) but not in cells expressing DN-PYK2 (lane 6). (C) PYK2, p85, and Src bind Plexin-B1 upon receptor activation. Lysates of endothelial cells stably expressing myc-tagged Trk-A/Plexin-B1 chimeric receptors treated with 100 ng/ml NGF for the indicated periods of time were immunoprecipitated for the receptor (IP: myc) and immunoblotted for the presence of PYK2, p85, and Src. Each of these proteins is seen associating with the immunoprecipitated receptor complex at 1 min following treatment. Total PYK2, p85, Src, and myc immunoblots were used as loading controls. As a negative control, a hemagglutinin immunoprecipitation was performed (IP: HA; right panels) which showed no proteins after 0 or 5 min treatment with NGF when probed for PYK2, p85, Src, and myc. Total PYK2, p85, Src, and myc were used as loading controls (WB lanes; lower panels).
We next wanted to determine if PYK2 could associate with the intracellular portion of Plexin-B1 in endothelial cells. We treated endothelial cells stably expressing Trk-A/Plexin-B1 chimeric receptors with NGF and immunoprecipitated the receptor complex, this time blotting for the presence of PYK2. PYK2 was, in fact, observed in association with the receptor following NGF treatment (Fig. 7C). The p85 regulatory subunit of PI3K also associated with the receptor (Fig. 7C), which correlates well with the proposed model of PI3K activation. Src, which is often found associated with FAK family members, was also seen in the complex soon after stimulation of Plexin-B1, although not as strongly at later time points (Fig. 7C). An immunoblot for the myc tag revealed the presence of equal amounts of the receptor at all time points (Fig. 7C). As a negative control, a hemagglutinin immunoprecipitation was performed (Fig. 7C), which showed no proteins before or 5 min after treatment with NGF when probed for PYK2, p85, or Src. Total PYK2, p85, and Src are shown in each case (WB lanes). These results provide further evidence for the involvement of PYK2 in Plexin-B1 signaling and suggest that after receptor activation, PI3K and Src are recruited to the cytoplasmic portion of Plexin-B1, thereby participating in Plexin-B1 initiated signal transduction.
DISCUSSION
The process of axon guidance could be considered a specialized version of invasive growth and branching morphogenesis, since it involves growth of neurites and axons through the extracellular matrix and the establishment of contacts with distant cells and matrix components (59). It is therefore not surprising that scatter factors and semaphorins are involved in the chemoattraction of motor neuron axons (18). The role of plexin-semaphorin signaling, however, may not be restricted to the central nervous system. For example, we have recently shown high levels of Plexin-B1 expression in endothelial cells and that activation of Plexin-B1 by Semaphorin 4D influenced angiogenic responses in these cells (5). In fact, the gene expression pattern of plexins demonstrates that they are found in numerous adult tissues where plexin-semaphorin interactions have now been implicated in a host of responses, including loss of cell-cell contacts and branching morphogenesis in epithelium, regulation of angiogenesis, growth and metastasis of tumors, and immune responses (9, 59). Their function and mechanism of action in many of these tissues remains poorly understood. Here we show that Semaphorin 4D, acting through its receptor Plexin-B1, causes the activation of the PI3K pathway in endothelial cells by a mechanism that involves the sequential activation of the cytoplasmic tyrosine kinases PYK2 and Src, which are necessary for the stimulation of PI3K and Akt and for endothelial cell migration. We also provide evidence that these effects occur independently on residues involved in the Ras GAP activity of Plexin-B1. PYK2 appears to be the most upstream tyrosine kinase in this novel signaling route, as overexpression of dominant-negative PYK2 blocked Akt and Src activation as well as prevented endothelial cell chemotaxis. Furthermore, we obtained evidence that PYK2 is necessary for Plexin-B1 tyrosine phosphorylation, which likely leads to the subsequent recruitment of PYK2, Src, and the p85 subunit of PI3K to the activated receptor, resulting in transmission of intracellular signals required for endothelial cell chemotaxis.
These findings are aligned with the prior observation that the intracellular portion of Plexin-B1 is phosphorylated on tyrosine residues upon binding to Semaphorin 4D (11), collectively suggesting that activation of a protein tyrosine kinase phosphorylation cascade may take place as part of the Plexin-B1-initiated signal. However, it was not known how Plexin-B1 or its downstream proteins are phosphorylated, since Plexin-B1 is devoid of intrinsic tyrosine kinase activity. In this regard, recent evidence indicates that semaphorins can promote the oligomerization of tyrosine kinase receptors with plexins (55). For example, treatment of MLP29 liver progenitor cells with Semaphorin 4D results in the formation of a receptor signaling complex that includes Plexin-B1 and c-Met, leading to c-Met phosphorylation and activation, which, in turn, phosphorylates and activates Plexin-B1 (21). In human embryonic kidney 293 cells, Plexin-B family members can interact with the transmembrane tyrosine kinase ErbB-2, resulting in the activation of both ErbB-2 and plexin upon semaphorin binding (51). In endothelial cells, however, these interactions may not be detectable at biologically active levels of Semaphorin 4D (5). Instead, using Semaphorin 4D and chimeric Plexin-B1 molecules that cannot associate with c-Met or ERB2 because they lack the Plexin-B1 extracellular region, our present data implicate the FAK family member PYK2 in the initiation of Semaphorin 4D-Plexin-B1 signaling in endothelial cells, which eventually leads to activation of Src, PI3K, and Akt and a proangiogenic response exemplified by cell migration.
To date, several studies have implicated PYK2 in the regulation of endothelial cell motility (29, 38, 56) and vascular smooth muscle growth and differentiation (42), functions that are essential for angiogenesis. Once tyrosine phosphorylated, PYK2 localizes to focal adhesions and activates downstream effectors such as Src, mitogen-activated protein kinases, and PI3K, allowing it to participate in the transfer of signals from the cell surface to the cytoskeleton (4). Through the use of chimeric receptor constructs including the extracellular region of the NGF receptor, Trk-A, fused to the intracellular portion of Plexin-B1, we can specifically elicit Plexin-B1 signaling by treatment with NGF. Using this system, we have recently shown that Plexin-B1 induces endothelial cell migration and stress fiber formation through the small G-protein RhoA, a key regulator of actin polymerization that is also found in association with the cytoskeleton at focal adhesions (5, 19, 40). In this study we show that NGF treatment of endothelial cells stably expressing these chimeric receptors results in Plexin-B1-mediated PYK2 phosphorylation, a condition necessary for cell migration and phosphorylation and activation of downstream targets. Interestingly, under identical experimental conditions we failed to detect changes in FAK phosphorylation in response to Semaphorin 4D. Although this cannot rule out the participation of FAK in Plexin-B1 signaling, FAK is expressed in almost all tissues but PYK2 is expressed mainly in the central nervous system and in cells and tissues derived from hematopoietic lineages, which is a pattern of expression closely resembling that of Plexin-B1 (23). On the other hand, the ability of Plexin-B1 to stimulate Rho GTPases and cytosolic tyrosine kinases may be interrelated, as both PDZ-RhoGEF and LARG can be phosphorylated by PYK2 and FAK, thereby prolonging their stimulating activity on RhoA (13), and the activation of RhoA-initiated pathways can promote the activation of FAK and PYK2 (13). These observations raise the possibility of the existence of an intimate interplay between the Rho-GTPases and the activation of PYK2 by Plexin-B1, whose full elucidation warrants further investigation.
Our present findings indicate that upon binding of Semaphorin 4D, Plexin-B1 can promote the activation of the cytoplasmic tyrosine kinase PYK2, which is required for phosphorylation of tyrosine residues on the intracellular portion of this receptor. This likely creates a docking site for the SH2 domains of the p85 regulatory subunit of PI3K, thus providing a mechanism for PI3K-Akt activation, which contributes to the proangiogenic phenotype observed in Semaphorin 4D-treated endothelial cells, namely, cytoskeletal polymerization and cell migration. Indeed, we detected p85 in the phosphotyrosine-immunoprecipitated fraction of lysates from Semaphorin 4D-treated cells, indicating that this PI3K subunit was, in fact, bound to its target proteins, most likely at a membrane-proximal area such as the focal adhesion complex where the p110 catalytic subunit can interact with its lipid substrates, resulting in Akt activation (10). We also show that PYK2-mediated activation of the Src pathway is necessary for chemotaxis of endothelial cells toward Semaphorin 4D and therefore necessary in mediating Plexin-B1 signaling. Thus, the emerging notion is that Plexin-B1 stimulates Rho-GTPases through the direct binding to Rho GEFs while concomitantly acting as a Ras GAP (35, 36), a Rac inhibitor (61, 62), and an activator of an intracellular tyrosine kinase cascade that controls PI3K-Akt and Src as well as other signaling events that are known to be regulated by cytoplasmic tyrosine kinases. These findings broaden the complexity of signaling mechanisms by which Plexin-B1 may achieve its numerous physiological functions, including those involved in axon guidance and endothelial cell migration and angiogenesis.
Acknowledgments
Special thanks go to Panomwat Amornphimoltham, Lynn Cross, Vyomesh Patel, Ana Barac, Silvia Montaner, Akrit Sodhi, Nathalia Velloso, and Valerie Perrot for support and critical comments.
REFERENCES
- 1.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 1999. Unified nomenclature for the semaphorins/collapsins. Semaphorin Nomenclature Committee. Cell 97:551-552. [DOI] [PubMed] [Google Scholar]
- 3.Aurandt, J., H. G. Vikis, J. S. Gutkind, N. Ahn, and K. L. Guan. 2002. The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG. Proc. Natl. Acad. Sci. USA 99:12085-12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avraham, H., S. Y. Park, K. Schinkmann, and S. Avraham. 2000. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 12:123-133. [DOI] [PubMed] [Google Scholar]
- 5.Basile, J. R., A. Barac, T. Zhu, K. L. Guan, and J. S. Gutkind. 2004. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 64:5212-5224. [DOI] [PubMed] [Google Scholar]
- 6.Bates, D., G. I. Taylor, J. Minichiello, P. Farlie, A. Cichowitz, N. Watson, M. Klagsbrun, R. Mamluk, and D. F. Newgreen. 2003. Neurovascular congruence results from a shared patterning mechanism that utilizes Semaphorin3A and Neuropilin-1. Dev. Biol. 255:77-98. [DOI] [PubMed] [Google Scholar]
- 7.Birchmeier, C., F. Bladt, and T. Yamaai. 1997. The functions of HGF/SF and its receptor, the c-Met tyrosine kinase, in mammalian development. Ciba Found. Symp. 212:169-182. [DOI] [PubMed] [Google Scholar]
- 8.Birchmeier, W., V. Brinkmann, C. Niemann, S. Meiners, S. DiCesare, H. Naundorf, and M. Sachs. 1997. Role of HGF/SF and c-Met in morphogenesis and metastasis of epithelial cells. Ciba Found. Symp. 212:230-246. [DOI] [PubMed] [Google Scholar]
- 9.Bismuth, G., and L. Boumsell. 2002. Controlling the immune system through semaphorins. Science STKE 2002:RE4. [DOI] [PubMed] [Google Scholar]
- 10.Cantley, L. C. 2002. The phosphoinositide 3-kinase pathway. Science 296:1655-1657. [DOI] [PubMed] [Google Scholar]
- 11.Castellani, V., and G. Rougon. 2002. Control of semaphorin signaling. Curr. Opin. Neurobiol. 12:532-541. [DOI] [PubMed] [Google Scholar]
- 12.Chen, C., and H. Okayama. 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7:2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chikumi, H., S. Fukuhara, and J. S. Gutkind. 2002. Regulation of G protein-linked guanine nucleotide exchange factors for Rho, PDZ-RhoGEF, and LARG by tyrosine phosphorylation: evidence of a role for focal adhesion kinase. J. Biol. Chem. 277:12463-12473. [DOI] [PubMed] [Google Scholar]
- 14.Comoglio, P. M., and L. Trusolino. 2002. Invasive growth: from development to metastasis. J. Clin. Investig. 109:857-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dikic, I., G. Tokiwa, S. Lev, S. A. Courtneidge, and J. Schlessinger. 1996. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature 383:547-550. [DOI] [PubMed] [Google Scholar]
- 16.Driessens, M. H., H. Hu, C. D. Nobes, A. Self, I. Jordens, C. S. Goodman, and A. Hall. 2001. Plexin-B semaphorin receptors interact directly with active Rac and regulate the actin cytoskeleton by activating Rho. Curr. Biol. 11:339-344. [DOI] [PubMed] [Google Scholar]
- 17.Driessens, M. H., C. Olivo, K. Nagata, M. Inagaki, and J. G. Collard. 2002. B plexins activate Rho through PDZ-RhoGEF. FEBS Lett. 529:168-172. [DOI] [PubMed] [Google Scholar]
- 18.Ebens, A., K. Brose, E. D. Leonardo, M. G. Hanson, Jr., F. Bladt, C. Birchmeier, B. A. Barres, and M. Tessier-Lavigne. 1996. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron 17:1157-1172. [DOI] [PubMed] [Google Scholar]
- 19.Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature 420:629-635. [DOI] [PubMed] [Google Scholar]
- 20.Fujisawa, H., T. Kitsukawa, A. Kawakami, S. Takagi, M. Shimizu, and T. Hirata. 1997. Roles of a neuronal cell-surface molecule, neuropilin, in nerve fiber fasciculation and guidance. Cell Tissue Res. 290:465-470. [DOI] [PubMed] [Google Scholar]
- 21.Giordano, S., S. Corso, P. Conrotto, S. Artigiani, G. Gilestro, D. Barberis, L. Tamagnone, and P. M. Comoglio. 2002. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat. Cell. Biol. 4:720-724. [DOI] [PubMed] [Google Scholar]
- 22.Hanke, J. H., J. P. Gardner, R. L. Dow, P. S. Changelian, W. H. Brissette, E. J. Weringer, B. A. Pollok, and P. A. Connelly. 1996. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271:695-701. [DOI] [PubMed] [Google Scholar]
- 23.Hanks, S. K., and T. R. Polte. 1997. Signaling through focal adhesion kinase. Bioessays 19:137-145. [DOI] [PubMed] [Google Scholar]
- 24.Hu, H., T. F. Marton, and C. S. Goodman. 2001. Plexin B mediates axon guidance in Drosophila by simultaneously inhibiting active Rac and enhancing RhoA signaling. Neuron 32:39-51. [DOI] [PubMed] [Google Scholar]
- 25.Igishi, T., and J. S. Gutkind. 1998. Tyrosine kinases of the Src family participate in signaling to MAP kinase from both Gq and Gi-coupled receptors. Biochem. Biophys. Res. Commun. 244:5-10. [DOI] [PubMed] [Google Scholar]
- 26.Kawakami, A., T. Kitsukawa, S. Takagi, and H. Fujisawa. 1996. Developmentally regulated expression of a cell surface protein, neuropilin, in the mouse nervous system. J. Neurobiol. 29:1-17. [DOI] [PubMed] [Google Scholar]
- 27.Kolodkin, A. L., D. V. Levengood, E. G. Rowe, Y. T. Tai, R. J. Giger, and D. D. Ginty. 1997. Neuropilin is a semaphorin III receptor. Cell 90:753-762. [DOI] [PubMed] [Google Scholar]
- 28.Kolodkin, A. L., D. J. Matthes, and C. S. Goodman. 1993. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 75:1389-1399. [DOI] [PubMed] [Google Scholar]
- 29.Kuwabara, K., T. Nakaoka, K. Sato, T. Nishishita, T. Sasaki, and N. Yamashita. 2004. Differential regulation of cell migration and proliferation through proline-rich tyrosine kinase 2 in endothelial cells. Endocrinology 145:3324-3330. [DOI] [PubMed] [Google Scholar]
- 30.Maina, F., M. C. Hilton, R. Andres, S. Wyatt, R. Klein, and A. M. Davies. 1998. Multiple roles for hepatocyte growth factor in sympathetic neuron development. Neuron 20:835-846. [DOI] [PubMed] [Google Scholar]
- 31.Merlot, S., and R. A. Firtel. 2003. Leading the way: directional sensing through phosphatidylinositol 3-kinase and other signaling pathways. J. Cell Sci. 116:3471-3478. [DOI] [PubMed] [Google Scholar]
- 32.Miyazawa, K., T. Shimomura, D. Naka, and N. Kitamura. 1994. Proteolytic activation of hepatocyte growth factor in response to tissue injury. J. Biol. Chem. 269:8966-8970. [PubMed] [Google Scholar]
- 33.Neufeld, G., T. Cohen, N. Shraga, T. Lange, O. Kessler, and Y. Herzog. 2002. The neuropilins: multifunctional semaphorin and VEGF receptors that modulate axon guidance and angiogenesis. Trends Cardiovasc. Med. 12:13-19. [DOI] [PubMed] [Google Scholar]
- 34.Ohta, K., A. Mizutani, A. Kawakami, Y. Murakami, Y. Kasuya, S. Takagi, H. Tanaka, and H. Fujisawa. 1995. Plexin: a novel neuronal cell surface molecule that mediates cell adhesion via a homophilic binding mechanism in the presence of calcium ions. Neuron 14:1189-1199. [DOI] [PubMed] [Google Scholar]
- 35.Oinuma, I., Y. Ishikawa, H. Katoh, and M. Negishi. 2004. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science 305:862-865. [DOI] [PubMed] [Google Scholar]
- 36.Oinuma, I., H. Katoh, A. Harada, and M. Negishi. 2003. Direct interaction of Rnd1 with Plexin-B1 regulates PDZ-RhoGEF-mediated Rho activation by Plexin-B1 and induces cell contraction in COS-7 cells. J. Biol. Chem. 278:25671-25677. [DOI] [PubMed] [Google Scholar]
- 37.Oinuma, I., H. Katoh, and M. Negishi. 2004. Molecular dissection of the semaphorin 4D receptor plexin-B1-stimulated R-Ras GTPase-activating protein activity and neurite remodeling in hippocampal neurons. J. Neurosci. 24:11473-11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panetti, T. S. 2002. Tyrosine phosphorylation of paxillin, FAK, and p130CAS: effects on cell spreading and migration. Front. Biosci. 7:d143-d150. [DOI] [PubMed] [Google Scholar]
- 39.Perrot, V., J. Vazquez-Prado, and J. S. Gutkind. 2002. Plexin B regulates Rho through the guanine nucleotide exchange factors leukemia-associated Rho GEF (LARG) and PDZ-RhoGEF. J. Biol. Chem. 277:43115-43120. [DOI] [PubMed] [Google Scholar]
- 40.Raftopoulou, M., and A. Hall. 2004. Cell migration: Rho GTPases lead the way. Dev. Biol. 265:23-32. [DOI] [PubMed] [Google Scholar]
- 41.Raper, J. A. 2000. Semaphorins and their receptors in vertebrates and invertebrates. Curr. Opin. Neurobiol. 10:88-94. [DOI] [PubMed] [Google Scholar]
- 42.Rocic, P., G. Govindarajan, A. Sabri, and P. A. Lucchesi. 2001. A role for PYK2 in regulation of ERK1/2 MAP kinases and PI 3-kinase by ANG II in vascular smooth muscle. Am. J. Physiol. Cell. Physiol. 280:C90-C99. [DOI] [PubMed] [Google Scholar]
- 43.Rohm, B., A. Ottemeyer, M. Lohrum, and A. W. Puschel. 2000. Plexin/neuropilin complexes mediate repulsion by the axonal guidance signal semaphorin 3A. Mech. Dev. 93:95-104. [DOI] [PubMed] [Google Scholar]
- 44.Rohm, B., B. Rahim, B. Kleiber, I. Hovatta, and A. W. Puschel. 2000. The semaphorin 3A receptor may directly regulate the activity of small GTPases. FEBS Lett. 486:68-72. [DOI] [PubMed] [Google Scholar]
- 45.Scheffzek, K., A. Lautwein, W. Kabsch, M. R. Ahmadian, and A. Wittinghofer. 1996. Crystal structure of the GTPase-activating domain of human p120GAP and implications for the interaction with Ras. Nature 384:591-596. [DOI] [PubMed] [Google Scholar]
- 46.Schlaepfer, D. D., and T. Hunter. 1998. Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell. Biol. 8:151-157. [DOI] [PubMed] [Google Scholar]
- 47.Serini, G., D. Valdembri, S. Zanivan, G. Morterra, C. Burkhardt, F. Caccavari, L. Zammataro, L. Primo, L. Tamagnone, M. Logan, M. Tessier-Lavigne, M. Taniguchi, A. W. Puschel, and F. Bussolino. 2003. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 424:391-397. [DOI] [PubMed] [Google Scholar]
- 48.Shoji, W., S. Isogai, M. Sato-Maeda, M. Obinata, and J. Y. Kuwada. 2003. Semaphorin3a1 regulates angioblast migration and vascular development in zebrafish embryos. Development 130:3227-3236. [DOI] [PubMed] [Google Scholar]
- 49.Sieg, D. J., D. Ilic, K. C. Jones, C. H. Damsky, T. Hunter, and D. D. Schlaepfer. 1998. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK-cell migration. EMBO J. 17:5933-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swiercz, J. M., R. Kuner, J. Behrens, and S. Offermanns. 2002. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron 35:51-63. [DOI] [PubMed] [Google Scholar]
- 51.Swiercz, J. M., R. Kuner, and S. Offermanns. 2004. Plexin-B1/RhoGEF-mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J. Cell Biol. 165:869-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi, T., A. Fournier, F. Nakamura, L. H. Wang, Y. Murakami, R. G. Kalb, H. Fujisawa, and S. M. Strittmatter. 1999. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 99:59-69. [DOI] [PubMed] [Google Scholar]
- 53.Tamagnone, L., S. Artigiani, H. Chen, Z. He, G. I. Ming, H. Song, A. Chedotal, M. L. Winberg, C. S. Goodman, M. Poo, M. Tessier-Lavigne, and P. M. Comoglio. 1999. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99:71-80. [DOI] [PubMed] [Google Scholar]
- 54.Tamagnone, L., and P. M. Comoglio. 2000. Signalling by semaphorin receptors: cell guidance and beyond. Trends Cell. Biol. 10:377-383. [DOI] [PubMed] [Google Scholar]
- 55.Tamagnone, L., and P. M. Comoglio. 2004. To move or not to move? Semaphorin signalling in cell migration. EMBO Rep. 5:356-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang, H., Q. Hao, T. Fitzgerald, T. Sasaki, E. J. Landon, and T. Inagami. 2002. Pyk2/CAKbeta tyrosine kinase activity-mediated angiogenesis of pulmonary vascular endothelial cells. J. Biol. Chem. 277:5441-5447. [DOI] [PubMed] [Google Scholar]
- 57.Tessier-Lavigne, M., and C. S. Goodman. 1996. The molecular biology of axon guidance. Science 274:1123-1133. [DOI] [PubMed] [Google Scholar]
- 58.Thomas, S. M., and J. S. Brugge. 1997. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13:513-609. [DOI] [PubMed] [Google Scholar]
- 59.Trusolino, L., and P. M. Comoglio. 2002. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat. Rev. Cancer 2:289-300. [DOI] [PubMed] [Google Scholar]
- 60.Ueki, K., D. A. Fruman, S. M. Brachmann, Y. H. Tseng, L. C. Cantley, and C. R. Kahn. 2002. Molecular balance between the regulatory and catalytic subunits of phosphoinositide 3-kinase regulates cell signaling and survival. Mol. Cell. Biol. 22:965-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vikis, H. G., W. Li, and K. L. Guan. 2002. The plexin-B1/Rac interaction inhibits PAK activation and enhances Sema4D ligand binding. Genes Dev. 16:836-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vikis, H. G., W. Li, Z. He, and K. L. Guan. 2000. The semaphorin receptor plexin-B1 specifically interacts with active Rac in a ligand-dependent manner. Proc. Natl. Acad. Sci. USA 97:12457-12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vlahos, C. J., W. F. Matter, K. Y. Hui, and R. F. Brown. 1994. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 269:5241-5248. [PubMed] [Google Scholar]
- 64.Winberg, M. L., J. N. Noordermeer, L. Tamagnone, P. M. Comoglio, M. K. Spriggs, M. Tessier-Lavigne, and C. S. Goodman. 1998. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell 95:903-916. [DOI] [PubMed] [Google Scholar]