Abstract
The regulatory circuits that orchestrate mammalian myoblast cell fusion during myogenesis are poorly understood. The transcriptional activity of FoxO1a directly regulates this process, yet the molecular mechanisms governing FoxO1a activity during muscle cell differentiation remain unknown. Here we show an autoregulatory loop in which FoxO1a directly activates transcription of the cyclic GMP-dependent protein kinase I (cGKI) gene and where the ensuing cGKI activity phosphorylates FoxO1a and abolishes its DNA binding activity. These findings establish the FoxO1a-to-cGKI pathway as a novel feedback loop that allows the precise tuning of myoblast fusion. Interestingly, this pathway appears to operate independently of muscle cell differentiation programs directed by myogenic transcription factors.
Fusion of cell membranes is required for the proper function and morphogenesis of several mammalian cell types, including macrophages, trophoblasts, spermatozoa and oocytes, and myoblasts that form skeletal muscle. While the mechanisms regulating intracellular membrane fusion events have been studied in some detail (4, 17), very little is known regarding the mechanisms governing mammalian cell-to-cell fusion events.
Complex programs orchestrate myogenesis, which involve coordinated myogenic cell migration, recognition, adhesion, alignment, and finally membrane fusion. The FoxO family of transcription factors (FoxO1a, FoxO3, and FoxO4a) regulates diverse cellular responses, including apoptosis, cell cycle arrest, differentiation, DNA repair, and oxidative stress (1, 3, 7, 34). While the ability of FoxO's to regulate divergent processes such as apoptosis and differentiation seems somewhat paradoxical, some of the cellular alterations associated with skeletal muscle cell differentiation do share a high degree of similarity with many phenotypic changes usually associated with apoptosis. Indeed, cytoskeleton remodeling and actin fiber disassembly are features shared by these two processes (10, 14, 16, 29, 32), and myosin light chain kinase, a conserved regulator of muscle cell contraction, is also essential for the blebbing of cell membranes observed during apoptosis (23). Furthermore, matrix metalloproteinases are required for both apoptosis and muscle cell fusion (28, 36), and activation of caspase 3, a key effector apoptotic protease, is also required for skeletal muscle cell differentiation (11).
FoxO1a is essential for normal development, as evidenced by the fact that knockout mice lacking FoxO1a die at embryonic day 9.5 (E9.5) to E10.5 due to defects in vasculogenesis, whereas targeted deletions of the close FoxO1a homologs FoxO3 and FoxO4a lead to limited, if any, developmental phenotypes (8, 18). Interestingly, endothelial cells lacking FoxO1a fail to reorganize into vessel-like structures when stimulated with vascular endothelial growth factor (12), suggesting that FoxO1a directs cytoskeleton remodeling and cell-cell interactions during the formation of the vasculature. In addition, FoxO1a is both necessary and sufficient to regulate myoblast cell fusion ex vivo (5, 25).
The transcriptional activity of FoxO1a also appears to be regulated by phosphorylation events during myoblast differentiation (5, 25), and conventionally this has been thought to be directed by Akt serine/threonine kinases that phosphorylate FoxO transcription factors, which provokes their nuclear export and destruction by the proteasome (22). However, in primary myoblasts and in the myoblast cell line C2C12, phosphorylation of FoxO1a occurs independently of the phosphatidylinositol 3-kinase/Akt pathway, suggesting that other kinases harness FoxO1a transcriptional activity during myogenic differentiation (5, 25). Indeed, inactivation of the Rho-associated kinase ROCK has been demonstrated to be a prerequisite for FoxO1a nuclear translocation and fusion of C2C12 cells (25). In addition, FoxO3 is phosphorylated by IκB kinase independently of Akt, and this also causes its ubiquitin-dependent degradation (20). These findings support the concept that divergent signaling pathways impinge upon the transcriptional activity of FoxO transcription factors, thereby regulating their diverse and cell type-specific functions.
Here we report that cyclic GMP-dependent protein kinase I (cGKI) is a regulator of FoxO1a activity during muscle cell fusion. Interestingly, upon muscle cell differentiation, FoxO1a is shown to directly activate cGKI transcription. In turn, cGKI then harnesses FoxO1a activity by phosphorylating residues juxtaposed to the FoxO1a DNA binding domain, thus abolishing the ability of FoxO1a to bind to its response elements. cGKI also promotes relocalization of FoxO1a into the cytoplasm. These results therefore suggest that a FoxO1a-to-cGKI pathway fine-tunes the rates of myoblast cell fusion.
MATERIALS AND METHODS
Yeast two-hybrid screen.
The CytoTrap system (Stratagene) was used to perform the yeast two-hybrid screen following the supplier's protocol. Particular care was taken to avoid reversion of the cdc25H yeast strain. FoxO1a was used as bait and cloned into the pSOS vector (Stratagene). A stable pSOS-FoxO1a cdc25H yeast strain was first established before screening with the commercially available heart cDNA target library (Stratagene).
Mouse primary myoblast isolation and culture, and culture of C2C12 cells.
Primary myoblasts were isolated and cultured from 6-day-old cGKI wild-type and knockout littermates (35) as described previously (5). Cell fusion experiments using CellTracker probes (Molecular Probes) were performed following the supplier's protocol. The two dyes used were CellTracker Green CMFDA and CellTracker Orange CMTMR. The C2C12 cell line was obtained from the American Type Culture Collection. Passage-1 and -2 cells were used for the experiments, and particular care was taken to avoid overgrowth by passing the cells before they reached 50 to 60% confluence. Failure to do so quickly induced differentiation and a partial or complete loss of differentiation potential in subsequent passages. C2C12 cells were cultured in Dulbecco's modified Eagle medium (DMEM)-10% fetal bovine serum by following standard protocols. Differentiation was induced by either growing the cells to confluence or transferring them to DMEM containing 2% horse serum (25).
RT-PCR, protein analysis, and ChIP experiments.
Reverse transcription-PCR (RT-PCR) and Western blotting were performed using standard protocols. Sequences of primers are available upon request. An anti-FoxO1a (FKHR) antibody was purchased from Cell Signaling Technology, an anti-green fluorescent protein (GFP) antibody from Molecular Probes, activated vasodilator-stimulated phosphoprotein (VASP) (serine 239) from nanoTools, an anti-FLAG antibody from Sigma, and an anti-cGKI (PKG) antibody from StressGen; the MF20 (myosin heavy chain) antibody was a gift from D. A. Fischman. Chromatin immunoprecipitation (ChIP) experiments were performed using the chromatin immunoprecipitation assay kit from Upstate Biotechnology following the supplier's protocol. The primers used to amplify the regions containing the FoxO1a binding sites surrounding the cGKIα promoter area were cGKI-7.0kb/F (5′-GTGCCTTATGCTTAGCAATGAG-3′), cGKI-6.7kb/R (5′-CACTTGAAGACGGTGAGTCCT-3′), cGKI +6.5kb/F (5′-GTGCATGAGACTCATTTCATGG-3′), and cGKI-+6.8kb/R (5′-GACGCAGGG TGAAGGGTAGGT-3′).
Phosphorylation and gel retardation assays.
The various domains of FoxO1a protein used for phosphorylation assays were produced either in BL21 bacteria (Stratagene) or in a coupled transcription-reticulocyte lysate translation kit (Promega). cGKI kinase was purchased from Promega, and kinase assays were performed according to the supplier's protocol. Peptides were produced at the St. Jude Children's Research Hospital (SJCRH) Hartwell Center for Biotechnology. Peptide sequences (with phosphorylated sites underlined) were as follows: Thr24, RSCTWPL; Ser152-155, QPRKSSSSRRNA; Ser184, KRLTLSQIY; Ser256, RAASMDN; Ser266/268, KKASLQSGQE; Ser319, RTSSNAS; control peptide, RKRSRAE. Electrophoretic gel mobility shift assays (EMSA) were performed as described previously (13). These assays were performed using in vitro-translated FoxO1a proteins due to the high instability of recombinant FoxO1a proteins in bacteria.
Luciferase assays.
Luciferase reporters carrying the region from kb −7.0 to −6.7 or from kb +6.5 to +6.8 of the cGKIα promoter (referred to below as the −7.0/−6.7-kb or +6.5/+6.8-kb region, respectively) containing the FoxO1a binding sites were generated by inserting PCR-amplified fragments upstream of the minimal promoter (KpnI/XhoI sites) of the pGL3-basic firefly luciferase vector (Promega). Primers used were cGKI-7.0kb/F (5′-ggggtaccGTGCCTTATGCTTAGCAATGAG-3′), cGKI-6.7kb/R (5′-ccgctcgagCACTTGAAGACGGTGAGTCCT-3′), cGKI+6.5kb/F (5′-ggggtaccGTGCATGAGACTCATTTCATGG-3′), and cGKI-+6.8kb/R (5′-ccgctcgagGACGCAGGGTGAAGGGTAGGT-3′). KpnI (5′) and XhoI (3′) sites (lowercase) were added for cloning purposes. Myoblasts (primary myoblasts or C2C12 cells) were transiently transfected with either reporter construct using FuGene6 or Lipofectamine 2000 following the supplier's protocol. Luciferase detection was performed using the Luciferase Assay System from Promega by following the manufacturer's recommendations. A secreted alkaline phosphatase (SEAP) reporter was cotransfected (100 ng per transfection) to normalize for transfection efficiency, and SEAP activity was determined as described previously (5).
Virus production, myoblast transduction, and cell sorting.
D. Persons, SJCRH, provided the murine stem cell virus (MSCV)-internal ribosome entry site (IRES)-GFP vector. All FoxO1a point mutations were introduced using standard molecular biology protocols (2). Myoblasts were transduced using standard techniques, and 2 days after infection, cells expressing GFP were sorted by flow cytometry.
Mice.
cGKI+/− mice (35) were kindly provided by Franz Hofmann and Robert Feil (Pharmacology and Toxicology Institute, Munich, Germany). Mice were maintained at SJCRH under specific-pathogen-free conditions by following the Institutional Animal Care and Use Committee guidelines.
RESULTS AND DISCUSSION
FoxO1a interacts with cyclic GMP-dependent kinase I.
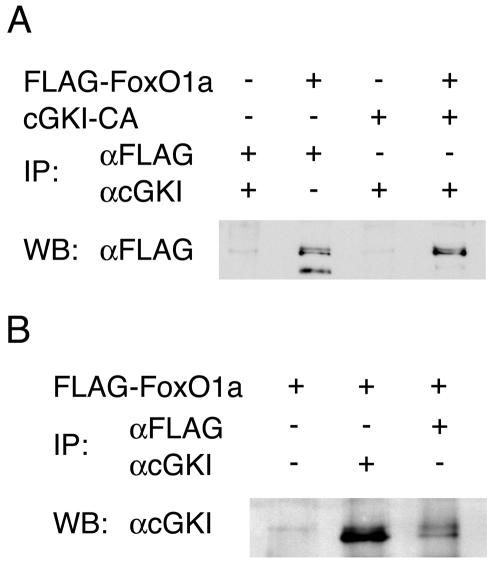
To identify possible regulators of FoxO1 activity during muscle cell fusion, we performed a yeast two-hybrid screen using full-length FoxO1a as bait. These analyses identified the catalytic subunit of cGKI as a prey for FoxO1a. Two independent clones encoding the C-terminal amino acids 414 and 353 of cGKI interacted strongly with FoxO1a in these assays. To determine whether FoxO1a-cGKI interactions were also detectable in muscle cells, we performed coimmunoprecipitation experiments with C2C12 cells engineered to overexpress Flag-FOXO1A and the kinase domain of cGKI. Notably, FoxO1a was coimmunoprecipitated with the kinase domain of cGKI by using an anti-cGKI antibody (Fig. 1A). Available antibodies to FoxO1a are poor at immunoprecipitating endogenous levels of FoxO1a, so we utilized Flag-tagged FoxO1a in our experiments. In differentiating C2C12 cells, endogenous cGKI was coimmunoprecipitated with overexpressed Flag-tagged FoxO1a by using an anti-Flag antibody (Fig. 1B). Therefore, FoxO1a and cGKI do form a complex in muscle progenitors.
FIG. 1.
cGKIα and FoxO1a interact. (A) Coimmunoprecipitation (IP) experiments in transiently transfected C2C12 cells demonstrate in vivo interaction between FoxO1a and a constitutively active form of cGKIα (cGKI-CA, where the cGMP regulatory domain has been deleted). Western blotting (WB) was performed with an anti-FLAG antibody. (B) Reverse coimmunoprecipitation experiments in transiently transfected C2C12 cells demonstrate the in vivo interaction between endogenous cGKIα and a FLAG-tagged FoxO1a. C2C12 cells were induced to differentiate for 3 days. Western blotting was performed with an anti-cGKI antibody.
These findings suggested that cGKI might regulate FoxO1a functions. Interestingly, after receiving differentiation cues, myoblasts show an essential and rapid spike in intracellular levels of cyclic GMP (cGMP) prior to fusion (9). Moreover, cGKI belongs to the AGC (cAMP-dependent protein kinase/protein kinase G/protein kinase C) family of protein kinases (26) and appears to contribute to the control of cytoskeleton remodeling by phosphorylating and inactivating the VASP (6, 15, 31). We therefore assessed the functional importance of the cGKI-FoxO1a interaction during myoblast fusion.
Cyclic GMP-dependent kinase I is a direct transcriptional target of FoxO1a.
During muscle cell differentiation. cGMP levels transiently increase in a temporal manner that follows the nuclear translocation and transcriptional activation of FoxO1a (5, 9). We therefore hypothesized that cGKI might be a direct transcription target of FoxO1a during myoblast fusion. The cGKI gene encodes two isoforms (α and β), which differ only in the sequences encoded by their respective first exons. An additional cGKI family member, cGKII, also possesses cGMP-dependent protein kinase activity (27), but cGKIβ and cGKII were not significantly expressed in proliferating or differentiating myoblasts (data not shown). In contrast, cGKIα mRNA and protein were robustly induced in primary myoblasts after the induction of differentiation (Fig. 2A to D). cGKIα induction was also observed in differentiating C2C12 cells (see Fig. 4A).
FIG. 2.
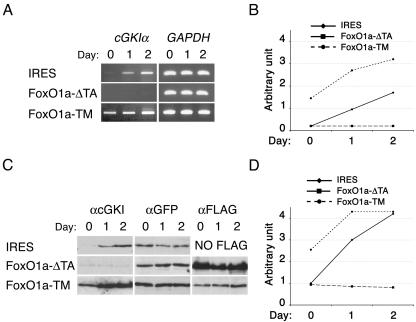
FoxO1a activation induces cGKIα transcription during primary myoblast differentiation. (A) RT-PCR analyses of cGKIα expression in primary myoblasts transduced with MSCV-IRES-GFP retrovirus vectors containing either no insert (IRES), FoxO1a-ΔTA (dominant negative), or FoxO1a-TM (dominant active). cGKIα was amplified using specific primers, and amplification of GAPDH mRNA was used to normalize mRNA levels. Following the induction of differentiation by transfer of the myoblasts to a low-serum medium (5, 30), cGKIα mRNA levels increased twofold in vector-transduced cells. Primary myoblasts expressing FoxO1a-ΔTA showed no increase in cGKIα expression, whereas myoblasts expressing FoxO1a-TM showed elevated expression of cGKIα. (B) Graph showing the induction of cGKIα transcripts in proliferating (day 0) and differentiating (days 1 and 2) myoblasts transduced with either the empty vector, FoxO1a-ΔTA, or FoxO1a-TM. Expression levels were normalized to GAPDH expression. (C) Western blot analysis of cGKI expression. Protein loading was controlled using an anti-GFP antibody. The expression of exogenous FoxO1a mutants was detected using an anti-FLAG antibody. (D) Graphic representation of the induction of cGKIα protein expression in the indicated myoblasts, normalized for expression of GFP.
FIG. 4.
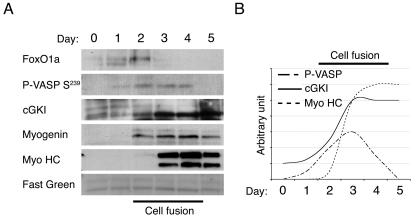
Timing of the FoxO1a-to-cGKIα pathway in differentiating C2C12 cells. (A) Western blot analysis of the indicated proteins in differentiating C2C12 cells over a 5-day time course. Uniform protein loading and homogeneous transfer of sodium dodecyl sulfate-polyacrylamide gel electrophoresis membranes were confirmed by staining with FastGreen. (B) Graphic representation of the induction of cGKIα, P-VASP (Ser239), and myosin heavy chain (Myo HC) protein expression in C2C12 cells, normalized for FastGreen staining.
The early-embryonic lethality of the FoxO1a knockout (at E9.5 to 10.5 [18]) precluded analysis of cGKIα expression in these knockout myoblasts, and repeated attempts to knock down endogenous FoxO1a expression using FoxO1a-directed short hairpin RNA failed to show effects on FoxO1a expression (data not shown). However, we were able to analyze the effects of a dominant-negative form of FoxO1a, FoxO1a-ΔTA (lacking the FoxO1a transactivation domain, which prevents myoblast fusion [5]), upon cGKIα expression in transduced myoblasts. Importantly, up-regulation of cGKIα was abolished in myoblasts expressing dominant-negative FoxO1a-ΔTA (Fig. 2A and B). Conversely, expression of the dominant-active FoxO1a-TM, which harbors alanine substitutions at the three known Akt sites of FoxO1a (Thr24, Ser256, and Ser319), and which dramatically accelerates the rate of myoblast cell fusion (5), led to 2.5-fold induction of cGKIα mRNA and cGKIα protein even in proliferating myoblasts and augmented this response following the induction of differentiation (Fig. 2C and D). Therefore, although the use of dominant versions of FoxO1a could have off-target effects, the data are consistent with the model where FoxO1a regulates, directly or indirectly, cGKIα expression.
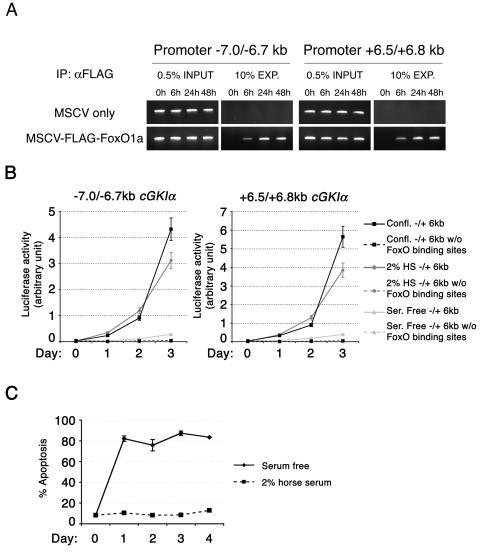
To address whether cGKIα is a direct transcriptional target of FoxO1a, we first surveyed the cGKI genomic sequence for the presence of FoxO1a consensus binding sites. Indeed, two regions, of 300 bp each (−7.0/−6.7 kb and +6.5/+6.8 kb), surrounding exon 1 of the cGKIα gene harbor several TTGTTTAC (13) FoxO DNA binding sites. These sites are also present at similar distances from exon 1 in the human, rat, and fugu cGKIα promoter-regulatory regions (data not shown). We therefore used ChIP assays to investigate whether FoxO1a occupied these elements in myoblasts in a differentiation-dependent manner. In primary myoblasts expressing Flag-tagged FoxO1a, ChIP with an anti-FLAG antibody demonstrated that FoxO1a was recruited to these sites in the cGKIα promoter region within 6 h following induction of differentiation (Fig. 3A). Furthermore, the activity of luciferase reporter constructs containing either of these elements upstream of a minimal promoter was dramatically induced in C2C12 myoblasts after induction of differentiation (Fig. 3B). However, the same regions deleted for the FoxO binding sites failed to show any induction of luciferase activity (Fig. 3B). Therefore, FoxO1a is recruited to FoxO binding sites in the cGKIα gene at the initial phase of myoblast differentiation, which coincides with the induction of cGKIα transcription. However, when we attempted to induce C2C12 muscle cell differentiation by complete serum withdrawal, as described by others (19), we were unable to detect significant increases in luciferase activity (Fig. 3B). This apparent discrepancy was due to a dramatic induction of apoptosis by following this method of differentiation (Fig. 3C). Thus, by following the conventional method for induction of differentiation ex vivo, i.e., placing the cells in 2% horse serum, FoxO1a is regulated in C2C12 cells in a fashion similar to that observed in primary myoblasts, a finding recently corroborated by others (25).
FIG. 3.
Chromatin immunoprecipitation demonstrates direct binding of FoxO1a to the cGKIα promoter region. (A) Direct binding of FoxO1a to cGKIα enhancer regions was assayed using ChIP. Since the FoxO1a-specific antibody performs poorly in immunoprecipitation assays, FLAG-tagged wild-type FoxO1a was used to perform the ChIP on transduced myoblasts. Upon differentiation, FoxO1a shows rapid binding to cGKIα enhancer regions (−7.0/−6.7 kb and + 6.5/+6.8 kb), which contain FoxO1a binding sites. Cells transduced with an empty MSCV-IRES-GFP vector were used as a negative control. (B) The cGKI promoter area harbors FoxO1a-responsive elements. C2C12 cells transfected with reporter luciferase constructs containing the FoxO1a binding regions (at −7.0/−6.7 kb or +6.5/+6.8 kb from the cGKI start codon) were induced to differentiate by (i) confluence in 10% DMEM (Confl.), (ii) transfer to a medium with 2% horse serum (2% HS), and (iii) transfer to a serum-free medium (Ser. Free). Constructs deleted for (w/o) the FoxO binding sites were used as a negative control. (C) Serum withdrawal did not provoke induction of the reporter due to a massive induction of apoptosis.
Cyclic GMP-dependent kinase I is activated during myoblast differentiation.
It has been demonstrated previously that a spike of the second messenger cGMP is observed prior to myoblast fusion (9). Using C2C12 cells as a model, we followed the timing of the expression of FoxO1a and cGKIα during differentiation and compared this with phosphorylation of a known cGKI target, the cytoskeletal protein VASP, by analyzing the status of VASP phosphorylation on serine-239, a consensus cGKI site (33). As expected (5), we observed a rapid increase in the steady-state levels of FoxO1a, which was immediately followed by a subsequent increase in cGKI levels, and then by increases in the muscle cell differentiation markers myogenin and myosin heavy chain and in muscle cell fusion (Fig. 4). Interestingly, after 3 days, the levels of FoxO1a rapidly diminished, whereas high levels of cGKI were sustained in differentiated myocytes; therefore, FoxO1a activity is required to initiate, but not sustain, cGKI expression during myoblast differentiation (Fig. 4). Further, while there was no detectable phospho-VASP-S239 in proliferating myoblasts, there was a striking increase in the phosphorylation of VASP on serine-239 2 days after the induction of differentiation, and this peaked at 3 days and declined thereafter (Fig. 4). These findings are consistent with a model where FoxO1a activity is required to induce cGKI expression, and cGKI activity is then induced by a spike of cGMP and is associated with subsequent down-regulation of FoxO1a expression. Since dominant-active FoxO1a greatly augments myoblast fusion (5), such a scenario would fine-tune FoxO1a activity to properly coordinate muscle cell fusion.
Cyclic GMP-dependent kinase I phosphorylates FoxO1a.
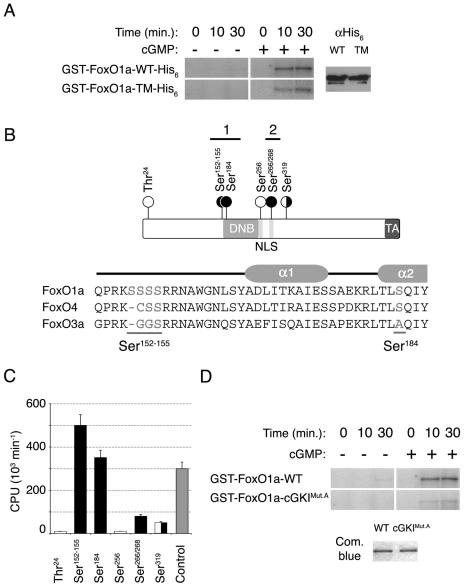
The timing of cGKIα expression and activity, and subsequent down-regulation of FoxO1a, during muscle cell differentiation and fusion suggested that FoxO1a itself might be a target of cGKIα. To address whether cGKIα might phosphorylate FoxO1a, we tested whether purified recombinant FoxO1a was phosphorylated by cGKIα. Indeed, in vitro kinase assays demonstrated that FoxO1a was robustly phosphorylated by cGKIα (Fig. 5A). Known kinases that phosphorylate FoxO1a include Akt, which phosphorylates FoxO1a on Thr24, Ser256, or Ser319 (22), but there is little evidence for Akt modifications of FoxO1a playing an important role during muscle cell differentiation, since Akt is not activated during myoblast cell differentiation, and dominant-negative Akt does not affect FoxO1 levels, phosphorylation, localization, or transcriptional activity, nor does it affect myoblast cell differentiation (5). Indeed, the dominant-active FoxO1a-TM mutant, which harbors alanine substitutions at these three Akt phosphorylation sites, was phosphorylated equally well by cGKIα (Fig. 5A). To define which regions of FoxO1a were phosphorylated by cGKIα, protein fragments covering the entire FoxO1a amino acid sequence were used as substrates in in vitro kinase assays with cGKIα. These analyses showed that fragments containing Thr24, Ser256, or Ser319 were not significantly phosphorylated by cGKIα (Fig. 5B and C), whereas fragments containing regions 1 and 2 were excellent phosphorylation substrates for cGKIα (Fig. 5C). To pinpoint the positions of these phosphorylation sites, we then used peptides spanning these two domains, using 9- to 12-mers or their nonphosphorylatable counterparts (with serine or threonine residues replaced by alanine) as in vitro cGKI substrates (data not shown). Peptides from region 1 containing Ser152-155 or Ser184 were robust cGKI substrates. Interestingly, the Ser152-155 residues are unique to FOXO1a (not present in FOXO3 and FOXO4a) and are located immediately N-terminal to the DNA binding domain, whereas Ser184 lies in the center of the second α-helix of the FoxO1a DNA binding domain and is absent in FOXO3 (Fig. 5B). A peptide containing Ser266 and Ser268, residing in the center of the second nuclear localization signal (NLS) of FoxO1a, a phosphorylation hot spot of FoxO transcription factors (34), was also weakly phosphorylated by cGKI (Fig. 5B and C). Therefore, FoxO1a can be phosphorylated by cGKIα.
FIG. 5.
cGKIα phosphorylates FoxO1a in vitro. (A) Full-length GST/His6 double-tagged wild-type (WT) FoxO1a and FoxO1a-TM show similar levels of in vitro phosphorylation by cGKIα. Equal amounts of protein were used in the kinase assays, as assessed by anti-His6 immunoblotting. cGKIα did not phosphorylate GST alone (data not shown). (B) Schematic of the FoxO1a transcription factor shows the locations of the residues phosphorylated by cGKIα (black circles) and Akt (white circles). The Forkhead DNA binding domain (DNB), NLS, and transactivation domain (TA) are also indicated (not drawn to scale). A sequence alignment of the N-terminal portions of the DNA binding domains of the three FoxO transcription factors is also shown. Residues phosphorylated by cGKIα are underlined. A schematic of helices α1 and α2 of the Forkhead DNA binding domain is shown above the sequences. (C) Quantification of in vitro phosphorylation of FoxO1a peptides. The bar graph shows the identified cGKIα sites (black) as well as Akt sites (white), in comparison with a positive-control peptide that is a substrate for cGKIα (gray). Means are given for at least three independent experiments, each performed in triplicate with each peptide. Error bars show the variance at each data point. (D) Equal amounts of full-length GST-tagged wild-type FoxO1a and FoxO1a-cGKIMut.A (Ser152-155/184Ala) were phosphorylated in vitro by cGKIα. Note the marked reduction in phosphorylation of FoxO1a-cGKIMut.A compared to that of wild-type FoxO1a, demonstrating that these serine residues are the major targets of cGKIα.
Phosphorylation of FoxO1a by cyclic GMP-dependent kinase I inhibits its DNA binding activity.
To evaluate the functional consequences of FoxO1a phosphorylation by cGKIα, FoxO1a mutants harboring nonphosphorylatable or phosphomimetic amino acid substitutions were generated. FoxO1a mutants with Ser266, Ser268, or Ser266/268 mutations showed no defects in nuclear-cytoplasmic shuttling or nuclear localization when expressed in differentiating primary myoblasts, and these cells also displayed no alterations in their rates of growth or differentiation or myoblast cell fusion (data not shown). However, we reasoned that cGKI phosphorylation sites in and around the FoxO1a DNA binding domain, Ser152-155 and Ser184, could have a major impact on its function, in view of the fact that phosphorylation of Ser256 by Akt appears to initiate a phosphorylation cascade that disables FoxO1a DNA binding activity and provokes its nuclear export and destruction by the proteasome (22). First, we confirmed that FoxO1a protein lacking these sites (FoxO1a-cGKIMut.A, where Ser152-155/184 were replaced by alanine) was poorly phosphorylated by cGKIα in vitro (Fig. 5D); therefore, these serines are the major cGKIα phosphorylation sites.
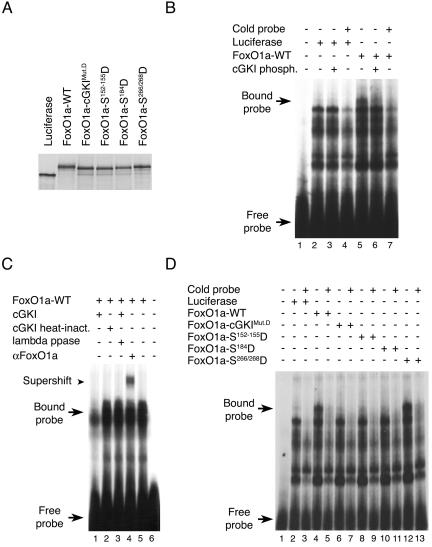
We next assessed the effects of cGKI phosphorylation on the ability of FoxO1a-cGKIMut.A to bind to an oligonucleotide containing a consensus FoxO1a DNA binding site (13). Importantly, while in vitro-translated FoxO1a readily bound to its cognate DNA binding site in EMSA, FoxO1a phosphorylated by cGKIα in vitro did not (Fig. 6A and B). To exclude the possibility that the cGKIα interaction might simply mask the FoxO1a DNA binding domain, we also tested whether heat-inactivated cGKIα blocked the ability of FoxO1a to bind to its recognition element. This was not the case, and furthermore, treatment of FoxO1a that had been previously phosphorylated by cGKIα with lambda phosphatase restored the ability of FoxO1a to bind to its recognition element (Fig. 6C). The inactivation of cGKI and the effectiveness of lambda phosphatase were confirmed using in vitro kinase assays (data not shown). Moreover, the phosphomimetic FoxO1a mutants FoxO1a-cGKIMut.D (FoxO1a-Ser152-155/184Asp), FoxO1a-Ser152-155Asp, and FoxO1a-Ser184Asp totally lacked DNA binding activity, while the FoxO1a-Ser266/268Asp mutant retained DNA binding activity (Fig. 6D). In addition, nonphosphorylatable versions of FoxO1a, both FoxO1a-TM and FoxO1a-cGKIMut.A, did not show any reduction in their DNA binding activity by EMSA after exposure to active cGKIα kinase (data not shown). Finally, the specific FoxO1a band shift could be competed by adding excess cold probe (Fig. 6B and D) and was supershifted by a FoxO1a-specific antibody (Fig. 6C). Therefore, cGKIα-mediated phosphorylation of FoxO1a abolishes its DNA binding activity.
FIG. 6.
Phosphorylation of FoxO1a by cGKIα abolishes FoxO1a DNA binding activity. (A) Production of equal amounts of in vitro-translated wild-type (WT) FoxO1a and FoxO1a mutants. (B) EMSA showing that DNA binding of FoxO1a is abolished upon phosphorylation (phosph.) by cGKIα. In vitro-translated luciferase was used as a negative control. (C) EMSA showing that DNA binding of in vitro-translated FoxO1a-WT is supershifted by binding to an anti-FoxO1a antibody. Heat inactivation (inact.) of cGKIα abolished its ability to prevent FoxO1a binding. In addition, FoxO1a phosphorylated by cGKIα and then treated with lambda phosphatase was capable of binding to its recognition element. (D) EMSA showing complete lack of DNA binding of FoxO1a-cGKIMut.D (Ser152-155/184Asp), FoxO1a-Ser152-155Asp, and FoxO1a-Ser184Asp, whereas DNA binding by FoxO1a-Ser266/268Asp was similar to that of wild-type FoxO1a. The FoxO1a-specific band shift was eliminated by addition of excess cold competitor oligonucleotide. The luciferase control shows no binding.
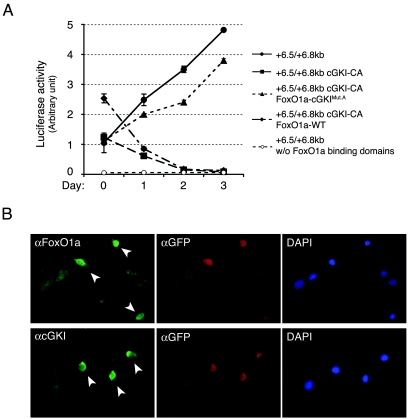
Together these findings suggested that cGKIα kinase activity might impair FoxO1a functions in directing muscle cell fusion. To test this notion, C2C12 cells were transiently transfected with a constitutively active form of cGKI (cGKI-CA, where the N-terminal cGMP regulatory domain has been deleted [21]). Constitutively active cGKI impaired the activation of the FoxO1a-responsive +6.5/+6.8-kb cGKIα luciferase reporter after induction of differentiation (Fig. 7A). By contrast, cGKI-CA failed to impair the induction of this promoter-reporter when cells were cotransfected with a vector expressing FoxO1a-cGKIMut.A (the cGKI unphosphorylatable form of FoxO1a) (Fig. 7A). As expected, the +6.5/+6.8-kb luciferase reporter deleted for the FoxO binding sites failed to show any activity and also was not affected by overexpression of FoxO1a or cGKI-CA (Fig. 3B and 7A; also data not shown). Identical findings were obtained by evaluating the regulation of a synthetic promoter-reporter that contained concatemerized FoxO1a binding sites upstream of a minimal promoter (data not shown) (5). We were unable to assess the long-term effects of forced overexpression of cGKI-CA on muscle cell differentiation or fusion, since it was toxic in both primary myoblasts and C2C12 cells. However, immunofluorescence analyses of C2C12 cells transiently expressing cGKI-CA suggested that activated cGKIα had rather dramatic effects on the morphology of these cells also overexpressing FoxO1a, with reductions in overall cell size relative to nontransduced cells (Fig. 7B). In addition, cells expressing cGKI-CA showed an accumulation of FoxO1a in the cytoplasm compared to untransfected cells (Fig. 7B), consistent with a model in which cGKIα-mediated phosphorylation of FoxO1a at residues within and around its DNA binding domain inhibits its binding to DNA.
FIG. 7.
In vivo inactivation of FoxO1a transcriptional activity by a constitutively active form of cGKIα. (A) Luciferase activity in proliferating or differentiating C2C12 myoblasts is shown after transient transfection with a luciferase reporter containing the +6.5/+6.8-kb cGKIα enhancer element alone or in the presence of cGKI-CA, wild-type FoxO1a (FoxO1a-WT) plus cGKI-CA, or FoxO1a-cGKIMut.A plus cGKI-CA. cGKI-CA does not inhibit the transcriptional up-regulation of the reporter by FoxO1a-cGKIMut.A, while it completely inhibits the up-regulation by endogenous or overexpressed FoxO1a-WT. The +6.5/+6.8-kb cGKIα enhancer element without (w/o) the FoxO binding sites is shown as a negative control. (B) Immunofluorescence of cGKI-CA-transduced C2C12 cells shows a reduction in overall cell size and dramatic changes in morphology as well as cytoplasmic accumulation of FoxO1a. Transduced cells are indicated by arrowheads, while wild-type cells are not. An anti-GFP antibody demarcates transduced cells. 4′,6′-Diamidino-2-phenylindole (DAPI) staining is used to show the cell nucleus.
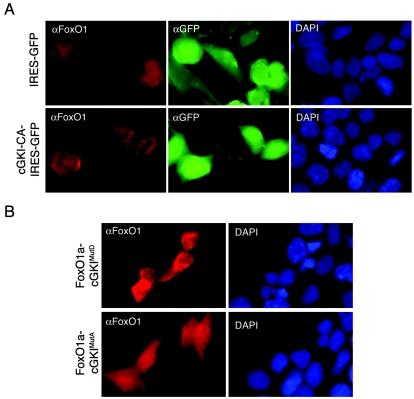
To confirm the effects of activated cGKIα on FoxO1a localization, 293T cells, which are resistant to the toxic effects of cGKI-CA, were transiently transfected with FoxO1a with or without cGKI-CA. In cells expressing FoxO1a alone, FoxO1a was distributed throughout the cell, but in those also overexpressing cGKI-CA, there was a clear exclusion of FoxO1a from the nucleus (Fig. 8A). These findings predicted that there would be clear differences in subcellular localization between the phosphomimetic FoxO1a-cGKIMutD mutant and the non-cGKIα-phosphorylatable mutant. Indeed, these experiments showed that that the alanine mutant FoxO1a-cGKIMutA was localized throughout the cell whereas the aspartic acid mutant FoxO1a-cGKIMutD was excluded from nuclei (Fig. 8B). Thus, cGKIα modifies FoxO1a functions at two levels, by directly impairing its ability to bind to its recognition element and by relocalizing FoxO1a into the cytoplasm, events that are both consistent with an important role for cGKIα in harnessing FoxO1a-driven myoblast fusion.
FIG. 8.
cGKI phosphorylation provokes exclusion of FoxO1a from the nuclei of 293T cells. (A) Immunofluorescence analyses of 293T cells stably expressing cGKI-CA and then transduced with a FoxO1a-expressing retrovirus show cytoplasmic accumulation of FoxO1A. GFP fluorescence demarks transduced cells. 4′,6′-Diamidino-2-phenylindole (DAPI) staining reveals cell nuclei. (B) Localization of FoxO1a in 293T cells expressing FoxO1a mutated at the cGKI phosphorylation sites. FoxO1a-cGKIMutD-expressing (phosphomimetic) cells show predominantly cytoplasmic staining, while FoxO1a-cGKIMutA (nonphosphorylatable) expression is diffuse, similar to that in cells overexpressing wild-type FoxO1a. DAPI staining again demarks all nuclei in the field.
Cyclic GMP-dependent kinase I-deficient primary myoblasts display excessive cell fusion.
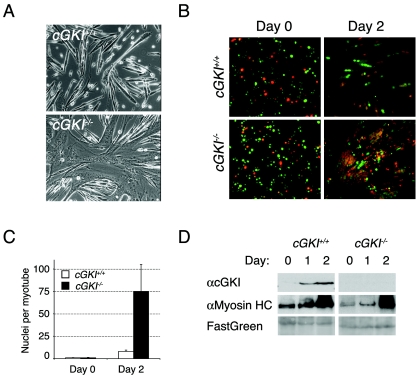
The timing of FoxO1a-dependent expression of cGKIα, together with the pre-fusion spike in the levels of intracellular cGMP that accompanies muscle cell differentiation (9), should trigger cGKIα kinase activity. In turn, cGKIα should then phosphorylate FoxO1a to harness its activity and control rates of myoblast fusion. To confirm that cGKI is an essential regulator of muscle fusion, we isolated P6 primary myoblasts from cGKI−/− mice and compared their ex vivo growth and differentiation characteristics with those of myoblasts of wild-type littermates. cGKI−/− myoblasts showed no difference in size or rate of proliferation (data not shown). However, within 2 days following the induction of differentiation, cGKI−/− myoblasts showed a markedly accelerated rate of fusion, with some myotubes containing well over 75 nuclei (Fig. 9A and C). This phenotype is strikingly similar to that of myoblasts expressing dominant-active FoxO1a-TM (5).
FIG. 9.
cGKI−/− primary myoblasts show an accelerated rate of myoblast fusion upon differentiation. (A) Representative photomicrographs of differentiated (day-2) cGKI+/+ and cGKI−/− primary myoblasts. cGKI−/− myoblasts display excessive fusion compared to control myoblasts and are similar to myoblasts engineered to overexpress dominant-active FoxO1a (5). (B) CellTracker probe analysis shows excessive fusion between red- and green-stained cGKI−/− myoblasts after 2 days of differentiation. (C) The number of nuclei per cell was counted at day 0 and day 2 for cGKI+/+ (white bars) and cGKI−/− (black bars) myoblasts. Means of at least three independent fields are given for each genotype. Error bars show the variance at each data point. (D) Western blot analyses of primary cGKI+/+ and cGKI−/− myoblasts showed that loss of cGKI has no effect on the levels and timing of expression of a late (myosin heavy chain) marker during muscle cell differentiation, nor does it affect expression of FoxO1a. FastGreen-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis membranes were used as loading and transfer controls.
To address whether the large multinucleated myotubes that formed during the differentiation of primary cGKI−/− myoblasts in vitro were the product of deregulated fusion rather than endo-reduplication events, cGKI−/− myoblasts were stained with two different cell tracker dyes, mixed, and then transferred to differentiation medium. All large cGKI−/− myotubes contained two-colored nuclei in an overall 1-to-1 ratio. Therefore, loss of cGKI specifically accelerated the rate of myoblast cell fusion (Fig. 9B), a phenotype remarkably akin to that of myoblasts engineered to overexpress dominant-active FoxO1a (5). Attempts to prove that the accelerated fusion phenotype of cGKI−/− myoblasts was intrinsic were unsuccessful, because overexpression of wild-type cGKIα, like that of cGKI-CA, was toxic to cGKI−/− (or wild-type) primary myoblasts (data not shown). Finally, cGKI loss was not associated with alterations in muscle cell differentiation per se, as evidenced by the facts that the temporal expression of the late muscle-specific marker myosin heavy chain was not perturbed (Fig. 9D) and the overall levels of FoxO1a were unaffected by loss of cGKI (data not shown). Therefore, cGKI functions downstream of FoxO1a to orchestrate myoblast cell fusion.
Conclusions.
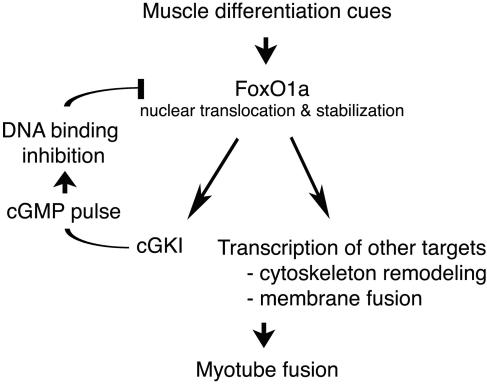
The molecular mechanisms mediating mammalian cell-to-cell fusion events have heretofore been enigmatic. In myoblasts, some of the initial events are now coming into focus with the identification of the FoxO1a-to-cGKIα regulatory pathway. We propose that this pathway acts as an essential rheostat of fusion events by maintaining a critical balance between a transcription factor (FoxO1a) and its negative regulator (cGKIα), resulting in proper fusion and muscle development. In differentiating myoblasts, FoxO1a accumulates in the nucleus and induces the transcription of target genes involved in membrane remodeling and fusion (Fig. 10) (5, 25). Its targets now also include cGKI, a kinase that in turn negatively regulates FoxO1a transcriptional activity. The essential nature of this negative-feedback loop is best illustrated by the in vitro overfusion phenotype observed in cGKI−/− myoblasts, which closely mimics the behavior of myoblasts expressing dominant-active FoxO1a. Following its induction by FoxO1a, cGKIα kinase activity is likely triggered by an essential spike in intracellular cGMP levels that accompanies myoblast cell fusion (9). cGKIα inhibits FoxO1a function in a rather unique manner, by phosphorylating residues that directly inhibit its DNA binding activity. This phosphorylation effectively squelches the transcriptional activity of FoxO1a to ensure the proper production of fusion components during differentiation (Fig. 10). This scenario classifies FoxO1a as an additional regulator of muscle cell differentiation, where it controls not only the expression of key genes involved in cytoskeletal remodeling and fusion (5) but also that of its dedicated negative regulator cGKI. Notably, this program occurs in parallel with those transcriptional programs directed by myogenic basic helix-loop-helix transcription factors (e.g., MyoD, myogenin, Myf4, and Myf6) (24), which are not affected by alterations in FoxO1a or cGKI activity. However, since both pathways are required for proper muscle development, an as yet unknown cross talk mechanism must exist that coordinates muscle cell differentiation and fusion.
FIG. 10.
Model of the FoxO1a-to-cGKIα regulatory feedback loop controlling myoblast fusion. Upon differentiation, FoxO1a activates cGKIα transcription as well as other target genes to set the stage for myoblast cell fusion. Upon the spike of cGMP secondary messenger, FoxO1a activity is inhibited by cGKIα phosphorylation, necessary for controlled cell fusion.
Acknowledgments
We thank Robert Feil and Franz Hofmann for providing the cGKI knockout mice.
This research was supported in part by NCI grant CA-71907, Cancer Center (CORE) Support Grant CA-21765, a grant from the Van Vleet Foundation, and the American Lebanese Syrian Associated Charities (ALSAC) of St. Jude Children's Research Hospital. P.R.J.B. is a fellow of the Van Vleet Foundation of Memphis, Tenn.
REFERENCES
- 1.Accili, D., and K. C. Arden. 2004. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117:421-426. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 2001. Current protocols in molecular biology. John Wiley and Sons, Inc., New York, N.Y.
- 3.Birkenkamp, K. U., and P. J. Coffer. 2003. Regulation of cell survival and proliferation by the FOXO (Forkhead box, class O) subfamily of Forkhead transcription factors. Biochem. Soc. Trans. 31:292-297. [DOI] [PubMed] [Google Scholar]
- 4.Blumenthal, R., M. J. Clague, S. R. Durell, and R. M. Epand. 2003. Membrane fusion. Chem. Rev. 103:53-69. [DOI] [PubMed] [Google Scholar]
- 5.Bois, P. R., and G. C. Grosveld. 2003. FKHR (FOXO1a) is required for myotube fusion of primary mouse myoblasts. EMBO J. 22:1147-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brindle, N. P., M. R. Holt, J. E. Davies, C. J. Price, and D. R. Critchley. 1996. The focal-adhesion vasodilator-stimulated phosphoprotein (VASP) binds to the proline-rich domain in vinculin. Biochem. J. 318:753-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgering, B. M., and G. J. Kops. 2002. Cell cycle and death control: long live Forkheads. Trends Biochem. Sci. 27:352-360. [DOI] [PubMed] [Google Scholar]
- 8.Castrillon, D. H., L. Miao, R. Kollipara, J. W. Horner, and R. A. DePinho. 2003. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 301:215-218. [DOI] [PubMed] [Google Scholar]
- 9.Choi, S. W., M. Y. Baek, and M. S. Kang. 1992. Involvement of cyclic GMP in the fusion of chick embryonic myoblasts in culture. Exp. Cell Res. 199:129-133. [DOI] [PubMed] [Google Scholar]
- 10.Coleman, M. L., and M. F. Olson. 2002. Rho GTPase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ. 9:493-504. [DOI] [PubMed] [Google Scholar]
- 11.Fernando, P., J. F. Kelly, K. Balazsi, R. S. Slack, and L. A. Megeney. 2002. Caspase 3 activity is required for skeletal muscle differentiation. Proc. Natl. Acad. Sci. USA 99:11025-11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuyama, T., K. Kitayama, Y. Shimoda, M. Ogawa, K. Sone, K. Yoshida-Araki, H. Hisatsune, S. I. Nishikawa, K. Nakayama, K. Ikeda, N. Motoyama, and N. Mori. 2004. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J. Biol. Chem. 279:34741-34749. [DOI] [PubMed] [Google Scholar]
- 13.Furuyama, T., T. Nakazawa, I. Nakano, and N. Mori. 2000. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem. J. 349:629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallo, R., M. Serafini, L. Castellani, G. Falcone, and S. Alema. 1999. Distinct effects of Rac1 on differentiation of primary avian myoblasts. Mol. Biol. Cell 10:3137-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gudi, T., J. C. Chen, D. E. Casteel, T. M. Seasholtz, G. R. Boss, and R. B. Pilz. 2002. cGMP-dependent protein kinase inhibits serum-response element-dependent transcription by inhibiting rho activation and functions. J. Biol. Chem. 277:37382-37393. [DOI] [PubMed] [Google Scholar]
- 16.Hall, A., and C. D. Nobes. 2000. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos. Trans. R. Soc. Lond. B 355:965-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12:627-661. [DOI] [PubMed] [Google Scholar]
- 18.Hosaka, T., W. H. Biggs III, D. Tieu, A. D. Boyer, N. M. Varki, W. K. Cavenee, and K. C. Arden. 2004. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc. Natl. Acad. Sci. USA 101:2975-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hribal, M. L., J. Nakae, T. Kitamura, J. R. Shutter, and D. Accili. 2003. Regulation of insulin-like growth factor-dependent myoblast differentiation by Foxo forkhead transcription factors. J. Cell Biol. 162:535-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu, M. C., D. F. Lee, W. Xia, L. S. Golfman, F. Ou-Yang, J. Y. Yang, Y. Zou, S. Bao, N. Hanada, H. Saso, R. Kobayashi, and M. C. Hung. 2004. IκB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 117:225-237. [DOI] [PubMed] [Google Scholar]
- 21.Landgraf, W., and F. Hofmann. 1989. The amino terminus regulates binding to and activation of cGMP-dependent protein kinase. Eur. J. Biochem. 181:643-650. [DOI] [PubMed] [Google Scholar]
- 22.Matsuzaki, H., H. Daitoku, M. Hatta, K. Tanaka, and A. Fukamizu. 2003. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc. Natl. Acad. Sci. USA 100:11285-11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills, J. C., N. L. Stone, J. Erhardt, and R. N. Pittman. 1998. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J. Cell Biol. 140:627-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molkentin, J. D., and E. N. Olson. 1996. Defining the regulatory networks for muscle development. Curr. Opin. Genet. Dev. 6:445-453. [DOI] [PubMed] [Google Scholar]
- 25.Nishiyama, T., I. Kii, and A. Kudo. 2004. Inactivation of Rho/ROCK signalling is crucial for the nuclear accumulation of FKHR and myoblast fusion. J. Biol. Chem. 279:47311-47319. [DOI] [PubMed] [Google Scholar]
- 26.Parker, P. J., and S. J. Parkinson. 2001. AGC protein kinase phosphorylation and protein kinase C. Biochem. Soc. Trans. 29:860-863. [DOI] [PubMed] [Google Scholar]
- 27.Pfeifer, A., P. Ruth, W. Dostmann, M. Sausbier, P. Klatt, and F. Hofmann. 1999. Structure and function of cGMP-dependent protein kinases. Rev. Physiol. Biochem. Pharmacol. 135:105-149. [DOI] [PubMed] [Google Scholar]
- 28.Powell, W. C., B. Fingleton, C. L. Wilson, M. Boothby, and L. M. Matrisian. 1999. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr. Biol. 9:1441-1447. [DOI] [PubMed] [Google Scholar]
- 29.Qu, G., H. Yan, and A. R. Strauch. 1997. Actin isoform utilization during differentiation and remodeling of BC3H1 myogenic cells. J. Cell. Biochem. 67:514-527. [PubMed] [Google Scholar]
- 30.Rando, T. A., and H. M. Blau. 1994. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 125:1275-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinhard, M., M. Rudiger, B. M. Jockusch, and U. Walter. 1996. VASP interaction with vinculin: a recurring theme of interactions with proline-rich motifs. FEBS Lett. 399:103-107. [DOI] [PubMed] [Google Scholar]
- 32.Sabourin, L. A., and M. A. Rudnicki. 2000. The molecular regulation of myogenesis. Clin. Genet. 57:16-25. [DOI] [PubMed] [Google Scholar]
- 33.Smolenski, A., C. Bachmann, K. Reinhard, P. Honig-Liedl, T. Jarchau, H. Hoschuetzky, and U. Walter. 1998. Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J. Biol. Chem. 273:20029-20035. [DOI] [PubMed] [Google Scholar]
- 34.Tran, H., A. Brunet, E. C. Griffith, and M. E. Greenberg. 2003. The many forks in FOXO's road. Sci. STKE 2003:RE5. [DOI] [PubMed] [Google Scholar]
- 35.Wegener, J. W., H. Nawrath, W. Wolfsgruber, S. Kuhbandner, C. Werner, F. Hofmann, and R. Feil. 2002. cGMP-dependent protein kinase I mediates the negative inotropic effect of cGMP in the murine myocardium. Circ. Res. 90:18-20. [DOI] [PubMed] [Google Scholar]
- 36.Yagami-Hiromasa, T., T. Sato, T. Kurisaki, K. Kamijo, Y. Nabeshima, and A. Fujisawa-Sehara. 1995. A metalloprotease-disintegrin participating in myoblast fusion. Nature 377:652-656. [DOI] [PubMed] [Google Scholar]