Abstract
Mutations of the thyroid hormone receptor β (TRβ) gene cause resistance to thyroid hormone (RTH). RTH is characterized by increased serum thyroid hormone associated with nonsuppressible thyroid-stimulating hormone (TSH) and impaired growth. It is unclear how the actions of TRβ mutants are modulated in vivo to affect the manifestation of RTH. Using a mouse model of RTH that harbors a knockin mutation of the TRβ gene (TRβPV mouse), we investigated the effect of the steroid hormone receptor coactivator 3 (SRC-3) on RTH. In TRβPV mice deficient in SRC-3, dysfunction of the pituitary-thyroid axis and hypercholesterolemia was lessened, but growth impairment of RTH was worsened. The lessened dysfunction of the pituitary-thyroid axis was attributed to a significant decrease in growth of the thyroid and pituitary. Serum insulin-like growth factor 1 (IGF-1) was further reduced in TRβPV mice deficient in SRC-3. This effect led to reduced signaling of the IGF-1/phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway that is known to mediate cell growth and proliferation. Thus, SRC-3 modulates RTH by at least two mechanisms, one via its role as a receptor coregulator and the other via its growth regulatory role through the IGF-1/PI3K/AKT/mTOR signaling.
Thyroid hormone receptors (TRs) are ligand-dependent transcription factors that mediate the biological activities of the thyroid hormone 3,5,3′-triiodo-l-thyronine (T3) in growth, development, and differentiation and in maintaining metabolic homeostasis. There are two TR genes, α and β, located on human chromosomes 17 and 3, respectively, that give rise to four T3-binding isoforms: α1, β1, β2, and β3 (2, 20). The expression of TRs is tissue dependent and developmentally regulated (2, 23). The transcriptional activity of TRs is regulated at multiple levels (2). In addition to the ligand (T3), the type of thyroid hormone response elements (TREs) located on the promoters of T3 target genes affects the magnitude and sensitivity of the transcriptional response. A host of coregulatory proteins plays a further critical role in modulating the gene regulatory functions of DNA-bound TRs (2, 14, 23). In the absence of T3, TRs repress basal transcription through association with a variety of corepressors. Binding of T3 induces structural changes to release the corepressors and allow for recruitment of coactivators. Corepressors harbor deacetylase activity that acts to modify the chromatin structure to limit the access of basal transcriptional machinery to the promoter. Coactivator complexes, in contrast, harbor the activities (e.g., histone acetyltransferase and histone methyltransferase) that trigger transcription by rendering chromatin more open to other transcription factors and that affect RNA splicing (14). The tissue- and time-dependent expression of coregulatory proteins provides an additional level of regulation by the T3-triggered dynamic exchange of corepressors and coactivators on TRs.
Mutations of the TRβ gene cause resistance to thyroid hormone (RTH), which is characterized by a reduced sensitivity of tissues to the action of thyroid hormone (19, 24). The elevated serum thyroid hormone levels of RTH patients are associated with nonsuppressible thyroid-stimulating hormone (TSH). Other clinical features are goiter, attention deficit hyperactivity disorder, decreased IQ, dyslexia, short stature, decreased weight, tachycardia, cardiac disease, and hypercholesterolemia (19, 24). RTH can appear sporadically, but most commonly it is a familial syndrome with autosomal dominant inheritance. TRβ mutants derived from RTH patients have reduced or no T3-binding activity and transcriptional capacities and act in a dominant-negative manner to cause the clinical phenotypes (19, 24).
To understand the molecular basis of RTH, we created a knockin mutant mouse by targeting the PV mutation to the TRβ gene locus via homologous recombination and the Cre-LoxP system (TRβPV mice) (7). The PV mutation was derived from a patient (PV) with severe RTH characterized by attention deficit hyperactivity disorder, short stature, low weight, goiter, and tachycardia. PV has an unusual mutation in exon 10, a C insertion at codon 448, that produces a frame shift of the carboxyl-terminal 14 amino acids of TRβ1 (11). In vitro characterization of the PV mutant showed a complete loss of T3-binding and transactivation activities. The PV mutation also strongly interferes with the transcriptional activity of wild-type TRs in vitro. TRβPV mice faithfully reproduce RTH in humans and therefore provide a valid mouse model to elucidate the molecular basis of RTH and to advance understanding of the molecular actions of TRβ mutants in vivo.
Although most RTH patients are clinically euthyroid, some are hypothyroid and some may appear thyrotoxic. Intriguingly, the same individual may present evidence of hypothyroidism in one tissue but show signs of thyrotoxicosis in other tissues (1, 19). The observation that clinical phenotype varies among families with the same mutation, and among individuals within a family, suggests complex regulation of the actions of TRβ mutants in vivo. We previously identified the steroid hormone receptor coactivator 1 (SRC-1) as one of the factors that modulates the target-tissue responsiveness in RTH (6). SRC-1 belongs to the p160 family of coactivators that includes SRC-2 and SRC-3 (9). Despite sequence similarity in the functional domains of these SRC coactivators, the phenotypes exhibited by mice deficient in SRC-1 (22) and SRC-3 (21) are distinct, suggesting that these two activators have preferential roles in different tissues in vivo. These observations prompted us to ascertain whether SRC-3 has different regulatory roles in the molecular actions of TRβ mutants in vivo, thereby contributing to the variable phenotypic manifestation of RTH. Indeed, we found that the lack of SRC-3 reduces the growth of both the pituitary and the thyroid in TRβPV mice, thereby lessening the dysregulation of the pituitary-thyroid axis. In contrast, the lack of SRC-3 exacerbates the growth impairment observed in TRβPV mice. Further studies indicated that this modulatory effect of SRC-3 on growth is mediated via the insulin growth factor 1 (IGF-1)/phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway. Therefore, SRC-3 modulates the manifestation of RTH via TR-dependent and TR-independent pathways.
MATERIALS AND METHODS
Mutant mice.
This animal study was carried out according to the protocol approved by the National Cancer Institute Animal Care and Use Committee. Genotyping was performed by using specific primers for TRβ and SRC-3 as described previously (7, 21). TRβPV mice deficient in SRC-3 were obtained by crossing TRβPV mice with SRC-3-null mice (21). Mice with different genotypes used in the present study were intercrossed several generations, and littermates were used in the comparison of phenotypes.
Hormone assays.
The serum levels of total T4 (TT4) and total T3 (TT3) were determined by using a Gamma Coat T4 and T3 assay RIA kit (Dia-Sorin, Stillwater, MN) according to the manufacturer's instructions. TSH levels in serum were measured as previously described (15, 16). IGF-I levels in serum were determined by using a DSL-2900 mouse/rat RIA (Diagnostic Systems Laboratories, Webster, TX) according to the manufacturer's instructions. A cholesterol E kit (Wako Diagnostics, Richmond, VA) was used for quantitative determination of total cholesterol in mouse serum according to the Wako cholesterol E microliter procedure provided by the manufacturer.
Quantitative real-time RT-PCR.
Total RNA was extracted from pituitary, thyroid, and liver of mice by using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Total RNA (200 ng) was used in real-time reverse transcription-PCR (RT-PCR) determinations as described previously (25). The specific primers were as follows: α-GSU, 5′-AGTGTATGGGCTGTTGCTTC-3′ (sense) and 5′-AGCTACGACTTGTGGTAGTAG-3′ (antisense); TSHβ, 5′-GGATAGGAGAGAGTGTGCC-3′ (sense) and 5′-AGCTTACGGCGACAGGGAA-3′ (antisense); GH, 5′-TTCGAGCGTGCCTACATT-3′ (sense) and 5′-GCATGTTGGCGTCAAACTTG-3′ (antisense); CYP7A, 5′-GGTCTCTGAACTGATCCGTC-3′ (sense) and 5′-GCCTCCTTGATGATGCTATC-3′ (antisense); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-ACATCATCCCTGCATCCACT-3′ (sense) and 5′-GTCCTCAGTGTAGCCCAAG-3′ (antisense).
Western blot analysis.
Thyroid glands dissected from TRβPV mice were washed with phosphate-buffered saline (PBS) and homogenized on ice in cell lysis buffer containing 50 mM Tris, 100 mM HCl, 0.5% Triton X-100, 0.2 μM okadaic acid, 100 mM NaF, 0.2 mM Na3VO4, and proteinase inhibitor tablet (Complete Mini EDTA-free; Roche). Next, the tissue was incubated on ice for 10 min with occasional shaking. The lysate was then centrifuged for 15 min at 12,000 × g at 4°C, and the supernatant was collected. The protein concentration was determined by the method of Bradford (Pierce Chemical Co., Rockford, IL) with bovine serum albumin (Pierce Chemical Co.) as the standard. For detection of p-IGF1-R, AKT, pAKT, TRO, pmTOR, S6K1, pS6K1, and S6, thyroid extracts (70 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The Western blot analysis was carried out as described by Furumoto et al. (5). All primary antibodies used in the Western blot analysis were from Cell Signaling Technology, Inc.: phospho-IGF-IR (antibody 3021), phospho-S473 Akt (antibody 9271), phospho-Thr308 Akt (antibody 9275), total Akt (antibody 9272), phospho-S2448 mTOR (antibody 2971), total mTOR (antibody 2972), phospho-Thr421/S424 p70 S6 kinase (antibody 9204), and total p70 S6 kinase (antibody 9202). Except for the detection of Akt, for which the dilution of antibody was 1:1,000, all other antibodies were diluted 1:500. For the control loading, protein disulfide isomerase (PDI) was determined with the use of anti-PDI antibody 3632 (3) in a manner similar to that described by Furumoto et al. (5).
Histological, immunohistochemical, and ultrastructural analysis.
Thyroid glands were fixed in 10% neutral buffered formalin and subsequently embedded in paraffin. Five-μm-thick sections were prepared and stained with hematoxylin and eosin. In addition, paraffin sections were processed for immunohistochemistry. After antigen retrieval with acid citrate buffer at 95°C for 20 min, sections were incubated with rabbit anti-Ki67 (NeoMarkers, Fremont, CA), followed by indirect labeling with horseradish peroxidase-labeled anti-rabbit immunoglobulin G (Jackson Immunoresearch, West Grove, PA) according to standard methods. For transmission electron microscopy, pituitaries were fixed in glutaraldehyde, postfixed in osmium tetroxide, and processed into Epon-Araldite by standard methods. Thin sections were counterstained with uranyl acetate and lead citrate.
Statistical analysis.
All data are expressed as mean ± the standard error of the mean (SEM). Statistical analysis was performed with the use of analysis of variance, and P < 0.05 was considered significant. Log-rank (Mantel-Cox) testing for statistical significance was carried out with the use of GraphPad Prism 4.0a (GraphPad Software, Inc., San Diego, CA).
RESULTS
Lack of SRC-3 lessens the dysfunction of the pituitary-thyroid axis in TRβPV mice.
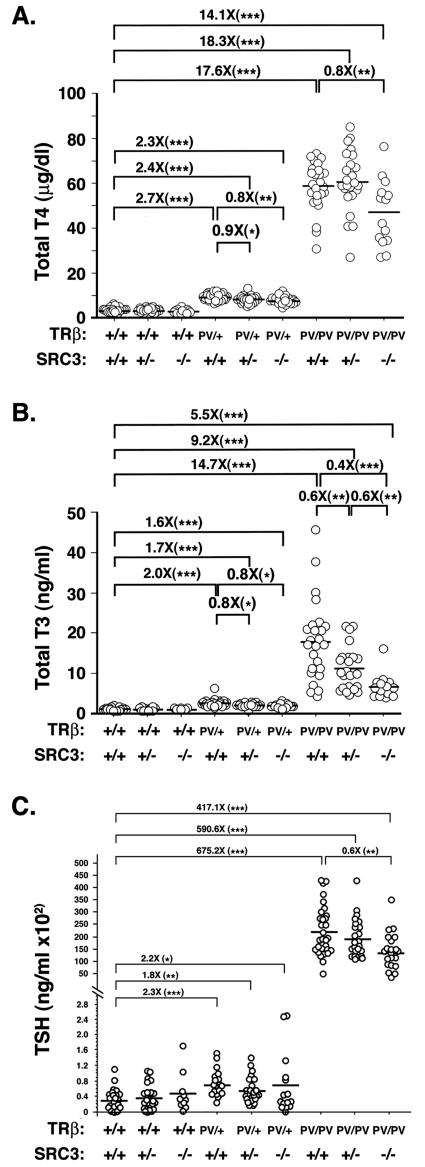
Thyroid hormone production is tightly regulated by serum TSH via the pituitary-thyroid feedback loop. To investigate the role of SRC-3 in the regulation of the pituitary-thyroid axis, we crossed TRβPV mice with mice carrying a SRC-3-null mutation (SRC-3−/− mice). We first compared the circulating levels of thyroid hormone in wild-type and TRβPV mice with or without SRC-3 (Fig. 1A and B). SRC-3-null mice did not exhibit resistance to thyroid hormone because their total T4 (TT4) concentrations did not significantly differ from those of wild-type mice (Fig. 1A; TRβ+/+ SRC-3+/+, 3.3 ± 0.2 μg/dl, n = 32; TRβ+/+ SRC3+/−, 3.2 ± 0.2 μg/dl, n = 24; TRβ+/+ SRC3−/−, 2.8 ± 0.3 μg/dl, n = 12). Consistent with earlier reports (7, 15, 16), TRβPV/+ SRC3+/+ mice had a 2.7-fold increase in TT4 (9.1 ± 0.3 μg/dl, n = 30) compared to the wild-type mice. Lack of SRC-3 significantly decreased TT4 by 10 and 20% in TRβPV/+ SRC-3+/− (8.1 ± 0.3 μg/dl, n = 24), and TRβPV/+ SRC-3−/− mice (7.7 ± 0.3 μg/dl, n = 23), respectively. TRβPV/PV SRC3+/+ mice had a 17.6-fold increase in TT4 (58.4 ± 2.1 μg/dl, n = 25) compared to the wild-type mice. Lack of SRC-3 did not change TT4 significantly in TRβPV/PV SRC3+/− mice but did decrease TT4 by 20% in TRβPV/PV SRC-3−/− mice (46.7 ± 3.9 μg/dl, n = 14). Thus, the extent of increase in TT4 concentrations in TRβPV/PV mice was reduced from 17.6- to 14.1-fold by the lack of SRC-3.
FIG. 1.
Comparison of serum thyroid hormones in TRβPV mice with or without SRC-3. Total serum T4 (A), total serum T3 (B), and TSH (C) were determined in adult mice (2 to 3 months old). Each circle represents the value for an individual mouse, and the horizontal bars represent the mean values (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Similar reduction in TT3 by the lack of SRC-3 in TRβPV mice was also detected (Fig. 1B). Consistent with earlier reports (7, 15, 16), TT3 concentrations were 2- and 14.7-fold higher in TRβPV/+ and TRβPV/PV mice (2.47 ± 0.14 ng/ml, n = 32; 17.85 ± 1.97 ng/ml, n = 25, respectively) than in wild-type mice (1.22 ± 0.06 ng/ml, n = 29). Lack of SRC-3 did not affect TT3 concentrations in TRβ wild-type mice (TRβ+/+ SRC3+/−, 1.15 ± 0.09 ng/ml, n = 14; TRβ+/+ SRC3−/−, 1.13 ± 0.09 ng/ml, n = 6) but did lower TT3 concentration in TRβPV/+ mice (∼23% reduction; 1.91 ± 0.16 ng/ml, n = 12 versus 2.47 ± 0.14 ng/ml, n = 32). Lack of SRC-3 further reduced the TT3 in TRβPV/PV mice by 40 and 60%, respectively, in mice lacking one or two alleles of SRC-3 (Fig. 1B; TRβPV/PV SRC-3+/+ mice, 17.85 ± 1.97 ng/ml, n = 25; TRβPV/PV SRC-3+/− mice, 11.14 ± 1.08 ng/ml, n = 24; TRβPV/PV SRC-3−/− mice, 6.64 ± 0.83, n = 14). Thus, highly elevated TT3 concentrations found in TRβPV/PV mice were significantly reversed by the lack of SRC-3 from a 14.7-fold increase in TRβPV/PV SRC-3+/+ mice to 5.5-fold in TRβPV/PV SRC-3−/− mice.
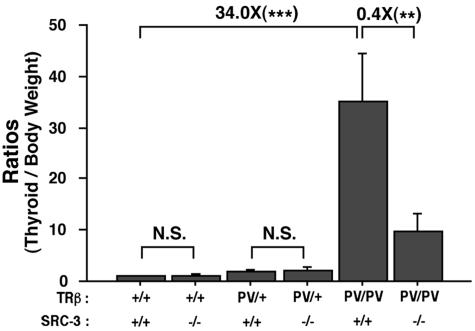
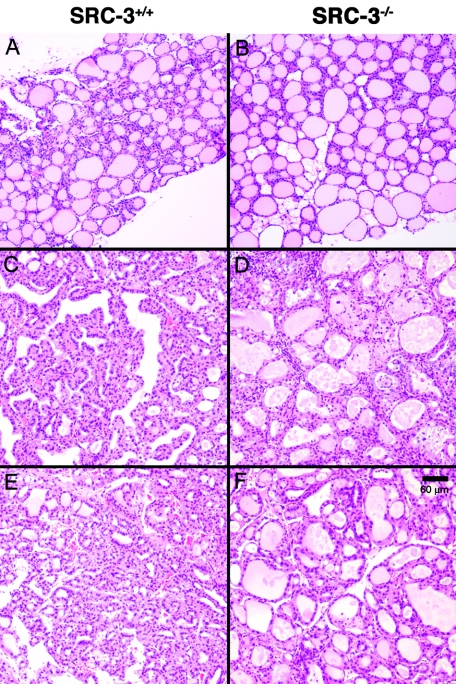
The reduced circulating thyroid hormone (Fig. 1A and B) in TRβPV mice lacking SRC-3 prompted us to examine the effect of lack of SRC-3 on the size of thyroid glands. Consistent with earlier observations (7), thyroid glands were enlarged in TRβPV/+ and TRβPV/PV mice (Fig. 2). Although the lack of SRC-3 had no significant effect on the size of the thyroid of wild-type mice and TRβPV/+ mice, SRC-3 deficiency reduced the size of thyroids in TRβPV/PV SRC-3−/− mice by 60%. The reduction in weight reflects the effect of SRC-3 on thyroid cell proliferation as shown by thyroid histology (Fig. 3). Morphological examination revealed that the thyroid glands of TRβPV/PV SRC-3+/+ mice had extensive hyperplasia (Fig. 3C and F) compared to wild-type thyroid (Fig. 3A), whereas the thyroid cells in TRβPV/PV SRC-3−/− mice had a more normal appearance with large follicles (Fig. 3D and F), compared to the normal histology in TRβ+/+ SRC-3−/− mice (Fig. 3B). These results indicate that the lack of SRC-3 ameliorates the abnormal proliferation of thyroid cells in TRβPV mice.
FIG. 2.
Comparison of thyroid weight in TRβPV mice with or without SRC-3. Thyroids were dissected from adult mice (2 to 3 months old). Relative ratios of thyroid weight per gram body weight (thyroid weight/body weight) were determined by setting that of wild-type mice as 1. Male mice at 4 to 5 months of age in each genotype were used (*, P < 0.05; **, P < 0.01; ***, P < 0.001; N.S., not significant, P > 0.05, n = 3 to 7).
FIG. 3.
Thyroids of TRβPV/PV SRC-3+/+ mice exhibit extensive hyperplastic morphology, whereas those of TRβPV/PV SRC-3−/− mice show nearly normal morphology with large follicles. Mouse genotypes: A, TRβ+/+ SRC-3+/+; B, TRβ+/+ SRC-3−/−; C and E, TRβPV/PV SRC-3+/+; D and F, TRβPV/PV SRC-3−/−. All panels are shown with the same magnification (bar = 60 μm). Panels A, B, C, and D show results for siblings at the ages of 6.8 months, and panels E and F show the results for siblings at the age of 9.7 months.
In RTH, the nonsuppressible serum TSH level is a critical indicator of the severity of the dysfunction of the pituitary-thyroid axis. We therefore compared serum TSH levels in mice with or without SRC-3 (Fig. 1C). Similar to our previous reports (7, 15, 16), TRβPV/+ and TRβPV/PV mice exhibited 2.3- and 675-fold higher TSH levels (74.7 ± 7.8 ng/ml, n = 20, for TRβPV/+ SRC-3+/+ mice; 21,995 ± 1,876 ng/ml, n = 30, for TRβPV/PV SRC-3+/+ mice) than wild-type mice (32.6 ± 5.7 ng/ml, n = 24). Consistent with TT4 and TT3 levels, the lack of SRC-3 did not significantly alter serum TSH concentrations in wild-type mice (38.3 ± 6.7 ng/ml, n = 24, for TRβ+/+ SRC-3+/− mice; 50.6 ± 16.2 ng/ml, n = 10, for TRβ+/+ SRC-3−/− mice). The lack of SRC-3 did not significantly affect the TSH levels in TRβPV/+ mice (compare 74.7 ± 7.8 ng/ml, n = 20, for TRβPV/+ SRC-3+/+ mice with 57.46 ± 6.7 ng/ml, n = 24, for TRβPV/+ SRC-3+/− mice or with 70 ± 21.2 ng/ml, n = 15, for TRβPV/+ SRC-3−/− mice). In TRβPV/PV mice, the lack of one allele of the SRC-1 gene did not significantly alter the TSH levels (compare 21,995 ± 1,876 ng/ml, n = 30, for TRβPV/PV SRC-3+/+ mice with 19,237 ± 1,608 ng/ml, n = 24, for TRβPV/PV SRC-3+/− mice), albeit the mean values tended to be lower. The lack of both alleles, however, significantly lowered the elevated TSH in TRβPV/PV mice by 40% (13,585 ± 1,614 ng/ml, n = 21, for TRβPV/PV SRC-3−/− mice). Taken together, results from thyroid function tests indicate that the lack of SRC-3 decreases the severity in the dysfunction of the pituitary-thyroid axis in TRβPV/PV mice.
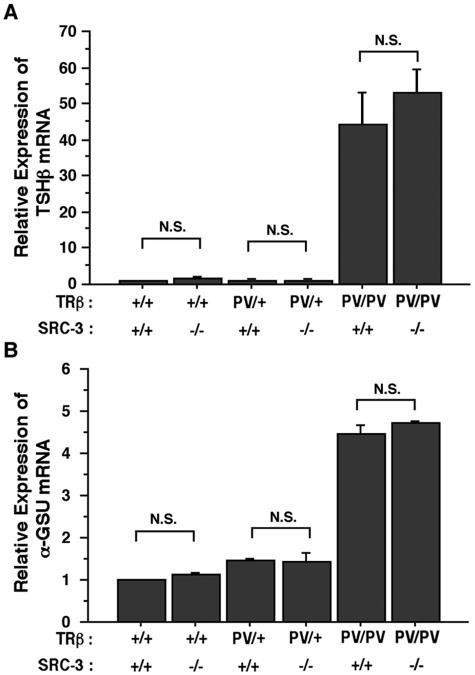
To understand how SRC-3 decreases the circulating TSH in TRβPV/PV SRC-3−/− mice, we examined the expression of the TSH β subunit gene (TSHβ) and α-glycoprotein common subunit (α-GSU) genes in TRβPV mice (Fig. 4). Previously, we reported that instead of being repressed by the elevated thyroid hormone, the expression of TSHβ and α-GSU genes is abnormally upregulated due to the interference of the transcriptional activity of wild-type TRs by the mutant PV (7). Figure 4 shows that the lack of SRC-3 did not alter the expression of the TSHβ (Fig. 4A) and α-GSU genes (Fig. 4B) in either the wild-type, TRβPV/+ SRC-3−/−, or TRβPV/PV SRC-3−/− mice (Fig. 4A). Therefore, the reduced circulating TSH level observed in TRβPV/PV SRC-3−/− mice (Fig. 1A) most likely is not due to the altered expression of TSHβ and α-GSU genes in the pituitary of TRβPV mice.
FIG. 4.
Comparison of the expression of TSHβ and α-GSU in the pituitary of TRβPV mice with or without SRC-3. The expression of TSHβ (Α) and α-GSU (B) mRNA was determined by using quantitative real-time RT-PCR as described in Materials and Methods. Relative quantification of each target mRNA was determined by arbitrarily setting the control value of wild-type mice as 1. Differences in total RNA input were normalized by signals obtained with specific primers for GAPDH. The data are expressed as means ± the SEM. (n = 3).
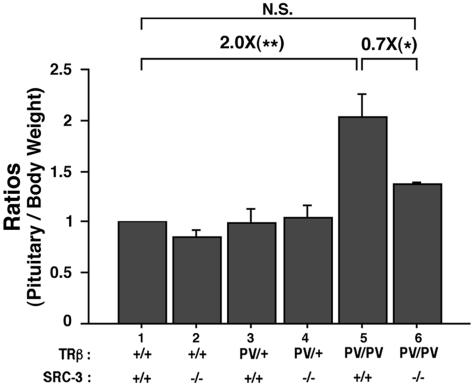
We further compared the effect of SRC-3 on the size of the pituitary of TRβPV mice (Fig. 5). Consistent with a previous report (5), the pituitary size of TRβPV/PV SRC-3+/+ mice was twice that of wild-type mice. The lack of SRC-3, however, decreased significantly (30%) the PV-induced enlargement of the pituitary in TRβPV/PV SRC-3−/− mice (Fig. 5). The lack of SRC-3 had no significant effect on the size of the pituitary in wild-type or TRβPV/+ SRC-3−/− mice. These results suggest that the decrease in the pituitary size may play a significant role in the overall reduction of the circulating TSH levels.
FIG. 5.
Comparison of pituitary weight in TRβPV mice with or without SRC-3. Relative ratios of pituitary weight per gram body weight (pituitary weight/body weight) were determined by setting the ratio of wild-type mice as 1. The pituitaries of adult male mice at the age of 3 to 5 months for each genotype were compared (*, P < 0.05; **, P < 0.01; ***, P < 0.001; N.S., not significant, P > 0.05; n = 3 to 7).
Lack of SRC-3 lessens the resistance of the liver in the regulation of serum cholesterol.
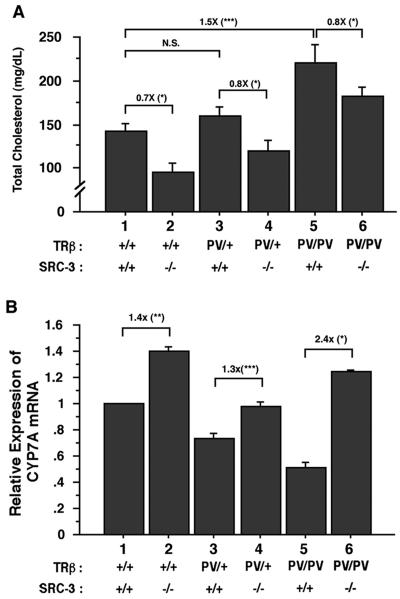
To understand whether the lack of SRC-3 alters the resistance to thyroid hormone in the liver, we measured the serum total cholesterol that is an in vivo marker of thyroid hormone action. The response of a decrease in serum cholesterol after administration of thyroid hormone is commonly used as a diagnostic test for assessing RTH in peripheral tissues (13). Figure 6A illustrates that the liver shows resistance to thyroid hormone by failing to respond properly to the elevated thyroid hormone as indicated by no repression of the cholesterol levels in TRβPV/+ SRC-3+/+ mice (compared to TRβ+/+ SRC-3+/+ mice; Fig. 6A, bar 3 versus bar 1; 142.5 ± 7.7 mg/dl, n = 13 and 159.35 × 10.3 mg/dl, n = 13 for TRβ+/+ SRC-3+/+ mice and TRβPV/+ SRC-3+/+ mice, respectively; P = 0.347). The resistance was intensified in TRβPV/PV SRC-3+/+ mice in that the serum cholesterol levels were significantly increased (Fig. 6A, bar 5 versus bar 1; 1.5-fold; 219.8 ± 20.9 mg/dl, n = 13) compared to TRβ+/+ SRC-3+/+ mice. Lack of SRC-3 lowered 20% of the serum cholesterol levels in both heterozygous and homozygous TRβPV mice (compare bars 3 and 4 and bars 5 and 6; 119.1 ± 12.2 mg/dl, n = 11, for TRβPV/+ SRC-3−/− mice and 182.6 × 10.1 mg/dl, n = 12, for TRβPV/PV SRC-3−/− mice). This reduction of serum cholesterol levels by the lack of SRC-3 also was apparent in mice without PV mutation (compare bars 1 and 2; 95.5 × 10.3 mg/dl, n = 12, for in TRβ+/+ SRC-3−/− mice).
FIG. 6.
Comparison of serum total cholesterol levels (A) and the expression of CYP7A mRNA in the liver (B) of TRβPV mice with or without SRC-3. (A) Serum total cholesterol levels were determined from adult mice (2 to 3 months old) as described in Materials and Methods. The data are expressed as means ± the SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001; n = 12 to 13). (B) Comparison of the expression of CYP7A mRNA in the liver of TRβPV mice with or without SRC-3. The expression of CYP7A mRNA was determined by using quantitative real-time RT-PCR as described in Materials and Methods. Relative quantification of each target mRNA was determined by arbitrarily setting the control value for wild-type mice as 1. Differences in total RNA input were normalized by signals obtained with specific primers for GAPDH. The data are expressed as mean ± the SEM. (n = 3).
T3 is important not only for the uptake of cholesterol by the liver but also for the hepatic degradation of cholesterol into bile acids. The rate-limiting enzyme in the latter process, cholesterol 7α hydroxylase (CYP7A), is induced by T3 at the transcriptional level (4, 10). That serum cholesterol levels were higher in TRβPV/+ SRC-3−/− and TRβPV/PV SRC-3−/− mice than in TRβ+/+ SRC-3−/− (bars 4 and 6 compared to bar 2; Fig. 6B) suggested that the lack of SRC-3 could affect the expression of the CYP7A gene. Indeed, Fig. 6B shows that whereas the expression of the CYP7A gene was repressed in TRβPV/+ SRC-3+/+ and TRβPV/PV SRC-3+/+ mice owing to the interference of the transcriptional activity of wild-type TRs by PV (compare bars 3 and 5 with bar 1), its expression was activated by the lack of SRC-3 (bars 2, 4, and 6 versus bar 1). Taken together, the lack of SRC-3 lessens the resistance of the liver in the regulation of serum cholesterol by overcoming the PV-mediated repression of the CYP7A gene as one of the mechanisms.
Lack of SRC-3 intensifies the impairment of postnatal growth in TRβPV mice.
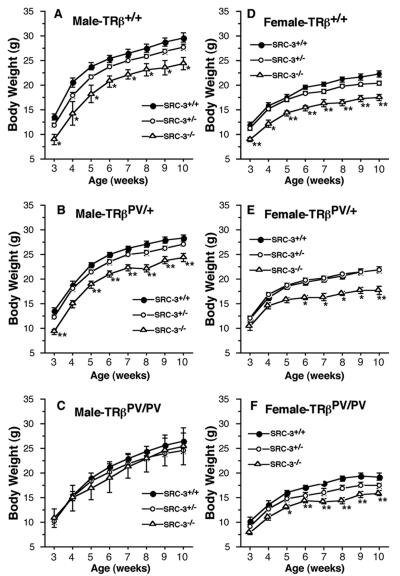
To understand the effect of SRC-3 on postnatal growth of TRβPV mice, we compared the growth curves of TRβPV mice with or without SRC-3 beginning at the age of 3 weeks until adulthood (Fig. 7). Consistent with an earlier report (21), the lack of both alleles of the SRC-3 gene impaired the weight gain of both male and female TRβ+/+ SRC-3−/− mice (Fig. 7A and D). Previously, we reported that TRβPV/PV SRC-3+/+ mice had impaired weight gain, but not TRβPV/+ SRC-3+/+ mice (17, 15, 16). Figure 7B and E show that both male and female TRβPV/+ SRC-3−/− mice had impaired weight gain. The defect in weight gain in homozygous TRβPV mice was further worsened by the lack of SRC-3, indicating that the mutation of the two alleles of the TRβ gene and the loss of both alleles of the SRC-3 gene contribute to the impairment in growth.
FIG. 7.
Comparison of weekly weight gain of TRβPV mice with or without SRC-3. The weights of male (A, B, and C) or female (D, E, and F) mice with or without SRC-3 were measured from 3 to 10 weeks old. The data are shown as means ± the SEM (n = 30 to 68).
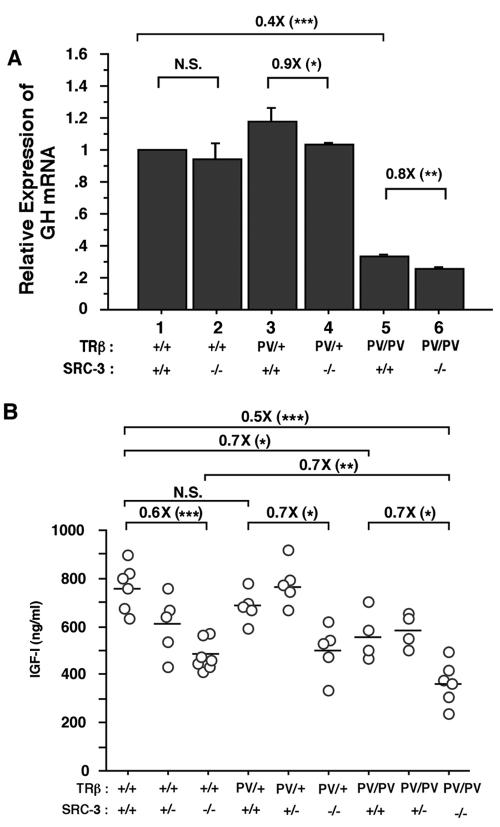
To understand how the lack of SCR-3 mediates the growth impairment, we first examined the expression of the growth hormone gene (GH) (Fig. 8). Previously, we reported that, instead of being activated by the elevated thyroid hormone, the expression of GH in TRβPV/PV mice was abnormally repressed because of interference with the transcriptional activity of wild-type TRs by the mutant PV (7). We hypothesized that the reduced GH expression could contribute to the impaired growth in TRβPV/PV mice. To understand whether the lack of SRC-3 had any effect on the expression of the GH gene, we compared the expression of the GH gene in TRβPV mice with or without SRC-3. Figure 8 shows that the lack of SRC-3 did not affect the expression of the GH gene in wild-type (TRβ+/+) mice, but it had a small effect (10% reduction) on TRβPV/+ SRC-3−/− mice. In TRβPV/PV SRC-3+/+ mice, the expression of the GH gene was reduced 60% (compare bar 5 with bar 1; Fig. 8), the lack of SRC-3 further lowered the expression of the GH gene (∼20%, bar 6 versus bar 5) in TRβPV/PV SRC-3−/− mice. These results suggest that SRC-3 could mediate the growth impairment, in part, via reduction in the expression of the GH gene.
FIG. 8.
Comparison of GH mRNA expression (A) and total serum IGF-I levels (B) in TRβPV mice with or without SRC-3. (A) The expression of GH was determined (200 ng of total RNA) in the pituitaries of mice with the genotypes indicated. Relative quantification of each target mRNA was determined by arbitrarily setting the control value of wild-type mice as 1. Differences in total RNA input were normalized by signals obtained with specific primers for GAPDH. The data are expressed as means ± the SEM (n = 3). (B). Serum levels of IGF-I in mice (2 to 3 months old) were determined as described in Materials and Methods. Each circle represents the value for an individual mouse, and the horizontal bars represent the mean values (*, P < 0.05; **, P < 0.01; ***, P < 0.001; N.S., not significant, P > 0.05, n = 5 to 7).
Recently, studies found that SRC-3 played a critical role in growth regulation (18, 21). One of the pathways mediated by SRC-3 in growth regulation was via the IGF-1/PI3K/AKT pathway (17, 26). We therefore determined the serum levels of IGF-1 in TRβPV mice with or without SRC-3 (Fig. 8B). Consistent with reports by others (18, 21), we detected a significant reduction (40%) in serum IGF-1 in TRβ+/+ SRC-3−/− mice compared to TRβ+/+ SRC-3+/+ mice (Fig. 8B). This level of reduction was also observed in heterozygous TRβPV mice deficient in SRC-3 (Fig. 8B). A significant 30% reduction in IGF-1 was found in TRβPV/PV SRC-3+/+ mice. An additional 30% reduction in IGF-1 was detected in TRβPV/PV SRC-3−/− mice. These results are consistent with the impaired postnatal growth illustrated in Fig. 7 and suggest that the mutation of the TRβ gene and the deficiency in SRC-3 collaborate to impair growth.
SRC-3 modulates the growth of thyroid via the IGF-1/PI3K/AKT/mTOR pathway.
Our data indicate that lack of SRC-3 alters the manifestation of RTH. The hallmark of RTH is the dysfunction of the pituitary-thyroid axis. Therefore, to gain insights into the mechanisms by which SRC-3 modulates the phenotypic expression of RTH, we focused on understanding how the lack of SCR-3 affects the growth of the thyroid in TRβPV mice.
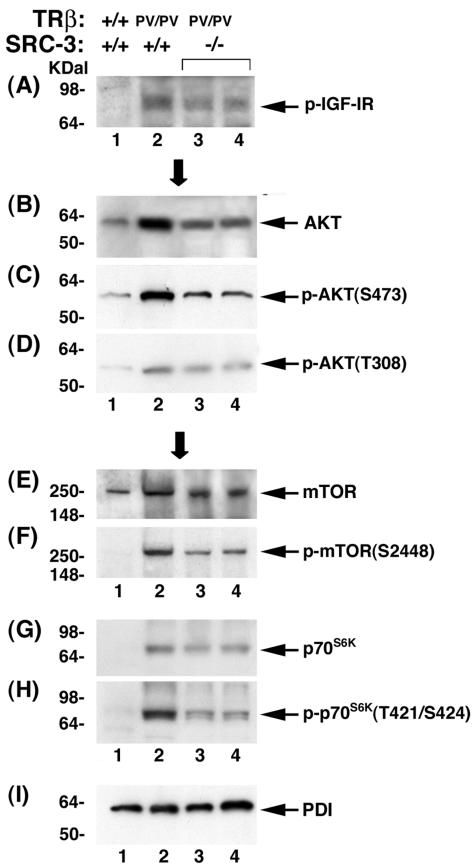
The weight of the thyroid was markedly reduced (Fig. 2), and the abnormal overproliferation of thyroid follicular cells was clearly suppressed by the lack of SRC-3 in TRβPV/PV SRC-3−/− mice (Fig. 3). Significantly reduced circulating IGF-1 levels in TRβPV/PV SRC-3−/− mice (Fig. 8B) prompted us to examine the expression of key regulators in the IGF-1/PI3K/AKT/mTOR pathway. This pathway was recently reported to mediate the SRC-3-induced growth of cultured prostate cancer cells and mammary tumor cells derived from transgenic mice overexpressing SRC-3/AIBI (17, 26). It is not known, however, whether the activation of the IGF-1/PI3K/AKT/mTOR pathway induced by SRC-3 contributes to the growth regulation of the thyroid. We reasoned that if this were the case, then the pathway would be repressed by the reduced phosphorylation of AKT and its downstream effectors in TRβPV mice deficient in SRC-3. We therefore determined the expression of the key regulators and their phosphorylation state in the IGF-1 signaling pathway by Western blot analysis (Fig. 9). Figure 9A shows that, compared to wild-type mice (lane 1), phosphorylated IGF-1 receptor (p-IGF-1R) was increased in TRβPV/PV SRC-3+/+ mice (lane 2). However, the lack of SRC-3 resulted in the reduction of the p-IGF-1R (Fig. 9A, lanes 3 and 4 versus lane 2). As shown in lane 2 of Fig. 9B, total AKT was increased in TRβPV/PV SRC-3+/+ mice compared to wild-type mice (Fig. 9B, lane 1). However, the lack of SRC-3 resulted in the reduction of total AKT in TRβPV/PV SRC-3−/− mice (Fig. 9B, lanes 3 and 4). Importantly, p-AKT was reduced by the lack of SRC-3 both at the phosphorylation site S473 (TRβPV/PV SRC-3−/− mice; Fig. 9C, lanes 3 and 4) and at the phosphorylation site T308 (Fig. 9D, lanes 3 and 4) compared to TRβPV/PV SRC-3+/+ mice (Fig. 9C and D, lane 2). The ratios of p(S473)-AKT/total AKT were decreased from 1.20 for TRβPV/PV SRC-3+/+ mice to 0.45 for TRβPV/PV SRC-3−/− mice and those of p(T308)-AKT/total AKT from 0.24 to 0.16. Moreover, the lack of SRC-3 decreased the expression of not only mTOR protein (Fig. 9E, lanes 3 and 4 versus lane 2) but also the phosphorylated mTOR (Fig. 9F). The ratios of p-mTOR/total mTOR were decreased from 1.27 for TRβPV/PV SRC-3+/+ mice to 0.67 for TRβPV/PV SRC-3−/− mice. The expression of p70S6K protein and its phosphorylation protein was also decreased by the lack of SRC-3 (Fig. 9G and H, lanes 3 and 4). The ratios of p-p70S6K/total p70S6K were decreased from 1.35 for TRβPV/PV SRC-3+/+ mice to 0.67 for TRβPV/PV SRC-3−/− mice. Figure 9I shows the loading control of PDI. These results clearly indicate that the signaling of IGF-1/PI3K/AKT/mTOR is reduced in TRβPV mice deficient in SRC-3, thereby resulting in reduced thyroid proliferation and cell growth.
FIG. 9.
Expression of key regulatory proteins of the IGF-1/PI3K/AKT/mTOR pathways in the thyroid of wild-type and mutant mice. Thyroid extract (70 μg) was used for determination of the expression of p-IGF-1R (A), total AKT (B), p-AKT(S473) (C), p-AKT(T308) (D), mTOR (E), p-mTOR (F), p70S6K (G), p-p70S6K (H), and PDI (I) as described in Materials and Methods. Experiments were repeated four times with similar results. Only representative results are shown.
DISCUSSION
The variable phenotypic manifestation among RTH patients with the same TRβ mutation suggests complex regulation of molecular actions of TRβ mutants in vivo. The present study identified SRC-3 as a modulator for the phenotypic manifestations of RTH. Lack of SRC-3 lessens the resistance in the pituitary-thyroid axis and ameliorates PV-induced hypercholesterolemia in TRβPV mice (Fig. 6A). However, the growth impairment induced by PV in TRβPV mice was further intensified by the lack of SRC-3 (Fig. 7). Therefore, the modulation of the phenotypic expression of RTH by the lack of SRC-3 is clearly target tissue dependent.
The target tissue-dependent modulation of the phenotypic manifestation of RTH caused by the lack of SRC-3 also has been observed for SRC-1, another member of the p160 coactivator family (6). However, the tissue-dependent modulation profiles caused by the lack of SRC-1 or SRC-3 in TRβPV mice are not identical (Table 1). In contrast to SRC-3, the lack of SRC-1 intensifies resistance in the pituitary-thyroid axis, increases thyroid cell proliferation, and has no effect on PV-induced hypercholesterolemia and the abnormal expression of the CYP7A gene in TRβPV mice (6) (Table 1). These contrasting effects of SRC-1 and SRC-3 on the manifestation of RTH cannot be explained by the redundant functions of these two coactivators. Thus, it is conceivable that, in addition to regulating nuclear receptor-dependent signaling, SRCs could also function independent of the nuclear receptor-signaling pathway. This notion is supported by the distinct phenotype exhibited by mutant mice deficient in SRC-1 or SRC-3. Mice deficient in SRC-1 exhibit normal growth, whereas mice deficient in SRC-3 display dwarfism (21, 22).
TABLE 1.
Comparison of RTH manifestation of TRβPV mice deficient in SRC-3 or SRC-1
| Nulled coactivator | Phenotype
|
||||
|---|---|---|---|---|---|
| Dysfunction of thyroid/pituitary axis | Impairment in wt gain | Thyroid proliferation | Liver function
|
||
| CYP7A gene expression | Hypercholesterolemia | ||||
| SRC-3 | Lessen | Intensify | Decrease | Upregulation | Reduced |
| SRC-1a | Intensify | Intensify | Increase | No effect | No change |
Kamiya et al. (6).
The altered resistance in target tissues of TRβPV mice caused by the lack of SRC-3 reveals the dual roles of SRC-3 in modulating phenotypic manifestation of RTH. SRC-3 could function as a classical coactivator that enhances the transcription of wild-type TR. Its deletion in TRβPV mice further intensifies the deleterious effect of PV because the transcriptional activity of TRα1 can no longer be activated as expected. This effect is exemplified by the additional reduction in the expression of the GH gene in TRβPV/PV SRC-3−/− mice compared to TRβPV/PV SRC-3+/+ mice (Fig. 8A). Even though it is unclear whether this is a direct or indirect effect, an additional drop in serum circulating IGF-1 levels was detected in TRβPV/PV SRC-3−/− mice compared to TRβPV/PV SRC-3+/+ mice (Fig. 8B) and thereby contributed to the intensification of growth impairment detected in such mice (Fig. 8F). The other role of SRC-3 in modulating the phenotypic manifestation is illustrated by the lessening of the dysfunction of the pituitary-thyroid axis in TRβPV/PV SRC-3−/− mice. This is accomplished through the action of SRC-3 as a regulator in cell growth and proliferation. We found that TSH levels were reduced (∼40%) in TRβPV/PV SRC-3−/− mice compared to TRβPV/PV SRC-3+/+ mice. However, the fact that this reduction is not due to the decreased expression of either TSHβ mRNA or α-GSU mRNA (Fig. 4) probably reflects the compensatory effect of other p160 family members at the transcriptional level for these genes in the pituitary (SRC-2 and SRC-3 are expressed in the pituitary; data not shown). However, the lack of SRC-3 clearly led to a smaller pituitary, and that could impact the synthesis and secretion of TSH. Similarly, the thyroid cells were not abnormally overproliferative (Fig. 3), and the size of the thyroid was significantly smaller (Fig. 2) in TRβPV/PV SRC-3−/− mice than in TRβPV/PV SRC-3+/+ mice, thereby promoting reduced synthesis of thyroid hormones. Therefore, SRC-3 modulates the manifestation of RTH both as a receptor coactivator and as a growth promoter independent of hormone-nuclear receptor signaling.
The present study identified SRC-3 as a novel regulator for thyroid proliferation. Although the precise mechanisms by which SRC-3 acts independent of hormone-nuclear receptor signaling are unclear, recent studies demonstrate that its growth-promoting effect is, at least in part, mediated by the IGF-1/PI3K/AKT/mTOR signaling pathway (17, 26). We found that serum IGF-1 levels in TRβPV/PV SRC-3+/+ mice were lower than in wild-type mice. The lack of SRC-3 further significantly lowered the serum IGF-1 in TRβPV/PV SRC-3−/− mice (Fig. 8). The lowered serum IGF-1 induced by the lack of SRC-3 led to a reduction in IGF-1 signaling pathway via PI3K-AKT-mTOR cascades that are known to regulate cell volume and organ size (8, 12). The contribution of thyroid growth induced by SRC-3 via the IGF-1/PI3K/AKT/mTOR pathway cannot be underestimated. TSH has long been recognized as a pivotal thyrocyte growth stimulator. In TRβPV/PV SRC-3−/−mice, however, despite highly elevated TSH levels (414-fold increase compared to wild-type mice), thyrocytes were clearly less hyperproliferative, and the size of thyroid was significantly smaller than in TRβPV/PV SRC-3+/+ mice, indicating that the lack of SRC-3 counteracts the proliferative effect of TSH. These observations suggest that under normal conditions, in addition to TSH, SRC-3 via the IGF-1/PI3K/AKT/mTOR pathway also contributes to maintaining the homeostasis of thyroid function.
The dual fashions by which SRC-3 modulates the manifestation of RTH have important implications for the molecular basis of RTH. RTH is caused by mutations of the TRβ gene. However, the present study reveals that other genetic changes independent of mutant TR actions could enhance or ameliorate the manifestation of RTH. It is not unreasonable to postulate that there are other such genetic factors waiting to be discovered. The SRC-3 identified in the present study and other unknown factors, together with their tissue-dependent expression, are likely to contribute to the tissue-heterogeneity of RTH that is frequently observed in RTH patients.
REFERENCES
- 1.Beck-Peccoz, P., and V. K. Chatterjee. 1994. The variable clinical phenotype in thyroid hormone resistance syndrome. Thyroid 4:225-232. [DOI] [PubMed] [Google Scholar]
- 2.Cheng, S. Y. 2000. Multiple mechanisms for regulation of the transcriptional activity of thyroid hormone receptors. Rev. Endocrinol. Metab. Disord. 1:9-18. [DOI] [PubMed] [Google Scholar]
- 3.Cheng, S. Y., S. Hasumura, M. C. Willingham, and I. Pastan. 1986. Purification and characterization of a membrane-associated 3,3′,5-triiodo-l-thyronine binding protein from a human carcinoma cell line. Proc. Natl. Acad. Sci. USA 83:947-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crestani, M., W. G. Karam, and J. Y. Chiang. 1994. Effects of bile acids and steroid/thyroid hormones on the expression of cholesterol 7 alpha-hydroxylase mRNA and the CYP7 gene in HepG2 cells. Biochem. Biophys. Res. Commun. 198:546-553. [DOI] [PubMed] [Google Scholar]
- 5.Furumoto, H., H. Ying, G. V. R. Chandramouli, L. Zhao, R. Walker, S. Meltzer, M. C. Willingham, and S. Cheng. 2005. An unliganded thyroid hormone receptor activates the cyclin D1/cyclin-dependent kinase/retinoblastoma/E2F pathway and induces pituitary tumorigenesis. Mol. Cell. Biol. 25:124-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamiya, Y., X. Y. Zhang, H. Ying, Y. Kato, M. C. Willingham, J. Xu, B. W. O'Malley, and S. Y. Cheng. 2003. Modulation by steroid receptor coactivator-1 of target-tissue responsiveness in resistance to thyroid hormone. Endocrinology 144:4144-4153. [DOI] [PubMed] [Google Scholar]
- 7.Kaneshige, M., K. Kaneshige, X. Zhu, A. Dace, L. Garrett, T. A. Carter, R. Kazlauskaite, D. G. Pankratz, A. Wynshaw-Boris, S. Refetoff, B. Weintraub, M. C. Willingham, C. Barlow, and S. Cheng. 2000. Mice with a targeted mutation in the thyroid hormone beta receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc. Natl. Acad. Sci. USA 97:13209-13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leevers, S. J., D. Weinkove, L. K. MacDougall, E. Hafen, and M. D. Waterfield. 1996. The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. EMBO J. 15:6584-6594. [PMC free article] [PubMed] [Google Scholar]
- 9.McKenna, N. J., R. B. Lanz, and B. W. O'Malley. 1999. Nuclear receptor coregulators: cellular and molecular biology. Endocrinol. Rev. 20:321-344. [DOI] [PubMed] [Google Scholar]
- 10.Pandak, W. M., D. M. Heuman, K. Redford, R. T. Stravitz, J. Y. Chiang, P. B. Hylemon, and Z. R. Vlahcevic. 1997. Hormonal regulation of cholesterol 7 alpha-hydroxylase specific activity, mRNA levels, and transcriptional activity in vivo in the rat. J. Lipid Res. 38:2483-2491. [PubMed] [Google Scholar]
- 11.Parrilla, R., A. J. Mixson, J. A. McPherson, J. H. McClaskey, and B. D. Weintraub. 1991. Characterization of seven novel mutations of the c-erbA betagene in unrelated kindreds with generalized thyroid hormone resistance. Evidence for two “hot spot” regions of the ligand binding domain. J. Clin. Investig. 88:2123-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel, S., P. A. Lochhead, G. Rena, S. Fumagalli, M. Pende, S. C. Kozma, G. Thomas, and C. Sutherland. 2002. Insulin regulation of insulin-like growth factor-binding protein-1 gene expression is dependent on the mammalian target of rapamycin, but independent of ribosomal S6 kinase activity. J. Biol. Chem. 277:9889-9895. [DOI] [PubMed] [Google Scholar]
- 13.Refetoff, S., R. E. Weiss, and S. J. Usala. 1993. The syndromes of resistance to thyroid hormone. Endocrinol. Rev. 14:348-399. [DOI] [PubMed] [Google Scholar]
- 14.Smith, C. L., and B. W. O'Malley. 2004. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocrinol. Rev. 25:45-71. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki, H., and S. Y. Cheng. 2003. Compensatory role of thyroid hormone receptor (TR) α1 in resistance to thyroid hormone: study in mice with a targeted mutation in the TR β gene and deficient in TRα1. Mol. Endocrinol. 17:1647-1655. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki, H., X. Y. Zhang, D. Forrest, M. C. Willingham, and S. Y. Cheng. 2003. Marked potentiation of the dominant-negative action of a mutant thyroid hormone receptor beta in mice by the ablation of one wild-type beta allele. Mol. Endocrinol. 17:895-907. [DOI] [PubMed] [Google Scholar]
- 17.Torres-Arzayus, M. I., J. F. De Mora, J. Yuan, F. Vazquez, R. Bronson, M. Rue, W. R. Sellers, and M. Brown. 2004. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell 6:263-274. [DOI] [PubMed] [Google Scholar]
- 18.Wang, Z., D. W. Rose, O. Hermanson, F. Liu, T. Herman, W. Wu, D. Szeto, A. Gleiberman, A. Krones, K. Pratt, R. Rosenfeld, C. K. Glass, and M. G. Rosenfeld. 2000. Regulation of somatic growth by the p160 coactivator p/CIP. Proc. Natl. Acad. Sci. USA 97:13549-13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss, R. E., and S. Refetoff. 2000. Resistance to thyroid hormone. Rev. Endocrinol. Metab. Disord. 1:97-108. [DOI] [PubMed] [Google Scholar]
- 20.Williams, G. R. 2000. Cloning and characterization of two novel thyroid hormone receptor beta isoforms. Mol. Cell. Biol. 20:8329-8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu, J., L. Liao, G. Ning, H. Yoshida-Komiya, C. Deng, and B. W. O'Malley. 2000. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc. Natl. Acad. Sci. USA 97:6379-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu, J., Y. Qiu, F. J. DeMayo, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1998. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 279:1922-1925. [DOI] [PubMed] [Google Scholar]
- 23.Yen, P. M. 2001. Physiological and molecular basis of thyroid hormone action. Physiol. Rev. 81:1097-1102. [DOI] [PubMed] [Google Scholar]
- 24.Yen, P. M. 2003. Molecular basis of resistance to thyroid hormone. Trends Endocrinol. Metab. 14:327-333. [DOI] [PubMed] [Google Scholar]
- 25.Ying, H., H. Suzuki, H. Furumoto, R. Walker, P. Meltzer, M. C. Willingham, and S. Y. Cheng. 2003. Alterations in genomic profiles during tumor progression in a mouse model of follicular thyroid carcinoma. Carcinogenesis 24:1467-1479. [DOI] [PubMed] [Google Scholar]
- 26.Zhou, G., Y. Hashimoto, I. Kwak, S. Y. Tsai, and M. J. Tsai. 2003. Role of the steroid receptor coactivator SRC-3 in cell growth. Mol. Cell. Biol. 23:7742-7755. [DOI] [PMC free article] [PubMed] [Google Scholar]