Abstract
Granzyme B (GrB) is a key effector of cytotoxic lymphocyte-mediated cell death. It is delivered to target cells bound to the proteoglycan serglycin, but how it crosses the plasma membrane and accesses substrates in the cytoplasm is poorly understood. Here we identify two cationic sequences on GrB that facilitate its binding and uptake. Mutation of cationic sequence 1 (cs1) prevents accumulation of GrB in a distinctive intracellular compartment and reduces cytotoxicity 20-fold. Mutation of cs2 reduces accumulation in this intracellular compartment and cytotoxicity two- to threefold. We also show that GrB-mediated cytotoxicity is abrogated by heparin and that target cells deficient in cell surface sulfate or glycosaminoglycans resist GrB. However, heparin does not completely prevent GrB internalization and chondroitin 4-sulfate does not inhibit cytotoxicity, suggesting that glycosaminoglycans are not essential GrB receptors. We propose that GrB enters cells by nonselective adsorptive pinocytosis, exchanging from chondroitin sulfate on serglycin to anionic components of the cell surface. In this electrostatic “exchange-adsorption” model, cs1 and cs2 participate in binding of GrB to the cell surface, thereby promoting its uptake and eventual release into the cytoplasm.
Antiviral and antitumor immunity depends on the recognition, engagement, and destruction of infected or malignant cells by cytotoxic lymphocytes (CLs). Killing of abnormal cells by CLs involves activation of target cell death receptor pathways or the release of CL cytotoxins into the target cell cytoplasm (reviewed in references 2 and 40). Among the cytotoxins are the serine proteases, granzyme A (GrA), and granzyme B (GrB). These enter the target cell cytoplasm through a process that is dependent on the pore-forming protein, perforin (50). Once in the cytoplasm, granzyme A causes caspase-independent death and GrB causes caspase-dependent death (reviewed in references 27 and 52).
Granzymes are stored in secretory lysosomes (granules) bound to the chondroitin proteoglycan, serglycin (reviewed in reference 36). GrB-serglycin complexes are secreted from CLs (31), and postsecretion expression of GrB cytotoxicity involves uptake by the target cell, perforin-mediated release into the cytoplasm, and the induction of apoptosis via degradation of specific substrates such as Bid or procaspase 3 (32, 47). Serglycin is apparently not required for GrB trafficking or function, because uncomplexed GrB is efficiently internalized by target cells (13, 35, 37), and there is no difference between free or complexed GrB in activity or cytotoxicity (14).
Although perforin was originally proposed to provide a channel through the plasma membrane for granzymes (reviewed in references 6 and 50), it is now thought that granzymes are first internalized via receptor-mediated endocytosis into an undefined intracellular compartment (13). Simultaneous or subsequent exposure of the cell to perforin releases the protease into the cytoplasm from this compartment. The first GrB receptor identified on target cells was the cation-independent mannose-6-phosphate receptor (M6PR) (38), which internalizes ligands via clathrin-dependent endocytosis (54). This is consistent with the binding of nascent GrB to the M6PR during trafficking to CL granules (16) and the ability of the M6PR to retrieve mannose-6-phosphorylated lysosomal proteins from the cell surface. However, other receptors and routes of GrB uptake must exist, because target cells lacking M6PR remain sensitive to GrB killing (10, 53), and GrB internalization continues in the absence of the key endocytic pathway component, dynamin (53, 54). Recent evidence suggests that electrostatic interactions between GrB and cell surface glycosaminoglycans (GAGs) can contribute to uptake (37, 41).
The nature of other receptors and pathways remains unclear, and regions on GrB that bind receptors or the cell surface have not been identified. GrB uptake has also been proposed to occur by binding to the serglycin receptor CD44 (36) or via fluid-phase endocytosis. Fluid-phase endocytosis of GrB is evident when dynamin-dependent endocytosis is blocked (53, 54), but it is not known whether this is a parallel or compensatory mechanism. The perforin-sensitive compartment from which GrB exits the endocytic pathway, and the mechanism of its release, have yet to be elucidated.
Here we identify two heparin-binding cationic sequences on GrB that play a role in its perforin-mediated cytotoxic function. Mutation of either of these sites suppresses GrB cytotoxicity by hampering its uptake into target cells without affecting its proteolytic activity or proapoptotic potential. Analysis of the binding and trafficking of wild-type (wt) and mutated GrB shows that uptake may involve—but does not require—cell surface glycosaminoglycans and is likely to occur via release from serglycin followed by adsorptive pinocytosis.
MATERIALS AND METHODS
Cells and antibodies.
Human Jurkat and HL60 cells were maintained in RPMI 1640 medium containing 2 mM glutamine, 1 mM pyruvate, 55 μM mercaptoethanol, and 10% heat-inactivated fetal calf serum (GIBCO). Chinese Hamster Ovary (CHO) lines K1, pgsA-745 (11), and pgsD-677 (26) were obtained from the American Type Culture Collection and maintained in F12K (Kaighn's modification) medium (GIBCO) containing 2 mM glutamine and 10% heat-inactivated fetal calf serum. The low-density lipoprotein receptor-like protein (LRP)-deficient CHO line 13-5-1 (12) was obtained from S. Leppla (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD). The anti-GrB monoclonal antibody 2C5 has been described previously (49).
Proteins.
Recombinant streptolysin O (SLO) (56) was a generous gift of S. Bhakdi (Institut fur Medizinische Mikrobiologie und Hygiene, Johannes-Gutenberg-Universitat Mainz, Mainz, Germany). Recombinant human GrB and mouse GrA were produced from Pichia pastoris following strategies described in references 9 and 44. Recombinant mouse perforin was produced according to reference 28. Analysis of the mannose 6-phosphate (man 6-P) content of GrB was carried out by GlycoSolutions Corporation (Worcester, MA) according to reference 58. Conjugation of granzymes to fluorescein isothiocyanate (FITC) (Sigma) was carried out according to Hermanson (19). Labeling conditions were empirically determined to yield an A498/A280 (F/P) ratio of 0.3 to 0.7. Granzymes labeled to this extent showed limited degradation in catalytic activity. At least two batches of each protein were independently labeled and used in these studies.
Glycosaminoglycans.
Chondroitin sulfate A (bovine chondroitin 4-sulfate [CS]); chondroitin sulfate B (porcine dermatan sulfate); chondroitin sulfate C (shark chondroitin 6-sulfate); heparan sulfate (HaS) (from bovine intestinal mucosa; molecular weight [MW] = ∼7,500); and heparin (from porcine intestinal mucosa; MW = ∼15,000) were obtained from Sigma.
Heparin-Sepharose pull-down assays.
“Bound” samples consisted of 200 ng of GrB (∼20 nM) incubated in 400 μl phosphate-buffered saline (PBS) containing 30 μl of 50% (vol/vol) heparin-Sepharose (Amersham) for 1 h at ambient temperature. To monitor loss of beads during subsequent steps, 10 μl of a separate aliquot of beads bound to human antithrombin was added to each tube immediately before washing. Beads were collected and washed three times in PBS. Bound material was removed by boiling in 50 μl sample buffer (62.5 mM Tris [pH 6.8], 2% sodium dodecyl sulfate [SDS], 10% glycerol, 100 mM dithiothreitol, 0.1% bromophenol blue) and analyzed by 12% SDS-polyacrylamide gel electrophoresis (PAGE) followed by immunoblotting with monoclonal antibody 2C5 and a monoclonal antibody against human antithrombin. “Prebound” samples consisted of antithrombin tracer beads and 200 ng GrB, mixed in 50 μl sample buffer.
Granzyme activity and cytotoxicity assays.
The activity of GrB and mutants was assessed using the peptide substrate Ac-IETD-pNA (Calbiochem) or Abz-IEPDSSMESK-dnp (46), as previously described (46). A unit of GrB activity is defined as the mass of granzyme required to cause an increase of 0.001 A405/min in the hydrolysis of Ac-IETD-pNA (50 μM) in a 200 μl reaction mixture. Specific activity of granzyme preparations ranged from 20 to 40 U/μg.
Cell killing assays using purified, recombinant perforin and GrB were carried out as described previously (45). Death was assessed by measuring loss of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction or by visually scoring viable cells via trypan blue exclusion. There was excellent concordance between the two assays.
EC50 is defined as the amount of GrB (in units/milliliter) required to kill 50% of the cells. Data were fitted to a sigmoidal dose response curve (variable slope) using GraphPad Prism version 4.0. The perforin was titrated every time a cytotoxicity assay was performed. The optimal amount of perforin was determined empirically and defined as that required to lyse 10 to 15% of target cells, independently of GrB. Validation experiments showed that a 1.5-fold dilution of this amount of perforin did not significantly alter the EC50 of GrB in the cytotoxicity assay, whereas a twofold dilution of this dose of perforin markedly increased the EC50, indicating that perforin had become limiting.
In SLO-mediated cytotoxicity assays, a given amount of GrB was added at each point to 4 × 105 HL60 cells in 100 μl Hybridoma-SFM (GIBCO) containing an empirically determined concentration of SLO. The optimal concentration of SLO caused lysis of 10 to 20% of cells and transiently permeabilized 60 to 80% of cells in Hybridoma-SFM (as determined by fluorescence-activated cell sorting [FACS] analysis of propidium iodide uptake). The rest of the experiment was performed as described previously (45).
Binding and localization analysis.
Sublytic perforin in HE (150 mM NaCl, 10 mM HEPES [pH 7.4], 0.4% fraction V bovine serum albumin, 5 mM CaCl2) was added to an equal volume of Hank's Balanced Salt Solution (HBSS) containing 10 mM HEPES (pH 7.4), 0.4% fraction V bovine serum albumin, 600 nM granzyme, and 2 × 105 cells. After 15 min at 4°C or 37°C, cells were washed twice with ice-cold HBSS and resuspended in 0.5 ml HBSS. Cells were analyzed by FACS using a Becton-Dickinson FACscan instrument. The remaining cells were attached to coverslips by use of Cell-Tak (Becton-Dickinson) and fixed in 4% formaldehyde in PBS for 20 min at 4°C. Cells were washed in PBS and mounted in PERMAFLUOR (Beckman-Coulter).
Confocal laser scanning microscopy (CLSM) was carried out using a Leica TCS NT upright microscope and Leica TCS software with a 100× oil immersion lens (numerical aperture, 1.4). Where z stacks were acquired the section step was approximately 1 μm. Differential interference contrast images were acquired simultaneously, and images were collected using constant settings. The images presented did not require additional correction for brightness or contrast.
RESULTS
GrB-mediated cytotoxicity is not dependent on mannose 6-phosphate.
It has been demonstrated by many groups that the CL-mediated killing of target cells via the perforin/granzyme pathway can be mimicked in vitro by incubating purified GrB and perforin with cultured cells. At sublytic concentrations of perforin, GrB is internalized and is necessary and sufficient to induce target cell apoptosis. (High concentrations of perforin alone induce plasma membrane permeabilization and necrosis.) This system has been used to implicate the M6PR in GrB uptake and killing (38, 53) and to investigate the function of perforin in GrB release (13).
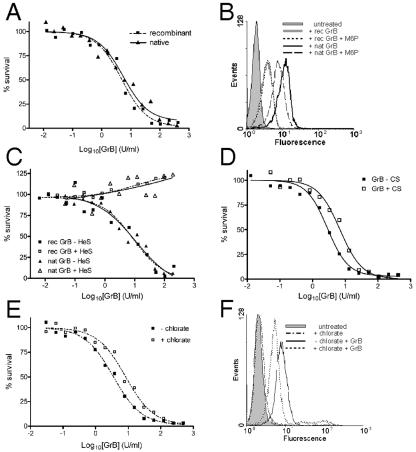
Since recombinant protein produced in Pichia pastoris is not mannose 6 phosphorylated (3) and yeast does not have an M6PR (reviewed in reference 8), it can be inferred that our recombinant GrB does not carry mannose 6-phosphate (man 6-P) and will not bind the M6PR. Indeed, recombinant GrB is essentially indistinguishable from native GrB in perforin-mediated killing of human Jurkat cells, as indicated by superimposable dose response curves and equivalent EC50 values (Fig. 1A and C) (45). Thus, it appears that binding to the M6PR is not required for granzyme B-mediated cytotoxicity.
FIG. 1.
Influence of man 6-P and electrostatic interactions on GrB uptake and cytotoxicity. (A) Native GrB and recombinant GrB kill target cells equally well. Enzymatic activities of the GrB preparations were matched by IETD-pNA cleavage assay. In the in vitro killing assay, Jurkat cells were incubated for 1 h at 37°C with a constant amount of perforin (empirically determined to lyse 10 to 15% of the cells) and increasing concentrations of GrB. Cells were washed and returned to complete medium, and survival was assessed 16 h later by MTT assay. The EC50 for recombinant GrB was 4.8 U/ml; for native GrB it was 5.4 U/ml. (B) Soluble man 6-P reduces uptake of native GrB but not recombinant GrB by Jurkat cells. Cells were incubated in 100 nM of FITC-GrB (recombinant) or 50 nM Alexa 488-GrB (native) in the absence or presence of 100 μM man 6-P at 37°C for 1 h and then washed and analyzed by FACS. (C) Heparin abrogates GrB-mediated cytotoxicity. GrB was preincubated at 37°C for 30 min with a 50-fold molar excess of heparin and then used in the in vitro killing assay. Heparin was maintained at at least a fivefold excess over GrB levels during exposure to cells. (D) Chondroitin 4-sulfate (CS) does not abrogate GrB-mediated cytotoxicity. Recombinant GrB was preincubated at 37°C for 30 min with a 50-fold molar excess of CS and then used in the in vitro killing assay. CS was maintained at at least a fivefold excess over GrB levels during exposure to cells. (E) Sodium chlorate treatment reduces killing of Jurkat cells by recombinant GrB. Target cells were cultured in the presence of 75 mM sodium chlorate for 48 h prior to and during the in vitro killing assay. The EC50 without chlorate was 3.4 U/ml; with chlorate it was 8.2 U/ml. (F) Chlorate treatment reduces uptake of FITC-GrB (recombinant) by Jurkat cells. Cells were cultured in the presence of 75 mM sodium chlorate for 48 h prior to GrB binding for 1 h at 37°C, washing, and FACS.
To investigate this further, we assessed the uptake of fluorescently labeled native and recombinant GrB by Jurkat cells in the presence and absence of mannose 6-phosphate (man 6-P), which antagonizes binding of ligands to the M6PR (22). Previous work has demonstrated that in the absence of perforin fluorescently labeled GrB accumulates in an intracellular compartment and can be detected by microscopy or FACS analysis (13, 35, 42, 53). As shown in Fig. 1B, we could demonstrate accumulation of native or recombinant GrB in Jurkat cells. (In these experiments recombinant GrB was labeled with FITC whereas native GrB was labeled with Alexa 488, which gives a brighter fluorescent signal.) We found that man 6-P reduced the uptake of native GrB without abrogating it but had no effect on the uptake of recombinant GrB. This indicates that although a proportion of native GrB can interact with the M6PR, recombinant GrB accumulates and kills independently of the M6PR.
To extend these observations we analyzed the man 6-P content of native GrB according to Zhou et al. (58) and found 0.2 mol of man 6-P per mole of protein (data not shown). A man 6-P peak was not evident in the recombinant material. Given that GrB has two Asn-linked oligosaccharide side chains (39), this indicates that only 10 to 20% of native GrB is available for binding to the M6PR.
Taken together, these results suggest that a proportion of native GrB may internalize via the M6PR, but this mode of entry is not essential for cytotoxicity. Since recombinant GrB cannot bind the M6PR, it provides a convenient tool to study M6PR-independent uptake pathways involved in GrB cytotoxicity.
GrB cytotoxicity is completely abrogated by heparin.
GrB is a cationic protein that binds negatively charged molecules like DNA and the glycosaminoglycan (GAG) heparin (51). It therefore has the potential to interact nonselectively with the negatively charged cell surface and be internalized by adsorptive pinocytosis or to specifically bind negatively charged protein or proteoglycan and internalize by receptor-mediated pinocytosis (7). Potential receptors for GrB internalization include CD44 or LRP. CD44 is involved in internalization of GAGs and GAG-associated protein and has been suggested as a receptor for serglycin-complexed GrB (36). LRP is a scavenger involved in the uptake of a wide range of ligands, including growth factors, lipoprotein, proteases, protease-serpin complexes, and microbial toxins (reviewed in reference 43). Cell surface heparan sulfate proteoglycans (HSPG) are implicated in internalization of ligands via LRP (57). Thus, if electrostatic interactions or HSPG are involved in GrB uptake, GAGs such as heparin should block GrB-mediated cytotoxicity by neutralizing its positive charge or by preventing binding to cell surface GAGs.
Jurkat cells were therefore exposed to perforin and increasing concentrations of native or recombinant GrB, in the presence or absence of heparin (MW = 15,000) (Fig. 1C). GrB killed Jurkat cells in a dose-responsive fashion, with an EC50 of 5 U/ml. However, preincubation with heparin totally inhibited GrB-mediated cytotoxicity (Fig. 1C). Preincubation with heparin did not inhibit the ability of perforin to permeabilize cells (data not shown), suggesting that the impact of heparin is on GrB rather than perforin. Since cytotoxicity depends on the catalytic activity of GrB, we also assessed whether heparin inhibits its ability to hydrolyze a standard peptide substrate (IETD-pNA). Heparin suppressed hydrolysis by a maximum of 30% but did not abrogate it, indicating that the lack of GrB cytotoxicity in heparin is not caused by inhibition of catalytic activity (data not shown).
Because GrB is delivered to target cells in vivo bound to chondroitin 4-sulfate on serglycin, we tested whether preincubation with free chondroitin 4-sulfate influences cytotoxicity. Killing was only slightly reduced in the presence of chondroitin 4-sulfate (Fig. 1D) compared to the total lack of killing in the presence of heparin (Fig. 1C). This indicates that serglycin does not interfere with GrB uptake.
One explanation for the inhibitory effect of heparin is that it inhibits binding of cell surface HSPG to sites on GrB. To test this idea, target cells were cultured in the presence of sodium chlorate, an inhibitor of sulfate adenylyltransferase, which reduces the sulfation of cellular protein and GAGs (1). As shown in Fig. 1E, cells treated with chlorate showed a 50% reduction in GrB-mediated killing. Binding and accumulation of FITC-GrB to cells was also reduced in the presence of chlorate (Fig. 1F). This is consistent with reports that chlorate treatment halves the number of sulfated GAGs on the cell surface of Jurkat cells (25). Taken together, the above-described results suggest that interaction with cell surface GAGs is part of the cytotoxic mechanism of GrB. The complete block by heparin and the relative insensitivity to chondroitin 4-sulfate are consistent with a mechanism involving exchange of GrB from serglycin to cell surface HSPG.
GAGs and LRP are not essential for GrB-mediated killing.
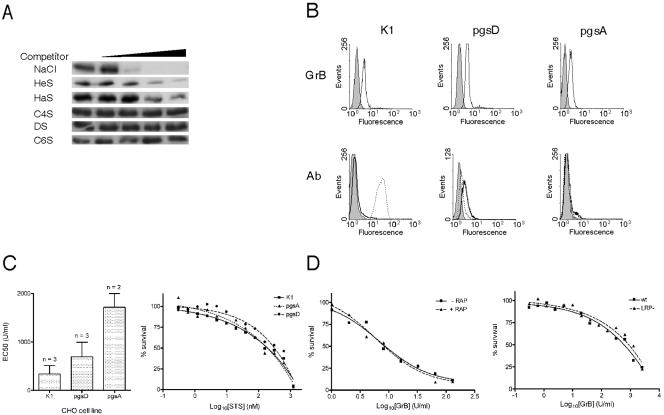
To show that GrB can bind GAGs we used a simple “pull-down” assay in which GrB is incubated with heparin-Sepharose beads. Bound GrB is pelleted with the beads, and material eluted from the beads is analyzed by immunoblotting. As shown in Fig. 2A, GrB bound efficiently to heparin and was eluted by NaCl, indicating an electrostatic interaction. Binding was inhibited by coincubation with excess soluble heparin or heparan sulfate (HaS) but not by less-anionic chondroitins. This suggests that GrB binds with greater efficiency to more anionic GAGs and supports the exchange mechanism.
FIG. 2.
Contribution of GAGs and LRP to GrB-mediated cytotoxicity. (A) GrB binds GAGs. Recombinant GrB (50 nM) was added to 0, 0.1, 0.2, 0.3, or 0.4 M NaCl or to 0, 0.1, 1, 10, or 100 μM of the indicated GAG competitor and then incubated with heparin-Sepharose beads and washed. Bound GrB was removed from the beads by boiling in SDS and assessed by SDS-PAGE and immunoblotting. HeS, heparin; HaS, heparan sulfate; C4S, chondroitin 4-sulfate; DS, dermatan sulfate; C6S, chondroitin 6-sulfate. (B) GrB uptake does not require GAGs. Upper panels: CHO GAG-deficient cell lines were incubated with 600 nM FITC-GrB (recombinant) for 1 h at 37°C, washed, and analyzed by FACS. Lower panels: FACS analysis of cell surface GAGs on the CHO cells by binding of anti-HaS antibody (dotted line) or anti-CS antibody (solid line). (C) GAG-deficient CHO lines are less sensitive than wt to GrB but are equally sensitive to staurosporine (STS). Shown are the EC50s derived from repeated GrB in vitro killing assays (left panel) and an STS dose-response experiment as assessed by MTT assay (right panel). Error bars indicate the standard deviation. (D) In vitro killing of Jurkat cells by GrB is not inhibited by the LRP chaperone, RAP (left panel). LRP-deficient CHO cells remain sensitive to GrB in the in vitro killing assay (right panel).
To determine whether interaction with cell surface GAGs contributes to GrB cytotoxicity, we assessed whether GrB can bind and kill well-characterized GAG-deficient CHO cells. The mutant CHO line pgsD-677 is deficient in N-acetylglucosaminyltransferase and glucuronosyltransferase (26) and, as shown in Fig. 2B, has no HaS but has more CS on the cell surface than the parental line CHO-K1. The mutant line pgsA-745 is deficient in xylosyltransferase and has no GAGs on the cell surface (11) (Fig. 2B). As shown in Fig. 2B, GrB accumulation levels were comparable in the three lines, suggesting that GAGs are not essential for GrB uptake.
Compared to Jurkat cells, the parental CHO cell line (K1) is intrinsically resistant to in vitro GrB and perforin-mediated killing, as indicated by higher EC50 values (Fig. 2C). Nevertheless, we were able to demonstrate that the GAG-deficient CHO cells are two- to fivefold more resistant to GrB than CHO-K1 cells (Fig. 2C). To rule out differences in apoptosis pathways arising from clonal variation, we tested the sensitivity of the lines to a different apoptosis inducer. As shown in Fig. 2C, the lines were similarly sensitive to staurosporine, which—like GrB—triggers caspase-dependent death. Thus, binding to GAGs may contribute to—but is not essential for—GrB-mediated cytotoxicity. Because cells totally deficient in GAGs still succumb to GrB, proteoglycans are unlikely to be the only GrB receptors. It is therefore likely that exchange also occurs to other anionic cell surface structures such as phospholipids, sulfated lipids, and gangliosides.
Binding to LRP is a common means of entry for a large number of proteins, including proteases (43), and binding of most ligands to LRP can be competed by its cationic chaperone receptor-associated protein (RAP) (34, 43). It is also known that HSPG act as coreceptors for the uptake of some ligands by LRP (57). Thus, GrB may enter cells by binding LRP and using HSPG as coreceptors. To test this idea, we investigated whether the LRP ligand, RAP (30), antagonizes GrB uptake or inhibits GrB-mediated killing and whether LRP-deficient CHO cells are resistant to GrB. In Jurkat cells RAP did not inhibit uptake of recombinant or native GrB (data not shown) or affect GrB-mediated killing (Fig. 2D). LRP-deficient CHO cells (12) were no more resistant to perforin and GrB than CHO-K1 cells (Fig. 2D). Thus, GrB-mediated cytotoxicity does not require LRP.
Identification of GAG-binding cationic sequences on GrB.
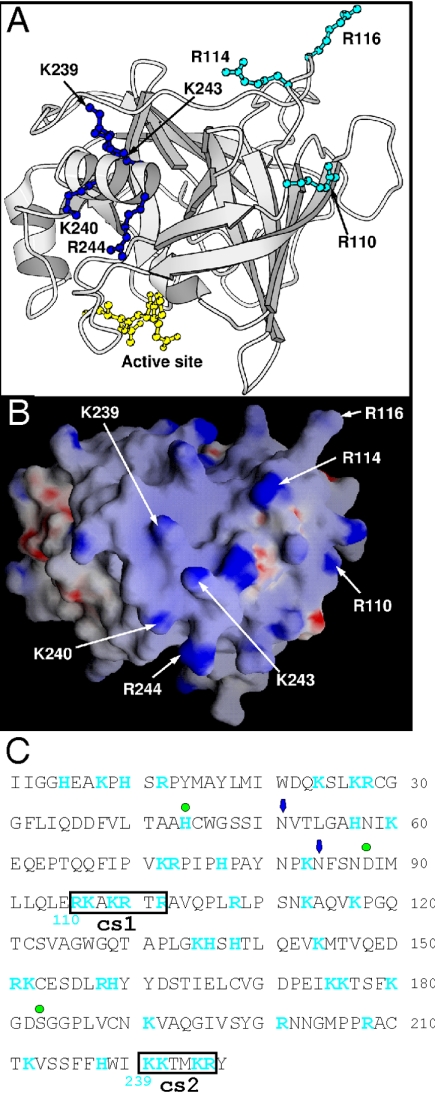
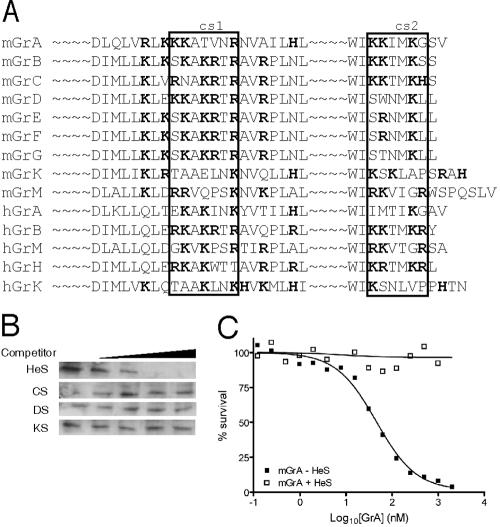
Many proteins bind GAGs, and consensus binding sites have been identified (5). Binding relies on electrostatic interaction between the negatively charged GAG and a positively charged motif in the protein that may adopt either an α-helical or β-strand conformation (XBBXBX or XBBBXXBX, where B is a basic amino acid and X is uncharged or hydrophobic) (5). As shown in Fig. 3, inspection of the GrB sequence and structure revealed a surface loop containing an exact match to the XBBXBX motif (110RKAKRTRA, where the underlined characters represent the putative heparin-binding motif) and a part of the amphipathic C-terminal helix that has paired basic residues also thought to bind GAGs (239KKTMKRY) (20). These were designated cationic sequence 1 (cs1) and cs2, respectively (Fig. 3C).
FIG. 3.
Location of two clusters of positively charged residues on human GrB. The structure of human GrB bound to a peptide inhibitor has been solved (39) (Protein Database accession number: 1IAU). (A) The residues mutated to alanine in the GrB cs mutants are labeled (numbering in 1IAU is based by convention on chymotrypsin). R110, R114, and R116 (cs1) are in cyan ball-and-stick, and K239, K240, K243, and K244 (cs2) are in blue ball-and-stick. The peptide inhibitor Ac-IEPD-CHO is in yellow ball-and-stick, and indicates the position of the catalytic site. (B) GRASP electrostatic potential surface of GrB in an orientation similar to the image shown in panel A. The positively charged residues selected for mutagenesis are labeled. (C) Sequence of mature GrB showing positively charged residues (blue) and the cationic sequences (boxed). Green dots mark the residues of the catalytic triad, and arrows mark the positions of Asn-linked carbohydrate side chains. Numbering in blue below the sequence is based on chymotrypsin.
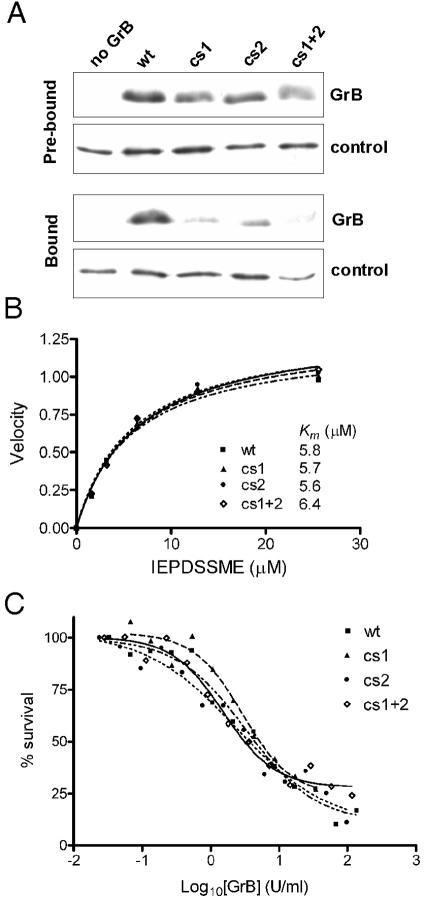
To test whether cs1 and cs2 contribute to GAG binding, we mutated them via alanine substitution (Fig. 3). In the “single” mutants, cs1 was changed to 110AKAKATAA or cs2 was changed to 239AATMAAY, where the boldface characters represent the mutated residues. The “double” mutant (cs1 plus cs2) carried the substitutions at both sites. In the pull-down assay both single mutants showed significantly impaired heparin binding compared to wt GrB, and the double mutant bound even less efficiently (Fig. 4A).
FIG. 4.
GrB cs mutants have impaired GAG binding but normal catalytic and apoptotic function. (A) Heparin-binding of cs mutants. Equal amounts of wt or mutant GrB were mixed with heparin-Sepharose beads. An irrelevant heparin-binding control protein (antithrombin) was included as a tracer to monitor loss of beads during the procedure. Samples were assessed by SDS-PAGE and immunoblotting. The Prebound panel compares the input GrBs used in the experiment, with no binding or washing steps, as assessed immediately after mixing with the tracer. The Bound panel shows the GrB remaining after binding and washing. Results are representative of five independent experiments. (B) The catalytic activity of wt and cs mutants on a quenched-fluorescence substrate Abz-IEPDSSMESK-dnp was measured as described previously (46). (C) The apoptotic function of the cs mutants was assessed using a modified in vitro killing assay, in which perforin was replaced by SLO. Enzymatic activities of the granzymes were matched via IETD-pNA cleavage assay. Jurkat cells were incubated for 1 h at 37°C with an empirically determined amount of SLO (permeabilizes 80% of cells) and increasing concentrations of GrB. Cells were washed, and survival was assessed 16 h later by MTT assay. The EC50 for wt GrB was 5 U/ml.
GrB cs mutants are catalytically active and proapoptotic.
Both cs1 and cs2 are located on the opposite side of the molecule to the catalytic pocket and are part of a larger cationic surface (Fig. 3A). This topology would minimize steric hindrance between a substrate and a GAG (or other negatively charged molecule) and would allow GrB to cleave substrates while bound to another molecule. It might also allow GrB activity to be allosterically regulated by a cofactor, as seen in other proteases such as those participating in blood coagulation.
None of the cs mutations altered the catalytic activity of GrB against a standard peptide substrate (Fig. 4B). To assess their ability to induce apoptosis via cleavage of natural cytoplasmic substrates, the mutants were incubated with cells transiently permeabilized with streptolysin O (SLO). Under the conditions used the SLO-generated plasma membrane pores do not cause lysis and are resealed by membrane repair processes (55). GrB entering the cytoplasm via these pores can directly access its proapoptotic substrates such as Bid and procaspase 3 and does not require perforin to cause death. As shown in Fig. 4C, the EC50 for GrB-mediated apoptosis in this system is approximately 5 U/ml. This is similar to the EC50 observed when purified perforin is used to deliver GrB (45) (Fig. 5), indicating that GrB can move without hindrance into the cytoplasm through the SLO pores and induce death. The EC50s obtained for all the mutants were similar to those of wt GrB, indicating that there is no impairment of their apoptotic potential (Fig. 4C).
FIG. 5.
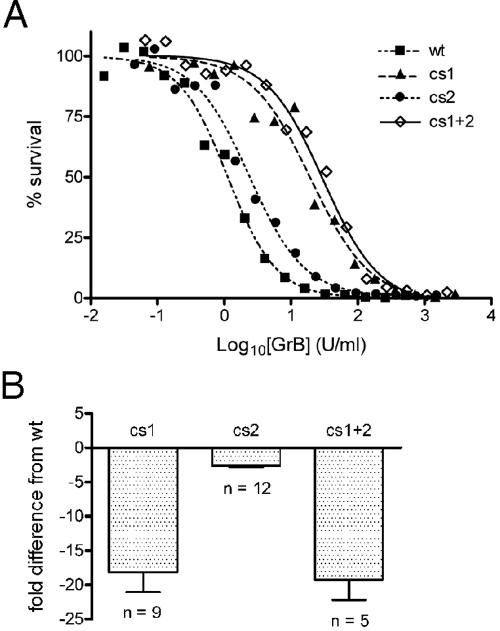
GrB cs mutants show impaired cytotoxicity in the presence of perforin. (A) Representative in vitro killing assay comparing wt and mutant GrBs. See Fig. 1A legend for details. (B) Severalfold differences in EC50s between the cs mutants and wt GrB in the in vitro killing assays. Error bars indicate the standard deviation. The EC50 for wt GrB was 3.9 ± 1.8 U/ml (n = 19).
GrB cs mutants have impaired perforin-mediated cytotoxicity.
GrB and perforin synergize to cause cell death. Perforin was originally proposed to operate like SLO and mediate GrB entry into cells through the plasma membrane, but it is now thought to release GrB from an intracellular compartment (13). To assess the impact of the cs mutations on perforin-mediated GrB function, target cells were incubated with sublytic concentrations of perforin and increasing concentrations of the different GrB cs mutants. As shown in Fig. 5, all of the cs mutants showed significantly impaired cytotoxicity, indicated by 2- to 20-fold increases in the EC50. Thus, cs1 containing the altered classical GAG-binding motif is 20-fold less cytotoxic than wt, cs2 containing the altered C-terminal helix is 2.5-fold less cytotoxic, and the double mutant is also 20-fold less cytotoxic.
Since these mutations have no impact on GrB catalytic activity or apoptotic potential, we conclude that the cationic sites play a role in uptake, intracellular trafficking, or perforin-mediated release into the cytoplasm. The absence of a multiplier effect on the EC50 of the double mutant suggests that cs1 and cs2 affect the same point in the pathway (the expected additive effect on the EC50 if this is the case would not be evident due to experimental variation). A simple explanation is that cs1 and cs2 both contribute to cell surface binding. Perturbation of either site reduces efficiency of binding and hence cytotoxicity.
Uptake and intracellular accumulation of GrB cs mutants.
To further dissect the function of the cationic sites we examined binding and accumulation of fluoresceinated (FITC) granzymes in Jurkat cells. The integrity of labeled protein and the extent of labeling was assessed by fluorography and immunoblotting (Fig. 6C). FITC-GrB and perforin were added to cells at 4°C or 37°C for 15 min, and then unbound material was removed by washing. Cells were assessed by FACS or confocal laser scanning microscopy (CLSM). Because pinocytosis is inhibited by low temperature (15, 21), we predicted binding but no accumulation of wt GrB at 4°C, contrasting with binding and accumulation at 37°C.
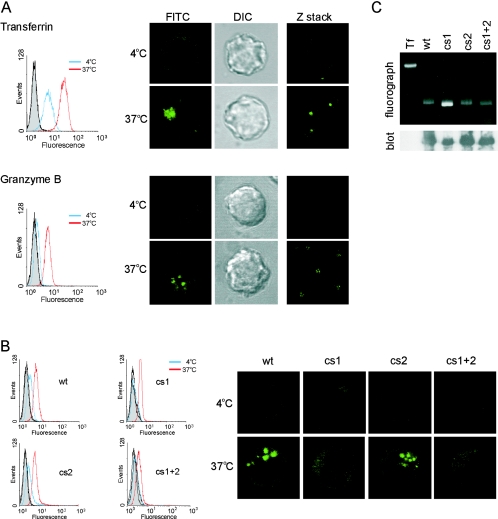
FIG. 6.
Uptake and intracellular localization of GrB and the cs mutants. Binding and accumulation of FITC-labeled transferrin (125 nM) or FITC-labeled granzymes (600 nM) in the presence of sublytic perforin. Jurkat cells were exposed to fluoresceinated protein and perforin at 4°C or 37°C for 15 min, washed, and analyzed by FACS. Autofluorescence levels of cells treated with perforin alone at 4°C (shaded histogram) or 37°C (black line) were identical. Cells from the same populations were also analyzed by CLSM. (A) Uptake and localization of wt GrB compared to transferrin. Image shows the distribution of fluoresceinated protein (FITC) with a corresponding differential interference contrast image. Shown are single optical sections from single cells (left and center panels) or z-stacks of multiple cells (right panels). (B) Uptake and localization of cs mutants at 4°C and 37°C was analyzed as described above. Images show single optical sections. (C) A fluorograph and an immunoblot probed with anti-GrB monoclonal 2C5 demonstrate integrity and equivalent FITC labeling of wt GrB and the cs mutants. The fluorograph was taken prior to transfer to a membrane for immunoblotting (Tf, transferrin).
We used well-characterized FITC-transferrin as a control in these experiments, expecting to see receptor-mediated cell surface binding but no intracellular accumulation at 4°C, and uptake and accumulation in endosomes at 37°C (29). As shown in Fig. 6A, transferrin was evident at the cell surface but not inside cells at 4°C and was mostly inside cells after 15 min at 37°C.
As shown in Fig. 6A and B, FACS analysis indicated very low binding of wt GrB to cells at 4°C. A pronounced shift was observed at 37°C, indicating that intracellular accumulation of wt GrB had occurred. To confirm that GrB had internalized, we analyzed cells by CLSM. At 4°C we observed very faint surface fluorescence, as well as a diffuse pattern of intracellular (cytoplasmic) staining which was not evident with transferrin. This may represent GrB that has accumulated in small endocytic vesicles via fluid-phase uptake, which is known to be reduced but not abrogated at 4°C (15). It is less consistent with binding to a sole, stationary, cell surface receptor, which would be indicated by staining of the plasma membrane (as is the case with transferrin). It is also unlikely to represent soluble GrB that has moved directly into the cell via a perforin channel or other nonpinocytic mechanism, since GrB released into the cytosol rapidly accumulates in the nucleus (51), and no nuclear GrB was evident. Furthermore, the same pattern was observed in the absence of perforin (data not shown).
At 37°C we observed wt GrB at the periphery with some diffuse cytoplasmic staining, but most of the material was localized in a large subcellular compartment(s) (Fig. 6A and B), which resembles structures described previously (13, 35, 53). For working purposes, we termed these structures “internalized GrB depots” (IGD). Similar patterns of GrB uptake and localization at 37°C were also observed using native GrB (Fig. 1 and data not shown). Identical results were obtained in the absence of perforin, indicating that it does not enhance uptake or distribution of wt GrB in this system (data not shown).
Next we examined the binding and accumulation of the cs mutants. As shown in Fig. 6B, the cs1 mutant showed little or no binding to cells at 4°C. At 37°C the mutant accumulated in cells 60% less efficiently than wt GrB. It appeared solely in the diffuse cytoplasmic pattern and showed no evidence of accumulation in IGD. This suggests that the classical GAG-binding motif mutated in cs1 is involved early in a pathway that directs GrB to IGD.
The cs2 mutant showed FACS and CLSM profiles similar to that of wt GrB, although qualitatively there appeared to be a decrease in the size of IGD within the cells (Fig. 7). This indicates that the GAG-binding C-terminal helix also contributes to trafficking of GrB to IGD but is less important than the classical GAG-binding motif. Consistent with this, the double mutant showed FACS and CLSM profiles identical to the cs1 mutant profiles.
FIG. 7.
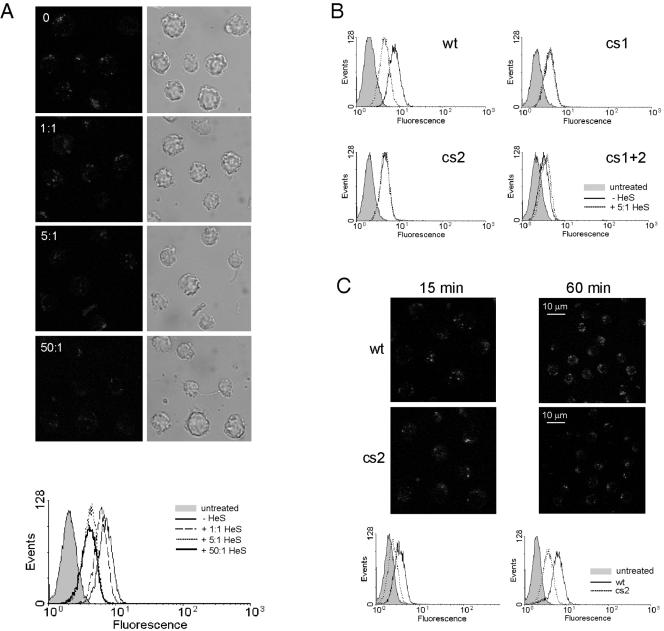
Effect of heparin on uptake and localization of granzymes, and the role of cs2. Jurkat cells were exposed to perforin and FITC-GrB at 37°C for 15 min or 60 min (C), washed, and analyzed by FACS and/or CLSM. (A) Heparin reduces but does not prevent GrB accumulation in intracellular vesicles. Images show z-stacks of multiple cells: the corresponding FACs profile is shown beneath. (B) A fivefold excess of heparin reduces GrB accumulation in Jurkat cells but does not further reduce accumulation of cs mutants. (C) cs2 shows reduced uptake and vesicular accumulation. Shown are z stacks of multiple cells.
Taken together, these results clearly indicate that the cationic sites on GrB are involved in uptake and trafficking of GrB to IGD in target cells. They also indicate that there is an uptake pathway that delivers GrB into cells independently of the cs sites, without accumulation in IGD. However, they do not explain the complete loss of cytotoxicity in the presence of heparin.
Influence of heparin on uptake and intracellular accumulation of GrB.
As indicated above (Fig. 1C), heparin completely blocks GrB-mediated cytotoxicity without affecting perforin. To determine whether GrB binding or accumulation is affected by heparin we incubated cells with GrB in the presence of increasing amounts of heparin. As shown in Fig. 7A and Table 1, even at 50-fold excess, heparin reduced but did not completely inhibit binding or accumulation of GrB in IGD. At the level used in cytotoxicity assays (5- to 50-fold excess), IGD were clearly evident. Thus, the heparin block of cytotoxicity cannot be explained solely by inhibition of GrB uptake or accumulation in IGD: it must also affect another step in the cytotoxic pathway.
TABLE 1.
Properties of GrB mutantsa
| Granzyme | Heparin bindingb | Activityc | Cytotoxicityd
|
Binding/accumulatione
|
Intracellular localizationf
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| SLO | Pfp | IGD
|
Cytoplasm
|
|||||||
| − HeS | + HeS | − HeS | + HeS | − HeS | + HeS | |||||
| wt | 1.00 | 1.0 | 1.0 | 1.00 | 1.00 | 0.38 | +++ | + | + | + |
| cs1 | 0.03 | 1.0 | 1.0 | 0.05 | 0.38 | 0.35 | − | − | + | + |
| cs2 | 0.1 | 1.0 | 1.0 | 0.5 | 0.63 | 0.57 | ++ | +/− | + | + |
| cs1+2 | 0.03 | 1.0 | 1.0 | 0.02 | 0.22 | NDg | − | − | + | + |
Results are normalized to wt GrB. Binding and localization of FITC-GrB was assessed in the presence of sublytic perforin for 15 min.
Assessed by heparin-Sepharose pull-down assay and densitometry (Fig. 4).
Enzymatic activity measured by hydrolysis of a synthetic peptide substrate (Fig. 4).
Target cell death measured by MTT assay following granzyme delivery by streptolysin O (SLO) (Fig. 4) or perforin (Pfp) (Fig. 5).
Binding and accumulation of FITC-GrB was assessed by FACS analysis, in the absence (−) or presence (+) of heparin (HeS). Averaged mean fluorescence intensities (MFI) were normalized to the averaged MFI for wt GrB without heparin. The MFI for wt GrB was 12.5 ± 1.9 (n = 6).
Intracellular localization of FITC-GrB was assessed by CLSM, in the absence or presence of heparin. For IGD: +++, multiple, large IGD in most cells; ++, fewer, smaller IGD in most cells; +, fewer, less intense IGD in many cells; +/−, rare, less intense IGD in occasional cells; −, no detectable IGD. For cytoplasm: +, diffuse cytoplasmic staining in most cells.
ND, not done.
Unlike wt GrB, uptake and accumulation of the cs mutants was essentially unaffected by the presence of heparin (Fig. 7B and Table 1). This suggests that heparin interferes with GrB uptake by binding to these sites, and this idea is further supported by the observation that heparin reduces wt GrB accumulation to mutant levels. It also suggests that cs1 and cs2 cooperate in heparin binding and GrB uptake, because if they worked independently heparin should further reduce uptake if only one site is mutated.
Mutation of cs2 influences GrB vesicular accumulation.
Mutation of cs2 yielded a reproducible 2.5-fold reduction in cytotoxicity (Fig. 5), but qualitatively its uptake and accumulation pattern resembled wt GrB results. One difference between the cytotoxicity and uptake assays is the time of exposure of cells to GrB (1 h and 15 min, respectively). Repeating the cytotoxicity assays with a 15-min exposure to cs2 resulted in a further reduction in apparent activity: it now showed a sixfold decrease in EC50 compared to wt GrB (data not shown). Comparison of the intracellular accumulation results obtained with cs2 and wt GrB at 15 min and 1 h showed fewer and smaller IGD in cells exposed to cs2 (Fig. 7C). Quantitation of the FACS data showed that cells accumulated about three times more wt GrB at both time points. This confirms that mutation of cs2 also perturbs accumulation of GrB in IGD.
Cationic sequences in other granzymes.
CLs produce other cytotoxic granzymes that cause perforin-mediated cell death (40). To assess whether cationic sequences are likely to play a role in the cytotoxic function of other granzymes, we examined the corresponding regions in all human and mouse granzymes. We noted a high density of cationic residues in most granzymes in both regions (Fig. 8A), suggesting conservation of heparin-binding ability. One exception is granzyme K, which in both human and mouse has a lower density of cationic residues in these regions.
FIG. 8.
Electrostatic interactions may contribute to the function of other granzymes. (A) Regions homologous to cs1 and cs2 (boxed) in other human and mouse granzymes. Positively charged amino acids are indicated in bold. (B) GrA binds GAGs. A 50 nM concentration of recombinant mouse GrA was used as described in the legend to Fig. 2A. (C) Heparin abrogates GrA and perforin-mediated killing of Jurkat cells. Recombinant GrA was preincubated at 37°C for 30 min with a 50-fold molar excess of heparin and then used in the in vitro killing assay (see Fig. 1A for details.) Heparin was maintained at at least a fivefold excess over GrA levels during exposure to cells.
To test whether cytotoxicity is heparin sensitive in another granzyme, we tested whether GrA can bind heparin and whether heparin abrogates GrA-mediated cell death. As shown in Fig. 8B, GrA binds heparin more tightly than other GAGs, as seen with GrB (Fig. 3). Furthermore, heparin totally abrogates perforin-dependent GrA-mediated killing (Fig. 8C). These results indicate that the uptake mechanisms studied here for GrB are likely to be similar for other granzymes.
DISCUSSION
Our study provides the first direct evidence for regions on GrB outside the active site that are important for cytotoxicity. These are not involved in modulating cleavage of the natural intracellular substrates of GrB but rather are sites that facilitate its uptake and trafficking in the target cell.
If GrB is considered as a roughly spherical protein, one hemisphere contains the active site and has an essentially neutral surface, while the other hemisphere has a highly cationic surface. Cs1 and cs2 form part of the cationic surface, and mutation of either sequence affects accumulation of GrB in the target cell without affecting cleavage of natural or artificial substrates. Mutation of cs1 results in a form of GrB that shows significantly reduced binding to heparin; reduced uptake into target cells; cytoplasmic distribution with no apparent accumulation in IGD; and significantly impaired cytotoxicity. Mutation of cs2 results in significantly reduced binding to heparin; slightly reduced uptake into target cells; cytoplasmic distribution with reduced accumulation in IGD; and slightly impaired cytotoxicity (see Table 1 for a summary of the properties of the cs mutants).
Because cs1 and cs2 independently have the features of characterized GAG-binding sites and since they behave differently, it is possible that each site has a discrete role in GrB uptake. However, at present we cannot rule out the possibility that they combine to form a single patch which interacts with the cell surface and that mutation of either site compromises the patch. The simplest interpretation of the data presented in this study is that both cs1 and cs2 contribute to a charge-dependent interaction with the cell surface that directs GrB into IGD. The failure of cs1 to accumulate in IGD indicates that either this site is more important for cell surface binding or it has an additional role in GrB trafficking to IGD.
Despite their significantly compromised cytotoxic potential and failure to accumulate in IGD, cs1 and the double mutant still internalize relatively efficiently at 37°C. They are localized in a diffuse “cytoplasmic” compartment, which is also observed with wt GrB and presumably reflects elements of a different (second) pinocytic pathway, as suggested previously (53). It is possible that GrB is contained within endocytic vesicles belonging to this second pathway that are too small be resolved by light microscopy. Release from this pathway by perforin must occur, since mutant GrBs that internalize this way can still kill, but their significantly compromised cytotoxicity suggests that such release is very inefficient.
Receptors for GrB.
Given the recent observation that GrB is released from CLs complexed to the proteoglycan serglycin (31), key issues that arise are the nature of the structure(s) or receptor on the target cell surface to which the GrB-serglycin complex binds and whether the complex itself is internalized. Ideally a selective GrB receptor would be ubiquitously expressed so that potentially every cell is sensitive to CLs and would be essential for viability so that it cannot be blocked or down-regulated in virus-infected or tumor cells. A candidate for such a receptor is the cation-independent M6PR. Through study of M6PR-null mouse cells engineered to overexpress the human M6PR, an early suggestion was that GrB enters cells primarily via the M6PR (33). Subsequent work using the same cells demonstrated a second uptake pathway that functions independently of M6PR internalization (53). However, more-recent findings indicate that M6PR-deficient cells remain susceptible to CLs and to purified GrB and that internalized GrB does not colocalize with internalized M6PR (10, 53). Thus, M6PR does not appear to be essential for GrB-mediated killing. Our results are consistent with this conclusion, because recombinant GrB from Pichia pastoris—which is not mannose 6-phosphorylated—kills cells equally as well as mannose 6-phosphorylated native GrB. Furthermore, we have demonstrated that no more than 20% of native GrB is mannose 6-phosphorylated and would be capable of binding the M6PR. These findings strongly support the existence of M6PR-independent routes of GrB internalization, perhaps involving other receptors. The existence of multiple receptors would ensure that uptake of GrB is not easily blocked by the target cell.
Several other receptors have been suggested for GrB. These include CD44, which is known to bind serglycin (48), and cell surface Hsp70, which binds GrB and mediates perforin-independent death (17). In this study we have also considered LRP, because of its broad distribution, the existence of functionally related family members, and its known capacity to internalize proteases. However, as demonstrated using LRP-deficient cells and the specific competitor, RAP, GrB uptake does not involve LRP.
GrB uptake by exchange and adsorption.
An alternate, emerging model for GrB uptake is that selective GrB receptors are not required and that the positively charged GrB adsorbs to the target cell through electrostatic interactions with negatively charged cell surface structures (Fig. 9) (24, 37, 41). These structures could include phospholipid headgroups, sulfated lipids, gangliosides, or HSPG. Uptake then follows bulk membrane flow and would not be easily blocked without compromising cell viability. Consistent with this “adsorption” model, our results show that GrB uptake and cytotoxicity is reduced in the less cationic cs mutants, in the presence of a sulfation inhibitor, and that cells lacking GAGs resist killing.
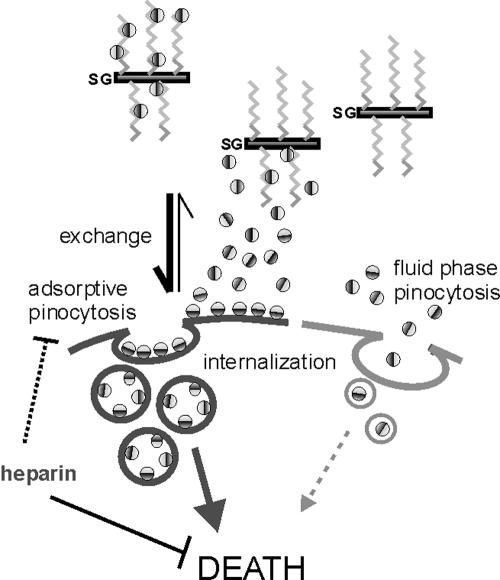
FIG. 9.
The exchange/adsorption model. Granzyme (indicated by two-tone sphere) is delivered into the synapse electrostatically bound to the CS chains (zigzag lines) of serglycin (SG). Granzyme exchanges from CS chains to more negatively charged components of the target cell surface and is internalized by adsorptive pinocytosis (7). Mutation of cs1 or cs2 compromises the cationic surface (indicated by darker hemisphere) of granzyme B and reduces adsorption. Nonadsorbed wt or mutant granzyme is internalized by fluid-phase pinocytosis. Perforin (not shown) releases granzyme from either pathway to cause cell death. Pretreatment of granzyme with excess heparin substantially reduces adsorption but not fluid-phase uptake and prevents death by interfering with an additional unidentified step(s) common to both pathways. Aspects of this model are consistent with recent studies showing that SG-GrB complexes are not internalized (37) and inhibition of granzyme uptake by anionic competitors (41).
One difficulty with this model is that the high positive charge of GrB is probably neutralized in vivo by serglycin, rendering adsorption to the cell surface much less efficient. It is therefore necessary to posit that GrB exchanges from serglycin to more negatively charged elements on the cell surface and is subsequently internalized (Fig. 9) (37). Our findings indicate that GrB binds less efficiently to chondroitin sulfate than to heparin, so such an exchange is possible. A further prediction of an “exchange/adsorption” model is that heparin—but not chondroitin sulfate (the GAG component of serglycin)—should inhibit GrB uptake and hence cytotoxicity. Consistent with this, we have clearly demonstrated that GrB-mediated killing is sensitive to heparin but is relatively insensitive to chondroitin sulfate (Fig. 1C and D).
On the other hand, if internalization of the serglycin-GrB complex is charge independent, heparin should not interfere with uptake or cytotoxicity of purified, uncomplexed GrB: it would simply mimic the chondroitin sulfate on serglycin and mask the positive charge of GrB without affecting binding to the cell. Also under this scenario, reduction of the cationicity of GrB via mutation would only affect its binding to serglycin, and such mutants should be silent in our in vitro systems. Thus, our finding that mutation of cs1 and cs2 affects both uptake and cytotoxicity of GrB supports the exchange/adsorption model, and weakens the selective receptor model, but does not exclude the possibility that both mechanisms operate in parallel.
The overall impact of heparin on GrB-mediated cytotoxicity is complex. FACS and imaging analysis shows that heparin reduces, but does not prevent, internalization of GrB. Yet it completely abrogates cytotoxicity, apparently without affecting perforin. This indicates that heparin also affects GrB trafficking or function within the cell by preventing an interaction with another (unidentified) component or target of the cytotoxic pathway.
Intracellular trafficking of GrB.
The uptake of solutes and macromolecules into cells is complex, and there are multiple portals into the cell (reviewed in reference 7). Pinocytosis or fluid-phase uptake encompasses the mechanistically distinct processes of macropinocytosis; clathrin-mediated endocytosis; caveolin-mediated endocytosis; and clathrin/caveolin-independent endocytosis. With the exception of macropinocytosis, these pathways offer selectivity of the cargos internalized, via interactions with dedicated receptors. Soluble molecules without receptors may enter nonspecifically by encasement in internalizing vesicles, in which uptake efficiency is dictated by the solute concentration and vesicle volume. Uptake efficiency may be enhanced by nonspecific binding of solutes to the cell membrane (adsorptive pinocytosis).
The GTPase dynamin functions in most pinocytic pathways to assist vesicle maturation. Dominant-negative dynamin blocks uptake via all pathways except macropinocytosis and some clathrin/caveolin-independent pathways. With the exception of caveolin-mediated endocytosis, the dynamin-dependent and independent pathways probably intersect in early endosomes where cargo and receptors are sorted to recycling endosomes or late endosomes. Internalized caveolae fuse with caveosomes, which have a neutral pH, and the caveosomes deliver their contents to subcellular compartments such as the endoplasmic reticulum and Golgi apparatus (7).
Which pinocytic pathway(s) does GrB utilize? Given the observation that GrB moves through early endosomes (35) and the proposed dependence on low pH for perforin function, it is unlikely that caveolae are involved in cytotoxicity. GrB uptake involves an energy- and temperature-dependent process that is blocked by cytochalasin (42). At 20°C GrB colocalizes with the early endosome markers rab4 and rab5; at 37°C it accumulates in compartments similar to the IGD observed here, which are not derived from lysosomes, the Golgi apparatus, or mitochondria (35). GrB uses both dynamin-dependent and -independent pathways to move into cells (53, 54), potentially via any pinocytic pathway. Our results are consistent with the presence of at least two pathways (Fig. 9), as demonstrated by the accumulation in IGD of wt GrB and cs2 but not cs1 and the diffuse cytoplasmic accumulation of wt GrB, cs1, and cs2. The fact that cs1 is still (weakly) cytotoxic suggests that the two pathways intersect or that both contain a perforin-sensitive compartment.
The role of perforin.
It remains to be determined how perforin acts to release GrB. Much of what we know about GrB and perforin stems from the use of in vitro systems using purified protein and cultured cells. These have shed light on the trafficking, substrates, and proapoptotic functions of GrB (2, 53). They have also provided insight into the structure and pore-forming function of perforin (reviewed in reference 6), indicating that perforin pores are too small to act as plasma membrane channels for GrB (4, 31). Perforin concentrations which allow formation of large pores that admit GrB directly to the cytoplasm are likely to be necrotic and proinflammatory.
Since the trafficking and molecular functions of GrB and perforin are not readily studied in the context of the CL-target cell synapse, it is still being debated whether granzymes move through a perforin pore at the plasma membrane or are released from endosomes (for discussions, see references 6 and 41). For example, it is argued that the concentration of perforin in the synapse greatly exceeds the concentration achievable in an in vitro system (6): this would be conducive to plasma membrane channel formation within the synapse and movement of GrB directly into the cytoplasm.
This view implies that GrB cationic sites, adsorptive pinocytosis, and endosomolysis are unnecessary for cytotoxicity in vivo. However, there is currently no evidence that has been presented, or is otherwise available, that supports the proposition that “addition of purified perforin and granzymes to the medium is a potentially useful model, but the dilute concentrations of these mediators may be many orders of magnitude smaller than their in vivo concentration after exocytosis into the junctional synapse between the cytotoxic cell and its bound target” (6). It cannot be assumed that the amount of perforin delivered into the synapse is uniform between CLs or that the same amount is released in every “hit” delivered by a particular CL. Indeed, it is evident that CL populations are heterogeneous with respect to granzyme and perforin expression (18, 23); thus, synaptic perforin and granzyme concentrations may differ considerably. Variation in the amount of perforin or granzyme delivered by particular CLs may explain recent observations that reduction of surface HSPG makes target cells less susceptible to CL killing in some contexts (37) but not others (24). In any event, the two models of perforin-mediated granzyme release (channel and endosomolysis) are not mutually exclusive.
Previous studies have suggested that bacterial pore-forming proteins like SLO and pneumolysin, or nonreplicating adenovirus, are functionally equivalent to perforin (4, 13). The pore-forming capabilities of SLO have recently been carefully characterized (55), and it has been shown to reversibly permeabilize the plasma membrane under the conditions described here. By contrast, perforin is thought to be endosomolytic (13, 31). Our results show an impact of the cs mutations on perforin-mediated cytotoxicity, but not on SLO-mediated cytotoxicity, strongly suggesting that SLO and perforin function differently. Results from studies that have depended on SLO delivery of granzymes should be reevaluated in this light.
Conclusion.
There is selective pressure on viruses and tumor cells to evade the immune system via camouflage, suppression, or resistance. In the case of resistance, target cells may down-regulate machinery required for death signal transduction, resist entry of cytotoxins, produce inhibitors of cytotoxins, or up-regulate endogenous antiapototic regulators. If the uptake of GrB by target cells depended on a single receptor or system, a potential chokepoint would be created. The work described here identifies specific noncatalytic regions of GrB involved in its uptake, trafficking, and cytotoxicity and indicates that GrB internalization is charge dependent and relatively nonselective. It also indicates that other cytotoxic granzymes may enter target cells via a similar mechanism.
Acknowledgments
This work was supported by Program grants to P.I.B., J.C.W., and J.A.T. from the National Health and Medical Research Council (Australia).
We thank C. Froelich (Evanston Northwestern Healthcare Research Institute, Evanston, IL) for discussions and Alexa-conjugated native GrB; R. Orlando (Department of Biochemistry and Molecular Biology, School of Medicine, Health Sciences Center, University of New Mexico, Albuquerque, NM) for the RAP expression system and LRP antibodies; S. Leppla (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD) for LRP-deficient cells; T. Brown (Department of Biochemistry and Molecular Biology, Monash University, Australia) for HS antibodies and CS antibodies; and S. Bhakdi (Institut fur Medizinische Mikrobiologie und Hygiene, Johannes-Gutenberg-Universitat Mainz, Germany) for purified SLO.
REFERENCES
- 1.Baeuerle, P. A., and W. B. Huttner. 1986. Chlorate—a potent inhibitor of protein sulfation in intact cells. Biochem. Biophys. Res. Commun. 141:870-877. [DOI] [PubMed] [Google Scholar]
- 2.Barry, M., and C. R. Bleackley. 2002. Cytotoxic lymphocytes: all roads lead to death. Nature Rev. Immunol. 2:401-409. [DOI] [PubMed] [Google Scholar]
- 3.Bretthauer, R. K., and F. J. Castellino. 1997. Glycosylation of Pichia pastoris-derived proteins. Biotechnol. Appl. Biochem. 30:193-200. [PubMed] [Google Scholar]
- 4.Browne, K. A., E. Blink, V. R. Sutton, C. J. Froelich, D. A. Jans, and J. A. Trapani. 1999. Cytosolic delivery of granzyme B by bacterial toxins: evidence that endosomal disruption, in addition to transmembrane pore formation, is an important function of perforin. Mol. Cell. Biol. 19:8604-8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardin, A. D., and H. J. Weintraub. 1989. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis 9:21-32. [DOI] [PubMed] [Google Scholar]
- 6.Catalfamo, M., and P. A. Henkart. 2004. Perforin and the granule exocytosis cytotoxicity pathway. Curr. Opin. Immunol. 15:522-527. [DOI] [PubMed] [Google Scholar]
- 7.Conner, S. D., and S. L. Schmid. 2004. Regulated portals of entry into the cell. Nature 422:37-44. [DOI] [PubMed] [Google Scholar]
- 8.Dell'Angelica, E. C., and G. S. Payne. 2001. Intracellular cycling of lysosomal enzyme receptors cytoplasmic tails' tales. Cell 106:395-398. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson, J. L., and T. M. Antalis. 1993. Tissue factor and plasminogen activator inhibitor expression in the differentiation of myeloid leukemic cells. Leukemia 7:864-871. [PubMed] [Google Scholar]
- 10.Dressel, R., S. M. Raja, S. Honing, T. Seidler, C. J. Froelich, K. von Figura, and E. Gunther. 2004. Granzyme-mediated cytotoxicity does not involve the mannose 6-phosphate receptors on target cells. J. Biol. Chem. 279:20200-20210. [DOI] [PubMed] [Google Scholar]
- 11.Esko, J. D., T. E. Stewart, and W. H. Taylor. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 82:3197-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FitzGerald, D. J., C. M. Fryling, A. Zdanovsky, C. B. Saelinger, M. Kounnas, J. A. Winkles, D. Strickland, and S. Leppla. 2004. Pseudomonas exotoxin-mediated selection yields cells with altered expression of low-density lipoprotein receptor-related protein. J. Cell Biol. 129:1533-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Froelich, C. J., K. Orth, J. Turbov, P. Seth, R. Gottleib, B. Babior, G. M. Shah, R. C. Bleackley, V. M. Dixit, and W. Hanna. 1996. New paradigm for lymphocyte granule mediated cytotoxicity: target cells bind and internalize granzyme B but an endosomolytic agent is necessary for cytosolic delivery and subsequent apoptosis. J. Biol. Chem. 271:29073-29079. [DOI] [PubMed] [Google Scholar]
- 14.Galvin, J. P., E. H. A. Spaeny-Dekking, B. Wang, P. Seth, C. E. Hack, and C. J. Froelich. 1999. Apoptosis induced by granzyme B-glycosaminoglycan complexes: implications for granule-mediated apoptosis in vivo. J. Immunol. 162:5345-5350. [PubMed] [Google Scholar]
- 15.Goldmacher, V. S., N. L. Tinnel, and B. C. Nelson. 1986. Evidence that pinocytosis in lymphoid cells has a low capacity. J. Cell Biol. 102:1312-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths, G. M., and S. Isaaz. 1993. Granzymes A and B are targeted to the lytic granules of lymphocytes by the mannose-6-phosphate receptor. J. Cell Biol. 120:885-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross, C., W. Koelch, A. DeMaio, N. Arispe, and G. Multhoff. 2003. Cell surface-bound heat shock protein 70 (Hsp70) mediates perforin-independent apoptosis by specific binding and uptake of granzyme B. J. Biol. Chem. 278:41173-41181. [DOI] [PubMed] [Google Scholar]
- 18.Grossman, W. J., J. W. Verbsky, B. L. Tollefsen, C. Kemper, J. P. Atkinson, and T. J. Ley. 2004. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood 104:2840-2848. [DOI] [PubMed] [Google Scholar]
- 19.Hermanson, G. T. 1996. Bioconjugate techniques. Academic Press, San Diego, Calif.
- 20.Hileman, R. E., J. M. Weiler, J. R. Fromm, and R. J. Linhardt. 1998. Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. BioEssays 20:156-167. [DOI] [PubMed] [Google Scholar]
- 21.Illinger, D., P. Poindron, and J. G. Kuhry. 1991. Fluid phase endocytosis investigated by fluorescence with trimethylamino-diphenylhexatriene in L929 cells; the influence of temperature and of cytoskeleton depolymerizing drugs. Biol. Cell 73:131-138. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan, A., D. Fischer, D. Achord, and W. Sly. 1977. Phosphohexosyl recognition is a general characteristic of pinocytosis of lysosomal glycosidases by human fibroblasts. J. Clin. Investig. 60:1088-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelso, A., E. O. Costelloe, B. J. Johnson, P. Groves, K. Buttigieg, and D. R. Fitzpatrick. 2002. The genes for perforin, granzymes A-C and IFN-gamma are differentially expressed in single CD8(+) T cells during primary activation. Int. Immunol. 14:605-613. [DOI] [PubMed] [Google Scholar]
- 24.Kurschus, F. C., R. Bruno, E. Fellows, C. S. Falk, and D. Jenne. 2005. Membrane receptors are not required to deliver granzyme B during killer cell attack. Blood 105:2049-2058. [DOI] [PubMed] [Google Scholar]
- 25.Legrand, D., P. H. van Berkel, V. Salmon, H. A. van Veen, M. C. Slomianny, J. H. Nuijens, and G. Spik. 1997. The N-terminal Arg2, Arg3 and Arg4 of human lactoferrin interact with sulphated molecules but not with the receptor present on Jurkat human lymphoblastic T-cells. Biochem. J. 327:841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lidholt, K., J. L. Weinke, C. S. Kiser, F. N. Lugemwa, K. J. Bame, S. Cheifetz, J. Massague, U. Lindahl, and J. D. Esko. 1992. A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in a Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc. Natl. Acad. Sci. USA 89:2267-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lieberman, J., and Z. Fan. 2003. Nuclear war: the granzyme A-bomb. Curr. Opin. Immunol. 15:553-559. [DOI] [PubMed] [Google Scholar]
- 28.Liu, C. C., P. M. Persechini, and J. D. Young. 1994. Characterization of recombinant mouse perforin expressed in insect cells using the baculovirus system. Biochem. Biophys. Res. Commun. 201:318-325. [DOI] [PubMed] [Google Scholar]
- 29.Mayor, S., J. F. Presley, and F. R. Maxfield. 1993. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J. Cell Biol. 121:1257-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melman, L., Z. F. Cao, S. Rennke, M. P. Marzolo, M. R. Wardell, and G. Bu. 2001. High affinity binding of receptor-associated protein to heparin and low density lipoprotein receptor-related protein requires similar basic amino acid sequence motifs. J. Biol. Chem. 276:29338-29346. [DOI] [PubMed] [Google Scholar]
- 31.Metkar, S. S., B. Wang, M. Aguilar-Santelises, S. M. Raja, L. Uhlin-Hansen, E. Podack, J. A. Trapani, and C. J. Froelich. 2002. Cytotoxic cell granule-mediated apoptosis: a multimeric delivery system where perforin delivers granzyme B-serglycin complexes into target cells without plasma membrane pore formation. Immunity 16:417-428. [DOI] [PubMed] [Google Scholar]
- 32.Metkar, S. S., B. Wang, M. L. Ebbs, J. H. Kim, Y. J. Lee, S. M. Raja, and C. J. Froelich. 2004. Granzyme B activates procaspase-3 which signals a mitochondrial amplification loop for maximal apoptosis. J. Cell Biol. 160:875-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motyka, B., G. Korbutt, M. J. Pinkoski, J. A. Heibein, A. Caputo, M. Hobman, M. Barry, I. Shostak, T. Sawchuk, C. F. B. Holmes, J. Gauldie, and C. R. Bleackley. 2000. Mannose 6-phosphate/insulin-like growth factor II receptor is a death receptor for granzyme B during cytotoxic T cell-induced apoptosis. Cell 103:491-500. [DOI] [PubMed] [Google Scholar]
- 34.Orlando, R. A., and M. G. Farquhar. 2004. Functional domains of the receptor-associated protein (RAP). Proc. Natl. Acad. Sci. USA 91:3161-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinkoski, M. J., M. Hobman, J. A. Heibein, K. Tomaselli, F. Li, P. Seth, C. J. Froelich, and R. C. Bleackley. 1998. Entry and trafficking of granzyme B in target cells during granzyme B-perforin-mediated apoptosis. Blood 92:1044-1054. [PubMed] [Google Scholar]
- 36.Raja, S. M., S. S. Metkar, and C. J. Froelich. 2003. Cytotoxic granule-mediated apoptosis: unraveling the complex mechanism. Curr. Opin. Immunol. 15:528-532. [DOI] [PubMed] [Google Scholar]
- 37.Raja, S. M., S. S. Metkar, S. Honing, B. Wang, W. A. Russin, N. H. Pipalia, C. Menaa, M. Belting, X. Cao, R. Dressel, and C. J. Froelich. 2005. A novel mechanism for protein delivery: granzyme B undergoes electrostatic exchange from Serglycin to target cells. J. Biol. Chem. 280:20752-20761. [DOI] [PubMed] [Google Scholar]
- 38.Rao, N. V., G. V. Rao, B. C. Marshall, and J. R. Hoidal. 1996. Biosynthesis and processing of proteinase 3 in U937 cells. J. Biol. Chem. 271:2972-2978. [DOI] [PubMed] [Google Scholar]
- 39.Rotonda, J., M. Garcia-Calvo, H. G. Bull, W. M. Geissler, B. M. McKeever, C. A. Willoughby, N. A. Thornberry, and J. W. Becker. 2001. The three-dimensional structure of human granzyme B compared to caspase-3, key mediators of cell death with cleavage specificity for aspartic acid in P1. Chem. Biol. 8:357-368. [DOI] [PubMed] [Google Scholar]
- 40.Russell, J. H., and T. J. Ley. 2002. Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 20:323-370. [DOI] [PubMed] [Google Scholar]
- 41.Shi, L., D. Keefe, E. Durand, H. Feng, D. Zhang, and J. Lieberman. 2005. Granzyme B binds to target cells mostly by charge and must be added at the same time as perforin to trigger apoptosis. J. Immunol. 174:5456-5461. [DOI] [PubMed] [Google Scholar]
- 42.Shi, L., S. Mai, S. Israels, K. A. Browne, J. A. Trapani, and A. H. Greenberg. 1997. Granzyme B (GraB) autonomously crosses the cell membrane and perforin initiates apoptosis and GraB nuclear localization. J. Exp. Med. 185:855-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strickland, D. K., and S. Ranganathan. 2003. Diverse role of LDL receptor-related protein in the clearance of proteases and in signaling. J. Thromb. Haemost. 1:1663-1670. [DOI] [PubMed] [Google Scholar]
- 44.Sun, J., C. H. Bird, M. S. Buzza, K. E. McKee, J. C. Whisstock, and P. I. Bird. 1999. Expression and purification of recombinant human granzyme B from Pichia pastoris. Biochem. Biophys. Res. Commun. 261:251-255. [DOI] [PubMed] [Google Scholar]
- 45.Sun, J., C. H. Bird, K. Y. Thia, A. Y. Matthews, J. A. Trapani, and P. I. Bird. 2004. Granzyme B encoded by the commonly occurring human RAH allele retains pro-apoptotic activity. J. Biol. Chem. 279:16907-16911. [DOI] [PubMed] [Google Scholar]
- 46.Sun, J., J. C. Whisstock, P. Harriott, B. Walker, A. Novak, P. E. Thompson, A. I. Smith, and P. I. Bird. 2001. Importance of the P4′ residue in human granzyme B inhibitors and substrates revealed by scanning mutagenesis of the proteinase inhibitor 9 reactive center loop. J. Biol. Chem. 276:15177-15184. [DOI] [PubMed] [Google Scholar]
- 47.Sutton, V. R., J. E. Davis, M. Cancilla, R. W. Johnstone, A. A. Ruefli, K. Sedelies, K. A. Browne, and J. A. Trapani. 2000. Initiation of apoptosis by granzyme B requires direct cleavage of bid, but not direct granzyme B-mediated caspase activation. J. Exp. Med. 192:1403-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toyama-Sorimachi, N., H. Sorimachi, Y. Tobita, F. Kitamura, H. Yagita, K. Suzuki, and M. Miyasaka. 1995. A novel ligand for CD44 is serglycin, a hematopoietic cell lineage-specific proteoglycan. Possible involvement in lymphoid cell adherence and activation. J. Biol. Chem. 270:7437-7444. [DOI] [PubMed] [Google Scholar]
- 49.Trapani, J. A., K. A. Browne, M. J. Dawson, and M. J. Smyth. 1993. Immunopurification of functional Asp-ase (natural killer cell granzyme B) using a monoclonal antibody. Biochem. Biophys. Res. Commun. 195:910-920. [DOI] [PubMed] [Google Scholar]
- 50.Trapani, J. A., and M. J. Smyth. 2002. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2:735-747. [DOI] [PubMed] [Google Scholar]
- 51.Trapani, J. A., M. J. Smyth, V. A. Apostolidis, M. Dawson, and K. A. Browne. 1994. Granule serine proteinases are normal nuclear constituents of natural killer cells. J. Biol. Chem. 269:18359-18365. [PubMed] [Google Scholar]
- 52.Trapani, J. A., and V. R. Sutton. 2004. Granzyme B: pro-apoptotic, antiviral and antitumor functions. Curr. Opin. Immunol. 15:533-543. [DOI] [PubMed] [Google Scholar]
- 53.Trapani, J. A., V. R. Sutton, K. Y. Thia, Y. Q. Li, C. J. Froelich, D. A. Jans, M. S. Sandrin, and K. A. Browne. 2003. A clathrin/dynamin- and mannose-6-phosphate receptor-independent pathway for granzyme B-induced death. J. Cell Biol. 160:223-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veugelers, K., B. Motyka, C. Frantz, I. Shostak, T. Sawchuk, and R. C. Bleackley. 2004. The granzyme B-serglycin complex from cytotoxic granules requires dynamin for endocytosis. Blood 103:3845-3853. [DOI] [PubMed] [Google Scholar]
- 55.Walev, I., S. C. Bhakdi, F. Hofmann, N. Djonder, A. Valeva, K. Aktories, and S. Bhakdi. 2001. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc. Natl. Acad. Sci. USA 98:3185-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weller, U., L. Muller, M. Messner, M. Palmer, A. Valeva, J. Tranum-Jensen, P. Agrawal, C. Biermann, A. Dobereiner, M. A. Kehoe, and S. Bhakdi. 1996. Expression of active streptolysin O in Escherichia coli as a maltose-binding-protein-streptolysin O fusion protein. The N-terminal 70 amino acids are not required for hemolytic activity. Eur. J. Biochem. 236:34-39. [DOI] [PubMed] [Google Scholar]
- 57.Wilsie, L. C., and R. A. Orlando. 2003. The low density lipoprotein receptor-related protein complexes with cell surface heparan sulfate proteoglycans to regulate proteoglycan-mediated lipoprotein catabolism. J. Biol. Chem. 278:15758-15764. [DOI] [PubMed] [Google Scholar]
- 58.Zhou, Q., J. Kyazike, T. Edmunds, and E. Higgins. 2002. Mannose 6-phosphate quantitation in glycoproteins using high-pH anion-exchange chromatography with pulsed amperometric detection. Anal. Biochem. 306:163-170. [DOI] [PubMed] [Google Scholar]