Abstract
SIT is a transmembrane adapter protein that modulates signals emanating from the T-cell receptor (TCR). Here, we have used gene-targeted mice to assess the role of SIT for T-cell development and peripheral T-cell functions. SIT−/− double-positive thymocytes show an upregulation of the activation markers CD5 and CD69, suggesting that SIT negatively regulates TCR-mediated signals at the CD4+ CD8+ stage of thymic development. This assumption is further supported by the observation that in female H-Y TCR transgenic mice, positive selection is enhanced and even converted to negative selection. Similarly, mature peripheral T cells are hyperresponsive towards TCR-mediated stimuli and produce larger amounts of T-helper 1 (TH1) cytokines, and SIT-deficient mice show an increased susceptibility to develop experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis. These results demonstrate that SIT is a critical negative regulator of TCR-mediated signaling and finely tunes the signals required for thymic selection and peripheral T-cell activation.
The recognition of specific antigen/major histocompatibility complex (MHC) complexes by the clonotypic T-cell receptor (TCR) initiates a number of signaling cascades which ultimately culminate in gene transcription. The proper function of these signal transduction pathways requires the coordinated action of many molecules. During the last years, the understanding of the membrane-proximal signaling events leading to T-cell activation has significantly advanced. For example, it has been demonstrated that the activation of protein tyrosine kinases belonging to the Src, Syk, and Tec families are among the earliest biochemical events that initiate cellular activation upon antigen recognition (reviewed in references 12 and 37) Also, major progress was made in elucidating how Src and Syk kinase-mediated signals are further processed to induce appropriate cellular responses. In this regard, the identification of a novel group of integral membrane molecules named transmembrane adapter proteins (TRAPs) was a major achievement (18).
All TRAPs known to date have a common molecular structure. They possess very short extracellular domains and comparatively long cytoplasmic tails containing several (up to 10) tyrosine-based signaling motifs (TBSMs) (18). After immunoreceptor engagement, the core tyrosine residues within the TBSMs become rapidly phosphorylated and thereby serve as docking sites for cytoplasmic signaling proteins carrying SH2 domains. By recruiting SH2 domain-containing proteins to the plasma membrane, the TRAPs function as molecular biochips which sort and coordinately propagate externally applied signals into the cell. Thus, the identification of the TRAPs has clarified the question of how signal-transducing receptors such as the TCR are connected with intracellular signaling pathways.
Presently, the family of TRAPs includes LAT, LAX, LIME, NTAL/LAB, PAG/Cbp, SIT, and TRIM. LAT represents the most well-characterized transmembrane adapter protein and appears to be primarily involved in positively regulating TCR-mediated signaling. By recruiting cytoplasmic signaling molecules such as Grb2, GADS, SLP-76, and phospholipase C-γ to the plasma membrane, LAT assembles a signaling complex that is required for coupling the T-cell receptor to the major intracellular signaling pathways leading to transcription of the T-cell growth factor interleukin-2 (16, 34, 48). The generation of LAT-deficient mice has further revealed the indispensable role of this transmembrane adapter protein for T-cell development (49).
Conversely to LAT, the transmembrane adapter proteins LAX and PAG seem to exert negative regulatory functions on TCR-mediated signaling either by recruiting the most important negative regulator of Src kinase activity, the cytosolic protein tyrosine kinase Csk, to the plasma membrane (PAG) (8, 22, 43) or by inhibiting TCR-mediated activation of p38 mitogen-activated protein kinase (LAX) (50).
In addition to the regulation of antigen receptor signaling, it appears that some transmembrane adapters may regulate signals initiated after coreceptor engagement. This has most recently been shown for the lipid raft-associated transmembrane adapter protein LIME, which exclusively becomes phosphorylated after external engagement of the CD4 or CD8 coreceptors (9, 21). However, the biological relevance of this phosphorylation has not yet been revealed.
Two transmembrane adapters, TRIM and SIT, differ from the other TRAPs known so far as they represent disulfide-linked homodimers. TRIM associates with the TCR complex, preferentially through an interaction with the TCR-ζ chain, and when overexpressed in Jurkat T cells, TRIM inhibits the spontaneous internalization of the TCR (10, 26). However, the precise function that TRIM plays during T-cell activation remains elusive.
SIT has been reported to act as a modulator of TCR-mediated signaling (29, 35). Indeed, when overexpressed in Jurkat T cells, wild-type (wt) SIT exerts strong negative regulatory effects upon TCR-mediated activation of the transcription factor NF-AT (29, 35) This inhibitory effect seems to be exclusively mediated via a single TBSM within the SIT cytoplasmic domain (YASV) that binds a still-to-be-defined ligand. In contrast, overexpression of a SIT mutant lacking the YASV motif strongly amplifies TCR signals. This effect is mediated via another tyrosine-based signaling motif, YGNL, which seems to control a Grb2-mediated signaling pathway (35). Collectively, the currently available data suggest that SIT serves as a multifunctional adapter that finely tunes signals emanating from the TCR.
The aim of the present study was to assess the role of SIT during T-cell development and T-cell activation using an in vivo model. To this end, we generated SIT-deficient mice. Our data confirm that SIT primarily acts as a negative regulator of TCR-mediated signals and sets signaling thresholds necessary for positive selection within the thymus. In addition, SIT seems to be involved in the maintenance of peripheral T-cell homeostasis, as demonstrated by the hypersensitivity of SIT-deficient T cells in response to TCR stimulation and increased susceptibility of SIT-deficient mice to autoimmunity in a murine model of multiple sclerosis.
MATERIALS AND METHODS
Mice.
The targeting construct was transfected into 129-derived embryonic stem cells. Targeted embryonic stem clones were selected and processed as previously described (2). Positive clones were injected into C57BL/6 blastocysts. Animals containing the disrupted SIT allele were crossed onto C57BL/6 (Charles River) for more than 10 generations. Genotype was determined by PCR using a mixture of three primers, 5′ SIT (CCT GAC TCT CAC ACC AGC AGC), 3′ SIT (GGT CCA CTG GGA CAA GAG TGC AGC C), and 3′ NEO (GAC GTG CTA CTT CCA TTT GTC ACG TCC) (BioTeZ Berlin-Buch GmbH). OT-I and OT-II TCR transgenic (tg) mice were kindly provided by Percy Knolle, P14 mice were provided by Thomas Kammerthoens, and H-Y TCR transgenic mice were kindly provided by Gary Koreztky. For TCR transgenic studies, SIT−/− mice × TCR tg mice were obtained by crossing SIT−/− mice with SIT+/− mice for TCR heterozygote transgenics. All mice analyzed were between 5 and 10 weeks of age.
Flow cytometry.
For each sample, 1 × 106 cells were stained with antibodies and analyzed on a FACSCalibur using the CellQuest software (Becton Dickinson). All antibodies were purchased from BD Biosciences except for H-Y-fluorescein isothiocyanate (clone T3.70; eBioscience). For flow cytometric determination of extracellular signal-regulated kinase (ERK) activation, thymocytes were surface labeled with anti-CD4-CyChr and anti-CD8-phycoerythrin, fixed, and permeabilized in phosphate-buffered saline (PBS) containing 3.7% formaldehyde and 0.2% saponin for 15 min at room temperature. After being washed in PBS containing 0.1% saponin, 0.1% NaN3, and 0.2% BSA/c, cells were stained with anti-phospho-p44/42 mitogen-activated protein kinase (Cell Signaling) in PBS supplemented with bovine serum albumin (10 mg/ml), 0.2% BSA/c, 0.1% saponin, and 0.1% NaN3 for 45 min at room temperature. Bound antibody was detected with fluorescein isothiocyanate-conjugated anti-rabbit immunoglobulin G (IgG) (Jackson ImmunoResearch Laboratories).
Apoptosis assay.
Thymocytes (5 × 105) were cultured in 96-well flat-bottomed plates coated with 10 μg/ml anti-CD3 (145-2C11; BD Biosciences) or 10 μg/ml anti-CD3 plus 10 μg/ml anti-CD28 (37.51; BD Biosciences). For concanavalin A (ConA) stimulation, a final concentration of 1 μg/ml was used. Samples were then incubated at 37°C for 24 h before analysis. After harvesting, the living cells were analyzed by forward scatter and side scatter flow cytometry parameters. Cells were also harvested, washed, and stained with annexin V and propidium iodide according to the manufacturer's instructions (Bender MedSystems).
Proliferation assay and cytokine determination.
T-cell purification was performed using a Pan T-cell Isolation kit and an AutoMacs magnetic separation system (Miltenyi Biotec). Purified T cells (2.5 × 104 cells/well) were cultured in RPMI medium (supplemented with 10% fetal calf serum, antibiotics, and 2-β-mercaptoethanol) in a U-bottomed 96-well plate (Costar) in the presence of plate-bound anti-mouse CD3ɛ (145-2C11; BD Biosciences) or phorbol myristate acetate (PMA) and ionomycin. Cells were cultured for 72 h and labeled with 0.5 μCi/well of [3H]thymidine during the last 6 h. Supernatants from anti-CD3-stimulated T cells were collected after 48 h and assessed for cytokines by cytometric bead array (BD Biosciences).
Induction of active EAE.
Myelin oligodendrocyte glycoprotein (MOG) p35-55, corresponding to the mouse sequence MEVGWYRSPFSRVVHLYRNGK, was synthesized by standard 9-fluorenylmethoxycarbonyl chemistry and purified by high-performance liquid chromatography. Active experimental autoimmune encephalomyelitis (EAE) was induced in 8- to 12-week-old mice by immunization with 200 μg of MOG p35-55 in complete Freund's adjuvant (Sigma-Aldrich, Taufkirchen, Germany) containing 800 μg of killed Mycobacterium tuberculosis (Difco Laboratories, Detroit, MI). In addition, 200 ng of pertussis toxin (List Biological Laboratories, Campbell, CA) dissolved in 200 μl PBS was injected intraperitoneally on the day of immunization and again 2 days postimmunization.
Statistics.
Statistical analyses were performed using GraphPad Prism (GraphPad Software Inc., San Diego, CA). P values were determined by an unpaired two-tailed Student's t test.
RESULTS
SIT is expressed predominantly in thymocytes.
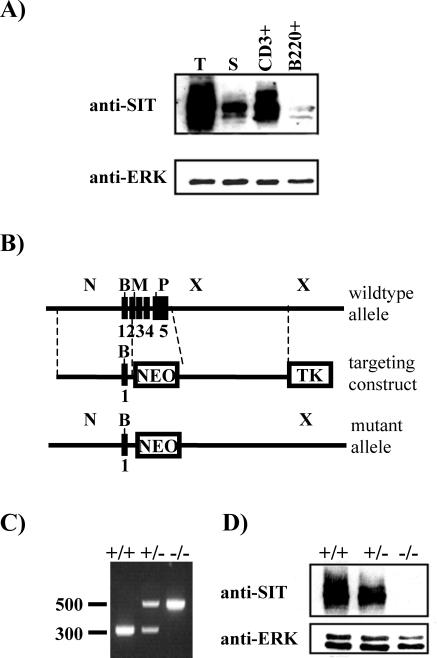
Previously, we have shown that SIT is selectively expressed in lymphoid cell lines (29). To assess the expression of SIT in primary cells from different murine lymphoid organs, anti-SIT Western blotting was performed. As shown in Fig. 1A, the highest levels of SIT protein expression are detectable in the thymus, and lower levels are detected in spleen. We next analyzed whether SIT expression is different in T lymphocytes compared to B lymphocytes and whether immature T cells express different amounts of SIT than mature T cells. Figure 1A shows that SIT expression is much higher in purified CD3+ splenic T cells than in B220+ B cells. In addition, the expression of SIT within the thymus (containing approximately 80% immature T cells) is much higher than in mature splenic T cells. Conversely, SIT is not expressed in the Gr-1+ myeloid lineage in the bone marrow (data not shown). In conclusion, SIT is expressed at the highest levels in immature thymocytes.
FIG. 1.
Expression of SIT in mouse lymphocytes and generation of SIT-deficient mice. (A) Lysates prepared from freshly isolated thymocytes (T), splenocytes (S), purified splenic T cells (CD3+), or purified splenic B cells (B220+) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted with an anti-SIT MAb. To demonstrate equal amounts of protein loading, the blot was stripped and reprobed with an anti-Erk polyclonal antibody. (B) Partial restriction map of the SIT locus and the targeting cassette. Filled boxes represent exons. Restriction sites are indicated as follows: N, NotI; B, BamHI; M, MluI; P, PstI; X, XbaI. Abbreviations: NEO, neomycin resistance cassette; TK, thymidine kinase. (C) PCR analysis of SIT wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mutant alleles. (D) Anti-SIT Western blot analysis showing absence of SIT protein in SIT−/− mice. Postnuclear lysates were prepared from thymocytes, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and blotted with an anti-SIT MAb. The blot was successively stripped and reprobed with an anti-ERK1/2 antibody as a loading control.
SIT−/− mice show normal B-cell but altered T-cell development.
To elucidate the function of SIT within the immune system, we generated SIT-deficient mice by homologous recombination. The SIT gene was disrupted in embryonic stem cells by using a standard gene-targeting strategy. As shown in Fig. 1B, the entire coding sequence of the SIT gene (19) was replaced with a neomycin resistance cassette, and the expected mutation was confirmed by PCR (Fig. 1C) and anti-SIT Western blotting (Fig. 1D).
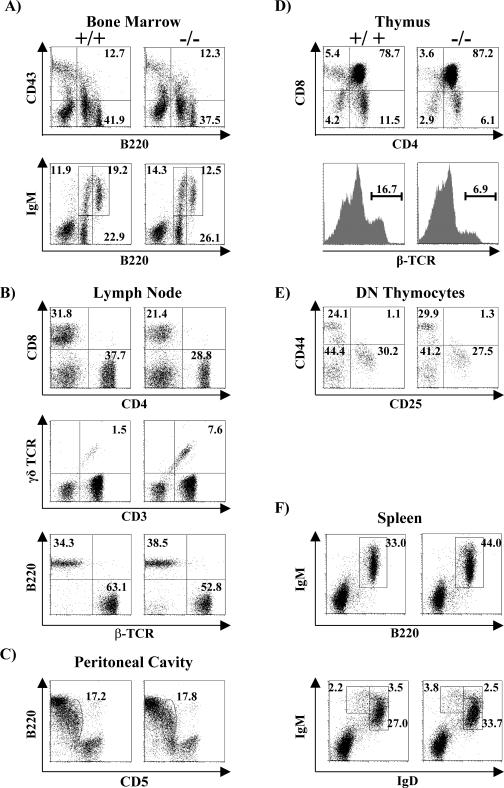
SIT-deficient mice are born at the expected Mendelian frequency, and they are viable, are fertile, show normal growth, and present no obvious abnormalities. Flow cytometry analysis further revealed that the distribution of B-cell subsets in the bone marrow, spleen, and lymph nodes is normal (Table 1 and Fig. 2). B-cell precursor fractions such as pro-B cells (B220+ CD43+), immature B cells (B220+ IgM+), and recirculating mature B cells (B220high IgM+) from SIT−/− bone marrow are comparable to those of wt mice (Fig. 2A). In the spleen, the total B-cell distribution (B220+ IgM+) as well as the percentages of B-cell subpopulations such as immature (IgMhigh IgDlow), transitional (IgMhigh IgDhigh), and mature (IgMlow IgDhigh) are also not affected by SIT deficiency (Fig. 2F). Moreover, B1 lymphocytes (CD5+ B220+) in the peritoneal cavity also show a normal distribution (Fig. 2C). In addition, basal immunoglobulin levels as well as those obtained after immunization of SIT-deficient mice were normal (data not shown). Collectively, these data suggest that SIT is dispensable for normal B-cell development and peripheral B-cell functions.
TABLE 1.
Cell numbers of lymphocyte subpopulations in thymus and lymph nodesa
| Genotype and P value | No. of mice examined | No. (106) of cells ± SD
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymus
|
Lymph nodes
|
|||||||||||
| Total | CD4− CD8− | CD4+ CD8+ | CD4+ CD8− | CD4− CD8+ | Total | CD4+ | CD8+ | γδTCR+ | B220+ | NK1.1+ | ||
| SIT+/+ | 15 | 135.2 ± 34.1 | 3.7 ± 1.7 | 109.2 ± 44.1 | 16.1 ± 6.1 | 5.3 ± 2.4 | 12.7 ± 3.8 | 4.9 ± 1.4 | 4.1 ± 1.0 | 0.14 ± 0.07 | 3.1 ± 1.5 | 0.15 ± 0.06 |
| SIT−/− | 15 | 174.3 ± 51.2 | 3.5 ± 1.0 | 154.6 ± 48.2 | 13.0 ± 3.2 | 3.4 ± 1.7 | 8.3 ± 3.3 | 2.9 ± 1.2 | 2.0 ± 0.7 | 0.43 ± 0.1 | 2.4 ± 1.2 | 0.20 ± 0.1 |
| P value | 0.03 | 0.8 | 0.02 | 0.1 | 0.03 | 0.0009 | 0.002 | <0.0001 | <0.0001 | 0.2 | 0.3 | |
Values that are indicated in boldface type are statistically significant.
FIG. 2.
Lymphocyte development in SIT-deficient mice. Bone marrow cells (A), lymph nodes cells (B), peritoneal cavity cells (C), thymocytes (D), double-negative thymocytes (E), and splenocytes (F) from 5- to 8-week-old mice were stained with MAbs for CD43, B220, IgM, CD4, CD8, TCR-β, CD44, CD25, γδTCR, IgD, and CD5 and analyzed by flow cytometry. Numbers represent the percentages of cells that fall into the indicated boxes or quadrant areas of total gated living cells.
To address the question of whether SIT plays a role in T-cell development, we investigated single-cell suspensions of thymocytes from wild-type animals and SIT-deficient littermates by flow cytometry. Table 1 shows that the cellularity of SIT-deficient thymi is moderately increased. Fluorescence-activated cell sorter analysis further revealed an alteration in the composition of thymocyte subpopulations (Fig. 2D), with clearly reduced proportions of both CD4+ and CD8+ mature single-positive (SP) thymocytes and an increase of CD4+ CD8+ (double-positive [DP]) immature thymocytes in SIT−/− mice. In agreement with this observation, a significant decrease of thymocytes expressing high levels of TCR (as a measure of terminal maturation) was found in the knockout animals (Fig. 2D). Due to the increase in total thymic cellularity, we calculated the absolute numbers of the individual thymocyte subsets. As summarized in Table 1, SIT−/− mice show an approximate 40% increase in the total number of immature DP thymocytes and a slight reduction in the number of mature SP thymocytes. In contrast, the numbers of double-negative thymocytes (Table 1) as well as their CD25/CD44 fluorescence-activated cell sorter profiles are normal (Fig. 2E). The latter finding suggests that SIT is dispensable during the earliest steps of thymic development. Thus, the higher total numbers of thymocytes in SIT-deficient mice are primarily caused by an increase in the DP subset. The alteration in the DP/SP ratio suggests a subtle defect in thymic selection.
Upregulation of cell surface molecules involved in thymic selection processes on SIT−/− DP thymocytes.
To obtain further insight into the molecular mechanism underlying the altered thymic development in SIT−/− mice, we analyzed the expression levels of surface markers whose expression is known to be regulated during thymic development.
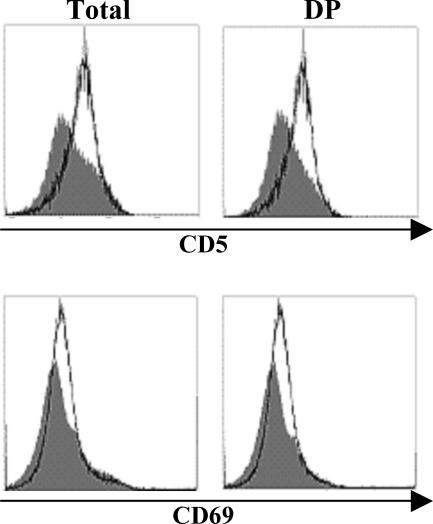
The expression of CD5 has been reported to directly correlate with the intensity of pre-TCR- and TCR-mediated signals (5, 6). Thus, high levels of CD5 expression are found in thymocytes of transgenic mice which express TCRs with a high affinity/avidity for their selecting ligands (5, 6) or on DP thymocytes of mice lacking negative regulatory cytosolic adapters such as c-Cbl or SLAP (32, 38). Therefore, it appears that CD5 serves as a negative-feedback regulator of TCR-mediated signals and that its expression is required to regulate signaling processes during thymocyte selection (5, 6, 42). As shown in Fig. 3, the expression of CD5 is upregulated on SIT-deficient DP thymocytes, indicating an enhanced signaling capability of the TCR in the absence of SIT.
FIG. 3.
Enhanced expression of CD5 and CD69 on SIT−/− DP thymocytes. Ungated (total) or CD4+ CD8+-gated (DP) thymocytes were assessed for surface expression of CD5 and CD69. The histograms represent profiles of cells from a representative SIT+/+ (shaded) and SIT−/− (thick lines) mouse. All data are representative of a minimum of 10 individual experiments.
CD69 is another maturation marker that becomes upregulated after TCR engagement by ligands presented by thymic stromal cells during both negative and positive selection (4, 31, 41, 47). Similar to CD5, DP thymocytes of SIT-deficient mice express higher levels of CD69 than wt littermates (Fig. 3). This further corroborates our hypothesis that TCR-mediated signaling is augmented in SIT-deficient thymocytes. In contrast to CD5 and CD69, the expression profiles of CD4, CD8, heat-stable antigen (HSA), CD45RB, and CD62L are normal in SIT−/− thymocytes (data not shown).
SIT deficiency alters positive selection in H-Y and P14 TCR transgenic mice.
Within the thymus, the majority of cells belong to the DP stage. Moreover, it is during this stage that autoreactive thymocytes are eliminated by negative selection, while thymocytes with the appropriate specificity for self-peptide/MHC complexes are positively selected and further develop to the SP stage. It is known from multiple studies that TCR transgenic mice expressing a defined αβTCR are well suited to study thymic selection processes (13, 27). Given the above-described findings, which suggested that loss of SIT affects thymic development by enhancing TCR-mediated signals, we wished to obtain a more detailed insight into SIT-mediated regulation of thymic selection processes. To this end, we crossed SIT−/− mice with mice expressing transgenic TCRs (P14, H-Y, OT-I, and OT-II) and evaluated the effect of SIT deficiency on selection processes.
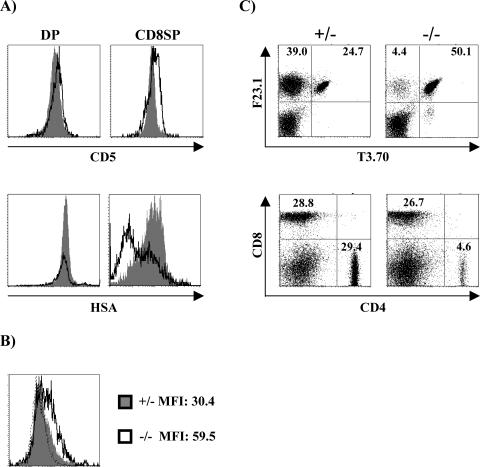
As shown in Fig. 4A, higher levels of CD5 are expressed on DP and in particular on positively selected CD8+ SP H-Y tg thymocytes of SIT−/− mice than in their SIT+/+ counterparts. Since CD5 expression on mature SPs has been proposed to result from TCR-mediated signals that are delivered during the selection processes (6), the enhanced expression of CD5 on SIT−/− SP cells indicates that, similar to the situation seen in the non-TCR tg mice, loss of SIT also enhances signaling via the TCR in the H-Y TCR tg model.
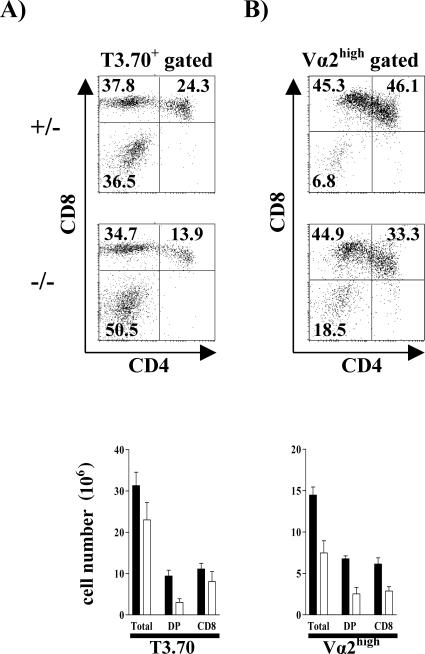
FIG. 4.
Enhanced positive selection in the absence of SIT. (A) Thymocytes from H-Y female mice were stained with CD4 and CD8 and assessed for the expression of CD5 and HSA on gated DP and CD8 SP cells. The histograms represent overlaid profiles of cells from a representative SIT+/− (shaded) and SIT−/− (thick lines) mouse. (B) Enhanced ERK1/2 activation in SIT−/− H-Y thymocytes. Single-cell suspensions from thymi were immediately fixed and stained for CD4, CD8, and phospho-ERK1/2. Numbers indicate MFI from one representative experiment out of four. Dotted lines indicate the levels of staining with the secondary antibody alone. (C) Representative dot plots of the expression of the clonotypic transgenic TCRα chain (T3.70) and transgenic TCRβ (F23.1) (upper panels) or CD4 and CD8 (lower panels) in lymph node cells. Numbers indicate the percentages of cells in each subset.
Figure 4A further demonstrates that the positively selected CD8+ SP TCR tg cells express strikingly lower levels of HSA than the corresponding cells from SIT+/+ animals. This indicates that SIT-deficient CD8 SP cells are more mature than SIT+/+ cells, likely because of enhanced positive selection (see below).
According to the currently proposed model, the activation/phosphorylation status of the dual-specificity kinase ERK directly correlates with the efficiency of positive selection (7, 23, 28, 33). Figure 4B depicts that ex vivo phosphorylation of ERK in DP thymocytes of SIT-deficient mice is stronger than in the corresponding SIT-positive animals (mean fluorescence intensity [MFI] of 59.5 versus 30.4). In contrast, the levels of Erk phosphorylation are similar after in vitro stimulation with PMA (MFI of 170 versus 183), which rules out the possibility that the cells express different amounts of phosphorylatable Erk. Note that the histograms shown in Fig. 4B were obtained from cells that had not been activated in vitro; thus, the expression levels of phospho-ERK indeed reflect the in vivo situation within the thymus. Since the H-Y TCR is known to signal rather inefficiently (36), it is therefore not surprising that the mean peaks of fluorescence (as a sign of Erk phosphorylation) are not very high. Nevertheless, both the more mature phenotype of SP cells and the enhanced activation of ERK on DP thymocytes strongly suggest that loss of SIT enhances the efficiency of positive selection in female H-Y TCR tg mice.
This assumption is even further supported by the finding that the proportions of non-TCR tg T cells are strongly decreased in SIT-deficient mice (Fig. 4C). The non-TCR tg pool in the H-Y mice is composed of CD4 cells in which the transgenic TCR-β chain is paired with an endogenous (non-tg) TCR-α chain (Fig. 4C). This pairing occurs in the H-Y tg mice because the transgenic TCR has only a weak affinity for the selecting ligands, and therefore, it transduces a weak signal that is not sufficient to properly suppress the rearrangement of the Tcra locus (20). The enhanced ratio of tg (CD8 H-Y+) to non-tg (CD4 H-Y−) T cells in SIT−/− mice therefore indicates that in the absence of SIT, the H-Y TCR signals stronger, resulting in a more efficient suppression of the rearrangement of the endogenous TCR-α chain.
Besides the above-described signs of enhanced positive selection, we also observed a significant reduction in the total number of H-Y-TCR tg DP thymocytes in SIT-deficient mice (Fig. 5A). This finding could indicate that the loss of SIT not only enhances the efficiency of positive selection but even partially converts positive selection to negative selection. Importantly, similar data as shown in Fig. 5A for the H-Y TCR tg model were found in a second TCR tg model, P14 (Fig. 5B). However, in the P14 TCR tg system, loss of SIT resulted in an even more dramatic reduction of thymic cellularity. This could suggest that in this system, the conversion from positive to negative selection caused by loss of SIT is even stronger than in the H-Y TCR tg model. In any case, both TCR tg models demonstrate that the loss of SIT alters thymic selection processes, likely by enhancing positive selection and also by partially converting positive selection to negative selection. In contrast to the H-Y and the P14 system, SIT deficiency did not alter the outcome of selection processes in a class I-restricted TCR tg model with higher affinity (OT-I) and class II-restricted TCR tg models (OT-II) (data not shown).
FIG. 5.
The absence of SIT partially converts positive selection to negative selection. Flow cytometric analysis of positive selection in thymus is shown. Thymocytes from H-Y (A) and P14 (B) TCR SIT−/− or SIT+/− transgenic mice were analyzed for transgenic TCR expression by staining with T3.70, a clonotypic antibody specific for H-Y TCRα chain, or Vα2, the P14 TCRα chain. The dot plots show CD4 and CD8 profiles of cells gated on TCRhigh. Numbers indicate percentages of cells in each subset. One representative animal for each group is shown. The graphs show the mean cellularity of TCR transgenic thymocyte subsets from seven SIT+/− and seven SIT−/− H-Y+ tg mice and three SIT+/− and three SIT−/− P14+ tg mice.
Normal negative selection in the absence of SIT.
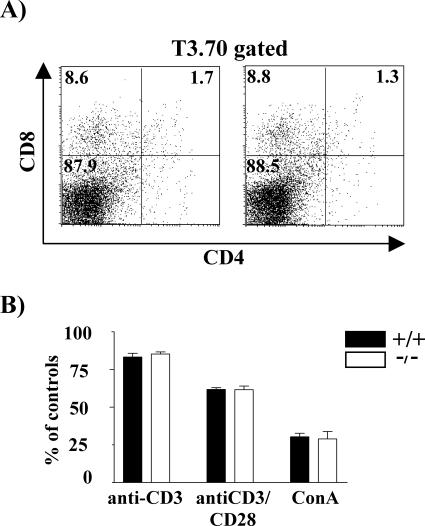
We also investigated whether SIT deficiency would directly influence negative selection. To assess this question in vivo, we took further advantage of the H-Y TCR transgenic model (27). In male H-Y TCR transgenic mice, the CD4+ CD8+ DP thymocytes are efficiently depleted by negative selection, and therefore, mature CD8 SP cells are barely detectable. However, some CD8 SP thymocytes downregulate the CD8 coreceptor and escape negative selection, thus permitting a small population of thymocytes to colonize the periphery. Figure 6A demonstrates that loss of SIT does not alter the cellular composition of the thymus in male H-Y TCR tg mice. Similarly, no differences were observed in the periphery of SIT-deficient H-Y TCR tg male mice (data not shown). These data strongly suggest that negative selection proceeds normally in the absence of SIT.
FIG. 6.
SIT is dispensable for negative selection. (A) Flow cytometric analysis of CD4/CD8 profiles of the T3.70+ population in H-Y TCR transgenic male mice. (B) DP thymocyte deletion in response to TCR cross-linking. Thymocytes were cultured in 24-well plates coated with anti-CD3 or anti-CD3 plus anti-CD28 or in the presence of ConA for 24 h, and their viability was determined. One of two independent experiments is shown, each using three pairs of mice. Black and open bars represent wild-type and knockout mice, respectively.
However, since the deletion process in the H-Y male model occurs at a very high efficiency and only extremely low numbers of DP cells are detectable in the mice, it is difficult to unmask augmented negative selection in this system. To circumvent this problem, we mimicked negative selection in vitro by stimulating freshly isolated thymocytes with CD3 and CD3 plus CD28 monoclonal antibodies (MAbs) or with the polyclonal mitogen ConA. Figure 6B shows that the survival rates of the in vitro-activated thymocytes were comparable between wild-type and SIT−/− mice. Thus, SIT seems to be dispensable for negative selection within the thymus as well as for in vitro deletion of immature T lymphocytes.
Alterations of the peripheral T-cell pools in SIT−/− mice.
To obtain further insight into the consequences of SIT deficiency, we examined the cellular composition of the peripheral lymphoid system. These experiments revealed normal sizes, architectures, and cellularities of spleen, Peyer's patches, and bone marrow (data not shown). Moreover, the numbers of B (B220+), αβT (TCRβ+), CD4, CD8, and NK (NK1.1+) cells were also unaffected in the absence of SIT (Fig. 2F and data not shown). In marked contrast, both superficial and mesenteric lymph nodes of SIT−/− mice show an approximate 50% decrease in total cellularity (Table 1). Anatomical analysis of these lymph nodes did not show alterations in global architecture. However, a significant reduction in the number of αβTCR+ lymphocytes in both the CD4 and CD8 subpopulations (Table 1 and Fig. 2B) was found by flow cytometry, whereas the numbers of B and NK cells as well as the expression levels of CD24, CD25, CD44, CD45RB, CD62L, and CD69 cells (Table 1, Fig. 2B, and data not shown) were all normal. Thus, the decrease in total cellularity of lymph nodes is due to a selective reduction in conventional αβTCR+ T lymphocytes.
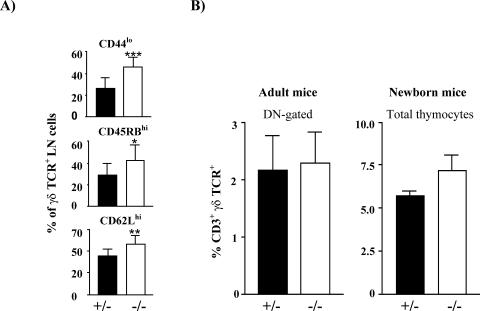
Further analysis of the secondary lymphatic organs revealed an increase in both the distribution and the absolute numbers of γδTCR+ lymphocytes in SIT-deficient lymph nodes, spleen, Peyer's patches, blood, and bone marrow (Table 1, Fig. 2B, and data not shown). Moreover, the SIT−/− γδ T-cell subset contains a higher proportion of CD44low, CD45RBhi, and CD62Lhi cells which correspond with a naive phenotype (25, 44) (Fig. 7A). γδ T cells develop very early in the thymus during embryonal life through a unique developmental pathway (3, 17). The first two waves of γδ T cells lack junctional diversity and migrate to the skin and to mucosal tissue. At the end of the fetal life, a third wave of γδ cells is produced. After exiting the thymus, these cells populate the spleen and the lymph nodes of adult mice. To assess whether SIT deficiency alters γδ T-cell development, we analyzed thymocyte suspensions from newborn and adult mice by flow cytometry. These experiments revealed no alteration of the numbers of CD3+ γδTCR+ cells within the thymus of SIT-deficient mice (Fig. 7B). Similarly, the distribution of intraepithelial gut lymphocytes, which are also mostly γδ T cells, was found to be unaffected in SIT-deficient mice (not shown). Collectively, these data suggest that SIT regulates the expansion of peripheral γδT cells rather than being involved in the homing of these cells to the gut or in their thymic development.
FIG. 7.
γδ T-cell populations in SIT−/− mice. (A) Proportion of CD3+ γδ TCR+ lymph node cells expressing CD44, CD45RB, and CD62L. Ten mice for each genotype were included in the groups. ***, P = 0.0003; **, P = 0.0038; *, P = 0.0352. (B) γδ T-cell distribution in thymi from adult or newborn mice. Black bars and open boxes represent wt and SIT-deficient mice, respectively.
SIT negatively regulates peripheral T-cell responses.
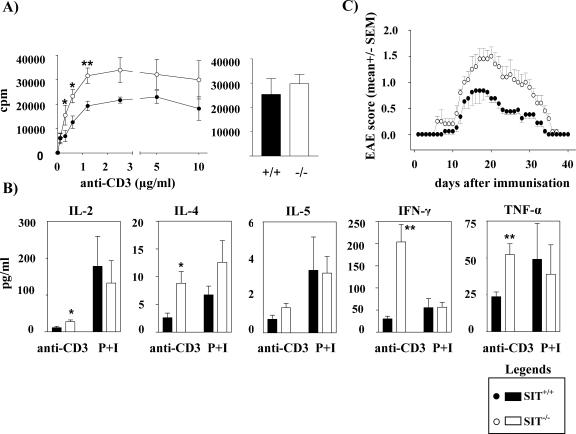
The altered distribution of peripheral T-cell subpopulations in SIT-deficient mice prompted us to investigate whether T cells function normally in the absence of SIT. To this end, we analyzed proliferation of purified splenic T cells that were stimulated with different concentrations of plate-bound anti-CD3 MAb by means of [3H]thymidine incorporation. As shown in Fig. 8A, compared to wild-type littermates, T cells of SIT−/− mice exhibit a ∼50% enhancement in proliferation upon CD3 stimulation that is most evident at intermediate antibody concentrations. Similarly, SIT-deficient T cells produce strikingly higher amounts of gamma interferon, interleukin-2, and tumor necrosis factor alpha after CD3 stimulation (Fig. 8B), strongly suggesting that SIT deficiency skews the peripheral T-cell repertoire towards the TH1 (T-helper 1) lineage.
FIG. 8.
Altered peripheral T-cell function in SIT−/− mice. (A) Enhanced T-cell proliferation. Purified splenic T cells were stimulated in 96-well plates coated with anti-CD3 antibody or PMA and ionomycin (P+I), pulsed with [3H]thymidine, and processed for standard scintillation counting. Results from seven wild-type and nine knock-out mice are shown as mean counts per minute (cpm). One of two independent experiments is shown. *, P = 0.01; **, P = 0.007. (B) Augmented cytokine production by anti-CD3-stimulated SIT−/− T cells. T cells were purified and stimulated with 0.3 μg/ml anti-CD3 or PMA and ionomycin (P+I). Results from 8 wild-type and 10 knockout mice are shown. IL-2, interleukin-2; IFN-γ, gamma interferon; TNF-α, tumor necrosis factor alpha. *, P = 0.02; **, P < 0.005. (C) Increased incidence of EAE in the absence of SIT. Mice were examined daily for signs of disease and graded on a scale of increasing severity from 0 to 5 as follows: 0, no signs; 0.5, partial tail weakness; 1, limp tail or slight slowing of righting from supine position; 1.5, limp tail and slight slowing of righting; 2, partial hind-limb weakness or marked slowing of righting; 2.5, dragging of hind limb(s) without complete paralysis; 3, complete paralysis of at least one hind limb; 3.5, hind-limb paralysis and slight weakness of forelimbs; 4, severe forelimb weakness; 5, moribund or dead.
To assess whether the altered peripheral T-cell function of SIT-deficient mice primes the organism for the development of autoimmune diseases, we investigated the clinical course of EAE in wild-type and SIT−/− mice. EAE is a T-cell-mediated autoimmune disease of the central nervous system that is characterized by inflammation, demyelination, and relapsing/remitting courses of paralysis (30). It represents a well-established animal model for multiple sclerosis and is believed to primarily be a TH1-mediated disease. As shown in Fig. 8C, analysis of the clinical EAE score following immunization with MOG shows a more severe disease in SIT−/− mice than in control animals. Thus, the absence of SIT not only affects selection processes within the thymus and peripheral T-cell functions but also aggravates the susceptibility of the organism to develop autoimmune diseases. Surprisingly, however, even a detailed biochemical analysis of most important membrane-proximal signaling events of SIT-deficient T cells (e.g., global tyrosine phosphorylation; TCR-mediated Ca2+ flux; LAT phosphorylation; activation of ERK, June N-terminal protein kinase, and p38; IκB degradation, etc.) did not reveal any significant changes compared to wt littermates (data not shown). Thus, the mechanisms by which SIT controls T-cell function remain elusive, although the biochemical analysis of TCR-mediated signals within the thymic compartment suggests that SIT primarily regulates TCR-mediated activation of Erk.
DISCUSSION
In this study, we report the characterization of mice lacking the transmembrane adapter molecule SIT. Our findings support our previous hypothesis that SIT primarily acts as a negative regulator of TCR-mediated signaling (29, 35). Because SIT is most highly expressed in thymocytes, we initially investigated the function of SIT during thymic selection processes. Non-TCR transgenic SIT-deficient thymocytes showed higher levels of CD5 and CD69 than wild-type animals. Upregulation of these two activation markers has been proposed to reflect the activation status of the cells within the thymus, and the expression levels of CD5 on thymocytes have been shown to directly correlate with the strength of TCR-mediated signals (6, 32, 38). Therefore, the enhanced expression of CD5 on SIT-deficient thymocytes suggests that not only in Jurkat T cells (29, 35) but also in vivo, SIT primarily acts as a negative regulator of T-cell activation. Furthermore, it indicates that one physiologic function of SIT is to finely tune signals that emanate from the TCR during thymic development. Clearly, the possibility that SIT directly regulates the expression levels of CD5 or CD69 cannot be completely ruled out. However, the observation that the expression levels of CD5 and CD69 are normal in SIT-deficient OT-I and OT-II TCR transgenic mice (data not shown) makes this possibility unlikely.
The enhanced expression of CD5 and CD69 (together with the slightly increased thymic cellularity) in non-TCR tg mice initially suggested that loss of SIT enhances TCR-mediated signals, thereby leading to subtle alterations of the thymic selection processes. This hypothesis was confirmed in the low-affinity H-Y TCR tg model system, where we demonstrated that in female H-Y TCR tg animals, loss of SIT apparently enhances the efficiency of positive selection. This hypothesis was deduced from different observations. First, SIT-deficient CD8+ SPs show enhanced expression of CD5 (as a sign of enhanced TCR-mediated signaling during the selection processes [6]). Second, the positively selected CD8+ SP cells in the female mice are more mature than their SIT-expressing counterparts (as assessed by lower levels of HSA expression). Third, ex vivo-isolated thymocytes show enhanced constitutive phosphorylation of the dual-specificity kinase ERK, whose activity has been reported to directly correlate with the efficiency of positive selection (1, 36, 40). Fourth, loss of SIT induces a more efficient silencing of the TCRα locus that results in a decrease of non-TCR tg CD4+ SP T-cells (20). Thus, the H-Y data are compatible with the hypothesis that loss of SIT enhances TCR-mediated signals and thereby facilitates positive selection. It is interesting that similar effects on positive selection as shown here for SIT have previously been observed in mice lacking expression of CD5 (in the H-Y and the P14 TCR tg models [5, 42]). Thus, it appears as if negative regulators such as SIT and CD5 serve to set the signaling thresholds for positive selection during thymic development.
In addition to enhanced positive selection, we found a decrease in DP cells in female H-Y TCR tg mice. This could suggest that in the H-Y system (and even more pronounced in the P14 system), loss of SIT not only enhances positive selection but even partially converts positive selection to negative selection. Although further experiments are required to prove this hypothesis, the idea that loss of SIT partially converts positive selection to negative selection is strongly supported by recent data that we obtained in our laboratory using female H-Y TCR tg SIT/TRIM double-knockout mice. TRIM is a second non-lipid-raft transmembrane adapter protein with unknown function that carries similar tyrosine-based signaling motifs in its cytoplasmic domain as SIT (10).
Importantly, the thymi of the female H-Y TCR tg TRIM/SIT double-knockout mice are almost indistinguishable from the thymi of wild-type male H-Y TCR tg animals, in which almost all DP thymocytes are eliminated by negative selection (due to expression of the endogenous male autoantigen). Thus, in the female H-Y TCR tg mice, concomitant loss of both TRIM and SIT completely converts positive selection to negative selection (L. Simeoni et al., unpublished data). The fact that this conversion is only incomplete in SIT-single-deficient mice (note that loss of TRIM by itself has a null effect on thymic development in either male or female H-Y TCR tg mice [Simeoni et al., unpublished]) suggests that the loss of SIT is partially compensated for by other transmembrane adapter proteins, TRIM being one potential candidate (see below).
It is also possible that SIT may regulate thymocyte survival. However, two observations argue against this hypothesis. First, the thymic cellularity in the OT-I and OT-II transgenic systems is normal. Second, we did not observed any differences in the survival rate of thymocytes after treatment with various apoptotic stimuli (e.g., dexamethasone, etoposide, and anti-Fas).
Compensatory mechanisms might also explain why SIT deficiency does not enhance positive selection (or convert positive selection to negative selection) in the OT-I and OT-II TCR transgenic models. Alternatively, it simply might suggest that the fine-tuning function of SIT becomes negligible beyond a certain strength of the TCR-mediated input signal. In line with a gatekeeper function of SIT only in low-strength signaling systems might be the finding that the loss of SIT apparently does not influence negative selection processes in any model that we investigated (i.e., the strong TCR-mediated signals that induce negative selection apparently can no longer be influenced by SIT).
Besides regulating thymic development, SIT seems to partially control the size of the peripheral T-cell pool. Thus, the loss of SIT leads to an expansion of γδ T cells in peripheral lymphoid organs without enhancing the production of γδ T cells within the thymus or impairing their homing to the gut. In addition, SIT-deficient mice show diminished numbers of αβ T cells, selectively in lymph nodes. The latter finding could be due either to a reduced production of αβ T cells within the thymus or to a defect in peripheral T-cell homeostasis or to both. The mild reduction in the numbers of SP thymocytes in nontransgenic SIT−/− mice could in fact be partially responsible for the reduced numbers of mature peripheral T cells. However, it is also known that similarly to immature thymocytes, naive T cells require continuous contact with self-peptide/MHC molecules (low-affinity signals) to prevent death by neglect (15, 39). Thus, low-affinity ligands dictate positive selection in immature thymocytes and survival in naive peripheral T cells. Loss of a negative regulatory molecule such as SIT could enhance the strength of TCR-mediated signaling in the presence of low-affinity peptides, thereby altering the survival/death rate of peripheral T cells.
Independently of the mechanism that underlies altered homeostasis of the peripheral T-cell pool, the absence of SIT results in hyperreactivity of peripheral T cells towards TCR-mediated stimuli, with the consequence of enhanced production of the TH1 cytokines tumor necrosis factor alpha and gamma interferon in vitro. In line with this, loss of SIT significantly enhances the clinical course of EAE, a TH1-mediated autoimmune disease of the central nervous system.
How SIT regulates peripheral T-cell functions and how it balances TH1 versus TH2 T-helper cells in the periphery is not known at present. Indeed, despite considerable effort, we could not reveal significant alterations of membrane-proximal signaling events in SIT-deficient peripheral T cells. One possibility to explain the hyperreactivity of peripheral T cells was based on our previous observation that in Jurkat T cells, SIT exerts its negative regulatory function via the tyrosine-based signaling motif YASV (29, 35). Moreover, in the Jurkat system, it was shown that upon pervanadate stimulation, the tyrosine kinase Csk, the major negative regulator of Src protein tyrosine kinases, is capable of binding to this motif. Therefore, Csk represented an attractive candidate for mediating the inhibitory function of SIT on TCR signaling. However, so far, we could not show an association between SIT and Csk in human or mouse T cells under more physiological conditions of stimulation (e.g., CD3 MAb instead of pervanadate [data not shown]). Similarly, we did not observe an upregulation of the enzymatic activity of Lck, a crucial substrate of Csk in peripheral T cells of SIT−/− mice. Furthermore, CD3-mediated phosphorylation of the TCR-ζ chain is not impaired in SIT-deficient thymocytes. Collectively, these data suggest that SIT is not involved in the inhibition of Src kinases (either directly or indirectly by recruiting Csk).
Another possibility to explain the negative regulatory role of SIT was based on our previous observation that SIT is capable of recruiting cytosolic protein tyrosine phosphatases (e.g., SHP2) to the plasma membrane (29). However, extensive biochemical analysis of SIT-deficient T cells did not reveal alteration of TCR-mediated membrane targeting of SHP1 or SHP2 (data not shown). This largely rules out the possibility that impaired membrane targeting of SHP1 or SHP2 underlies the enhanced signaling capacity of SIT−/− T cells. Nevertheless, the fact that SIT-deficient T cells react normally after stimulation with PMA and ionomycin suggests (and confirms our previous data) that SIT exerts its negative regulatory role upstream of activation of protein kinase C and production of IP3.
Although the molecular mechanism(s) underlying SIT-mediated inhibition of signaling is still unknown, it appears as if SIT would negatively regulate TCR-mediated activation of ERK in thymocytes. Indeed, ex vivo-isolated H-Y transgenic SIT-deficient thymocytes show enhanced phosphorylation of ERK1/2, and SIT−/− DP thymocytes display higher expression levels of CD5 and CD69, both molecules whose expression is regulated via the ERK-dependent transcription factors Ets-1 (14, 45) and AP-1 (11, 24). In light of these observations, it is important that we have previously demonstrated that SIT can bind the cytosolic adapter protein Grb2, an upstream activator of Erk, via a membrane-proximal YGNL motif. However, when overexpressed in Jurkat T cells, a CD8/CD8/SIT chimera (extracellular domain/transmembrane domain/intracellular domain) that carries an isolated YGNL motif does not inhibit T-cell functions (35). Rather, when this chimera is co-cross-linked with the TCR, it strongly upregulates TCR-mediated activation of the transcription factor NFAT. This suggests a positive rather than a negative regulatory role of the YGNL motif. However, further experiments are required to assess the role of the YGNL motif for SIT-mediated T-cell function.
Another possibility to explain enhanced TCR-mediated signaling in the absence of SIT would be to assume that SIT exerts its negative regulatory role in normal cells through sequestration of key signaling molecules from the lipid raft to the nonraft fraction, thereby limiting the numbers of signaling molecules in the lipid rafts. Loss of SIT within the nonraft fraction could then result in a redistribution of these signaling molecules to the lipid rafts. This would then permit enhanced signaling. A redistribution of molecules from nonrafts to rafts has most recently been proposed to cause the augmented FcɛRIII-mediated signaling in NTAL/LAB-deficient mast cells (46, 51).
However, another recent report suggested that when present in the nonraft fraction of chicken DT-40 cells, Grb2 inhibits B-cell receptor (BCR)-mediated signals via an unknown mechanism (38a). Targeting of Grb2 to the lipid rafts (e.g., by ectopic expression of the lipid raft-associated transmembrane adapter NTAL) rescues the BCR from the Grb2-mediated inhibitory signal, thus facilitating BCR-mediated responses. If this model would also apply for T cells, then it would be reasonable to assume that SIT inhibits TCR-mediated signaling by enhancing the inhibitory pool Grb2 in the nonraft fraction. The fact that we did not observe major alterations of biochemical signaling events following CD3 stimulation of SIT-deficient peripheral T cells does not necessarily contradict this hypothesis, simply because the subtle changes that are induced by loss of SIT might be too weak to be detected by standard biochemical techniques. In addition, loss of SIT might be to a large extent compensated by other non-raft-associated transmembrane adapter proteins exerting similar functions, such as TRIM and LAX.
Although the molecular mechanism(s) underlying SIT function is still elusive, it is obvious that SIT controls signaling pathways at different levels within the immune system. First, SIT-mediated signals are required to maintain a proper T-cell repertoire; second, they are mandatory to maintain the composition of the peripheral T-cell pools; third, they regulate the activation thresholds of peripheral T cells; and fourth, they control cytokine production/expansion of particular peripheral T-cell populations. In all cases, SIT seems to act as a fine tuner of TCR-mediated signaling processes.
The only mild alterations of thymic selection processes as well as the rather moderate hyperreactivity of peripheral T cells in SIT-deficient mice might be due to a functional redundancy among those transmembrane adapter proteins that serve as negative regulators during TCR-mediated signaling. In this regard, other nonraft transmembrane adapter proteins, for example, TRIM and LAX, might compensate for the loss of SIT. TRIM shares not only structural properties with SIT (both molecules are disulfide-linked homodimers) but also two tyrosine-based signaling motifs (YGNL and YASV, which is YASL in TRIM) (18). Similarly, LAX is also capable of binding, e.g., Grb2, and has been reported to negatively regulate TCR-mediated signals via an unknown mechanism (50). Therefore, the analysis of the SIT/LAX or SIT/TRIM double knockout or the SIT/LAX/TRIM triple knockout will possibly help to elucidate the biochemical pathways that are controlled by these transmembrane adapter proteins. Nevertheless, it is tempting to speculate that SIT as well as other transmembrane adapter proteins play important roles in priming the organism for the development of autoimmune diseases either by regulating peripheral tolerance or by altering the generation of an appropriate T-cell repertoire. In this regard, it will be important to assess the genetic status and the phosphorylation status of SIT and other negative regulatory transmembrane adapter proteins in patients suffering from autoimmune disease.
Acknowledgments
We are grateful to Jonathan Lindquist for critically reading the manuscript and helpful discussion; to Gary Koretzy, Thomas Kammerthoens, and Percy Knolle for TCR transgenic lines; to Reinhold Foerster and Oliver Pabst for investigation of gammadelta IEL; to Andrew Cope and Weiguo Zhang for reagents; and to the employees of the animal facility for maintenance of the animals. The help of Robert Enders, Evi Schaller, Agnes Fütterer, and Jennifer Meinecke is highly appreciated.
The work was supported by Deutsche Forschungsgemeinschaft (DFG)-funded research group 521 (research grants to L.S. and B.S.) and by DFG grants to K.P.
REFERENCES
- 1.Alberola-Ila, J., K. A. Hogquist, K. A. Swan, M. J. Bevan, and R. M. Perlmutter. 1996. Positive and negative selection invoke distinct signaling pathways. J. Exp. Med. 184:9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alimzhanov, M. B., D. V. Kuprash, M. H. Kosco-Vilbois, A. Luz, R. L. Turetskaya, A. Tarakhovsky, K. Rajewsky, S. A. Nedospasov, and K. Pfeffer. 1997. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc. Natl. Acad. Sci. USA 94:9302-9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison, J. P. 1993. Gamma delta T-cell development. Curr. Opin. Immunol. 5:241-246. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, G., K. J. Hare, and E. J. Jenkinson. 1999. Positive selection of thymocytes: the long and winding road. Immunol. Today 20:463-468. [DOI] [PubMed] [Google Scholar]
- 5.Azzam, H. S., J. B. DeJarnette, K. Huang, R. Emmons, C. S. Park, C. L. Sommers, D. El Khoury, E. W. Shores, and P. E. Love. 2001. Fine tuning of TCR signaling by CD5. J. Immunol. 166:5464-5472. [DOI] [PubMed] [Google Scholar]
- 6.Azzam, H. S., A. Grinberg, K. Lui, H. Shen, E. W. Shores, and P. E. Love. 1998. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J. Exp. Med. 188:2301-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bommhardt, U., Y. Scheuring, C. Bickel, R. Zamoyska, and T. Hunig. 2000. MEK activity regulates negative selection of immature CD4+CD8+ thymocytes. J. Immunol. 164:2326-2337. [DOI] [PubMed] [Google Scholar]
- 8.Brdicka, T., D. Pavlistova, A. Leo, E. Bruyns, V. Korinek, P. Angelisova, J. Scherer, A. Shevchenko, I. Hilgert, J. Cerny, K. Drbal, Y. Kuramitsu, B. Kornacker, V. Horejsi, and B. Schraven. 2000. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J. Exp. Med. 191:1591-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brdickova, N., T. Brdicka, P. Angelisova, O. Horvath, J. Spicka, I. Hilgert, J. Paces, L. Simeoni, S. Kliche, C. Merten, B. Schraven, and V. Horejsi. 2003. LIME: a new membrane Raft-associated adaptor protein involved in CD4 and CD8 coreceptor signaling. J. Exp. Med. 198:1453-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruyns, E., A. Marie-Cardine, H. Kirchgessner, K. Sagolla, A. Shevchenko, M. Mann, F. Autschbach, A. Bensussan, S. Meuer, and B. Schraven. 1998. T cell receptor (TCR) interacting molecule (TRIM), a novel disulfide-linked dimer associated with the TCR-CD3-zeta complex, recruits intracellular signaling proteins to the plasma membrane. J. Exp. Med. 188:561-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castellanos, M. C., C. Munoz, M. C. Montoya, E. Lara-Pezzi, M. Lopez-Cabrera, and M. O. de Landazuri. 1997. Expression of the leukocyte early activation antigen CD69 is regulated by the transcription factor AP-1. J. Immunol. 159:5463-5473. [PubMed] [Google Scholar]
- 12.Chu, D. H., C. T. Morita, and A. Weiss. 1998. The Syk family of protein tyrosine kinases in T-cell activation and development. Immunol. Rev. 165:167-180. [DOI] [PubMed] [Google Scholar]
- 13.Clarke, S. R., M. Barnden, C. Kurts, F. R. Carbone, J. F. Miller, and W. R. Heath. 2000. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol. Cell Biol. 78:110-117. [DOI] [PubMed] [Google Scholar]
- 14.Dittmer, J. 2003. The biology of the Ets1 proto-oncogene. Mol. Cancer 2:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst, B., D. S. Lee, J. M. Chang, J. Sprent, and C. D. Surh. 1999. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11:173-181. [DOI] [PubMed] [Google Scholar]
- 16.Finco, T. S., T. Kadlecek, W. Zhang, L. E. Samelson, and A. Weiss. 1998. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity 9:617-626. [DOI] [PubMed] [Google Scholar]
- 17.Hayday, A. C. 2000. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 18:975-1026. [DOI] [PubMed] [Google Scholar]
- 18.Horejsi, V., W. Zhang, and B. Schraven. 2004. Transmembrane adaptor proteins: organizers of immunoreceptor signalling. Nat. Rev. Immunol. 4:603-616. [DOI] [PubMed] [Google Scholar]
- 19.Hubener, C., A. Mincheva, P. Lichter, B. Schraven, and E. Bruyns. 2001. Complete sequence, genomic organization, and chromosomal localization of the human gene encoding the SHP2-interacting transmembrane adaptor protein (SIT). Immunogenetics 53:337-341. [DOI] [PubMed] [Google Scholar]
- 20.Huesmann, M., B. Scott, P. Kisielow, and H. von Boehmer. 1991. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell 66:533-540. [DOI] [PubMed] [Google Scholar]
- 21.Hur, E. M., M. Son, O. H. Lee, Y. B. Choi, C. Park, H. Lee, and Y. Yun. 2003. LIME, a novel transmembrane adaptor protein, associates with p56lck and mediates T cell activation. J. Exp. Med. 198:1463-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itoh, K., M. Sakakibara, S. Yamasaki, A. Takeuchi, H. Arase, M. Miyazaki, N. Nakajima, M. Okada, and T. Saito. 2002. Cutting edge: negative regulation of immune synapse formation by anchoring lipid raft to cytoskeleton through Cbp-EBP50-ERM assembly. J. Immunol. 168:541-544. [DOI] [PubMed] [Google Scholar]
- 23.Jameson, S. C., K. A. Hogquist, and M. J. Bevan. 1995. Positive selection of thymocytes. Annu. Rev. Immunol. 13:93-126. [DOI] [PubMed] [Google Scholar]
- 24.Karin, M. 1995. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 270:16483-16486. [DOI] [PubMed] [Google Scholar]
- 25.Kelly, K. A., M. Pearse, L. Lefrancois, and R. Scollay. 1993. Emigration of selected subsets of gamma delta + T cells from the adult murine thymus. Int. Immunol. 5:331-335. [DOI] [PubMed] [Google Scholar]
- 26.Kirchgessner, H., J. Dietrich, J. Scherer, P. Isomaki, V. Korinek, I. Hilgert, E. Bruyns, A. Leo, A. P. Cope, and B. Schraven. 2001. The transmembrane adaptor protein TRIM regulates T cell receptor (TCR) expression and TCR-mediated signaling via an association with the TCR zeta chain. J. Exp. Med. 193:1269-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kisielow, P., H. Bluthmann, U. D. Staerz, M. Steinmetz, and H. von Boehmer. 1988. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature 333:742-746. [DOI] [PubMed] [Google Scholar]
- 28.Mariathasan, S., S. S. Ho, A. Zakarian, and P. S. Ohashi. 2000. Degree of ERK activation influences both positive and negative thymocyte selection. Eur. J. Immunol. 30:1060-1068. [DOI] [PubMed] [Google Scholar]
- 29.Marie-Cardine, A., H. Kirchgessner, E. Bruyns, A. Shevchenko, M. Mann, F. Autschbach, S. Ratnofsky, S. Meuer, and B. Schraven. 1999. SHP2-interacting transmembrane adaptor protein (SIT), a novel disulfide-linked dimer regulating human T cell activation. J. Exp. Med. 189:1181-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin, R., H. F. McFarland, and D. E. McFarlin. 1992. Immunological aspects of demyelinating diseases. Annu. Rev. Immunol. 10:153-187. [DOI] [PubMed] [Google Scholar]
- 31.Merkenschlager, M., D. Graf, M. Lovatt, U. Bommhardt, R. Zamoyska, and A. G. Fisher. 1997. How many thymocytes audition for selection? J. Exp. Med. 186:1149-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naramura, M., H. K. Kole, R. J. Hu, and H. Gu. 1998. Altered thymic positive selection and intracellular signals in Cbl-deficient mice. Proc. Natl. Acad. Sci. USA 95:15547-15552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Shea, C. C., T. Crompton, I. R. Rosewell, A. C. Hayday, and M. J. Owen. 1996. Raf regulates positive selection. Eur. J. Immunol. 26:2350-2355. [DOI] [PubMed] [Google Scholar]
- 34.Paz, P. E., S. Wang, H. Clarke, X. Lu, D. Stokoe, and A. Abo. 2001. Mapping the Zap-70 phosphorylation sites on LAT (linker for activation of T cells) required for recruitment and activation of signalling proteins in T cells. Biochem. J. 356:461-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfrepper, K. I., A. Marie-Cardine, L. Simeoni, Y. Kuramitsu, A. Leo, J. Spicka, I. Hilgert, J. Scherer, and B. Schraven. 2001. Structural and functional dissection of the cytoplasmic domain of the transmembrane adaptor protein SIT (SHP2-interacting transmembrane adaptor protein). Eur. J. Immunol. 31:1825-1836. [DOI] [PubMed] [Google Scholar]
- 36.Priatel, J. J., S. J. Teh, N. A. Dower, J. C. Stone, and H. S. Teh. 2002. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity 17:617-627. [DOI] [PubMed] [Google Scholar]
- 37.Schaeffer, E. M., and P. L. Schwartzberg. 2000. Tec family kinases in lymphocyte signaling and function. Curr. Opin. Immunol. 12:282-288. [DOI] [PubMed] [Google Scholar]
- 38.Sosinowski, T., N. Killeen, and A. Weiss. 2001. The Src-like adaptor protein downregulates the T cell receptor on CD4+CD8+ thymocytes and regulates positive selection. Immunity 15:457-466. [DOI] [PubMed] [Google Scholar]
- 38a.Stork, B., M. Engelke, J. Frey, V. Horejsi, A. Hamm-Baarke, B. Schraven, T. Kurosaki, and J. Wienands. 2004. Grb2 and the non-T cell activation linker NTAL constitute a Ca2+-regulating signal circuit in B lymphocytes. Immunity 21:681-691. [DOI] [PubMed] [Google Scholar]
- 39.Surh, C. D., and J. Sprent. 2000. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J. Exp. Med. 192:F9-F14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swan, K. A., J. Alberola-Ila, J. A. Gross, M. W. Appleby, K. A. Forbush, J. F. Thomas, and R. M. Perlmutter. 1995. Involvement of p21ras distinguishes positive and negative selection in thymocytes. EMBO J. 14:276-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swat, W., M. Dessing, H. von Boehmer, and P. Kisielow. 1993. CD69 expression during selection and maturation of CD4+8+ thymocytes. Eur. J. Immunol. 23:739-746. [DOI] [PubMed] [Google Scholar]
- 42.Tarakhovsky, A., S. B. Kanner, J. Hombach, J. A. Ledbetter, W. Muller, N. Killeen, and K. Rajewsky. 1995. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science 269:535-537. [DOI] [PubMed] [Google Scholar]
- 43.Torgersen, K. M., T. Vang, H. Abrahamsen, S. Yaqub, V. Horejsi, B. Schraven, B. Rolstad, T. Mustelin, and K. Tasken. 2001. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J. Biol. Chem. 276:29313-29318. [DOI] [PubMed] [Google Scholar]
- 44.Tough, D. F., and J. Sprent. 1998. Lifespan of gamma/delta T cells. J. Exp. Med. 187:357-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tung, J. W., S. S. Kunnavatana, L. A. Herzenberg, and L. A. Herzenberg. 2001. The regulation of CD5 expression in murine T cells. BMC Mol. Biol. 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volna, P., P. Lebduska, L. Draberova, S. Simova, P. Heneberg, M. Boubelik, V. Bugajev, B. Malissen, B. S. Wilson, V. Horejsi, M. Malissen, and P. Draber. 2004. Negative regulation of mast cell signaling and function by the adaptor LAB/NTAL. J. Exp. Med. 200:1001-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamashita, I., T. Nagata, T. Tada, and T. Nakayama. 1993. CD69 cell surface expression identifies developing thymocytes which audition for T cell antigen receptor-mediated positive selection. Int. Immunol. 5:1139-1150. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, W., B. J. Irvin, R. P. Trible, R. T. Abraham, and L. E. Samelson. 1999. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int. Immunol. 11:943-950. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, W., C. L. Sommers, D. N. Burshtyn, C. C. Stebbins, J. B. DeJarnette, R. P. Trible, A. Grinberg, H. C. Tsay, H. M. Jacobs, C. M. Kessler, E. O. Long, P. E. Love, and L. E. Samelson. 1999. Essential role of LAT in T cell development. Immunity 10:323-332. [DOI] [PubMed] [Google Scholar]
- 50.Zhu, M., E. Janssen, K. Leung, and W. Zhang. 2002. Molecular cloning of a novel gene encoding a membrane-associated adaptor protein (LAX) in lymphocyte signaling. J. Biol. Chem. 277:46151-46158. [DOI] [PubMed] [Google Scholar]
- 51.Zhu, M., Y. Liu, S. Koonpaew, O. Granillo, and W. Zhang. 2004. Positive and negative regulation of FcepsilonRI-mediated signaling by the adaptor protein LAB/NTAL. J. Exp. Med. 200:991-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]