Abstract
Peptidylglycine α-amidating monooxygenase (PAM; EC 1.14.17.3) catalyzes the COOH-terminal α-amidation of peptidylglycine substrates, yielding amidated products. We have previously reported a putative regulatory RNA binding protein (PAM mRNA-BP) that binds specifically to the 3′ untranslated region (UTR) of PAM-mRNA. Here, the PAM mRNA-BP was isolated and revealed to be La protein using affinity purification onto a 3′ UTR PAM RNA, followed by tandem mass spectrometry identification. We determined that the core binding sequence is approximately 15-nucleotides (nt) long and is located 471 nt downstream of the stop codon. Moreover, we identified the La autoantigen as a protein that specifically binds the 3′ UTR of PAM mRNA in vivo and in vitro. Furthermore, La protein overexpression caused a nuclear retention of PAM mRNAs and resulted in the down-regulation of endogenous PAM activity. Most interestingly, the nuclear retention of PAM mRNA is lost upon expressing the La proteins that lack a conserved nuclear retention element, suggesting a direct association between PAM mRNA and La protein in vivo. Reporter assays using a chimeric mRNA that combined luciferase and the 3′ UTR of PAM mRNA demonstrated a decrease of the reporter activity due to an increase in the nuclear localization of reporter mRNAs, while the deletion of the 15-nt La binding site led to their clear-cut cytoplasmic relocalization. The results suggest an important role for the La protein in the modulation of PAM expression, possibly by mechanisms that involve a nuclear retention and perhaps a processing of pre-PAM mRNA molecules.
Posttranscriptional events including nuclear RNA processing and export, localization of RNA within the cell, degradation and stabilization of RNAs, and mRNA translation are increasingly seen as important cellular regulatory sites, whose alteration can contribute to disease states. The distribution of RNAs within various subcellular compartments results from a balance of export, import, and retention. These processes are largely mediated by RNA-binding proteins.
Many biologically active peptides are α-amidated at their COOH terminus, a structural feature that is often essential for their biological activity. These peptides are produced from larger inactive precursors that are cleaved to form peptides having a glycine residue at their COOH terminus (11, 26). Conversion of a peptidylglycine substrate into an α-amidated product is an enzymatic two-step reaction involving the copper, ascorbate, and molecular oxygen-dependent production of a peptidyl-α-hydroxyglycine intermediate; at physiological pH a second enzymatic activity catalyzes the subsequent formation of the α-amidated product (11, 35, 41). Both enzymes are derived from the bifunctional peptidylglycine α-amidating monooxygenase (PAM) precursor (EC 1.14.17.3) (11). Tissue-specific alternative splicing of the primary transcript of a single-copy gene located on human chromosome 5 (32) generates numerous PAM mRNA transcripts (10, 33, 40). The PAM proteins in any tissue reflect both the forms of PAM mRNA present and the co- or posttranslational modifications that occur. PAM has broad substrate specificity, is found in a variety of tissues, and is regulated in a tissue-specific fashion in response to endocrine manipulations (11, 26). This regulation often parallels the level of amidated peptides in vitro (43) and in vivo (31).
The elucidation of the mechanisms of regulation of PAM expression should bring new insights into the field of neuropeptide-processing enzyme regulation. Recently, we demonstrated that thyroid hormones and estrogen affect PAM gene expression posttranscriptionally by altering mRNA stability (12, 15). To further analyze the molecular mechanisms that are involved in PAM mRNA metabolism, our group identified a protein which binds specifically to a segment of the 3′ untranslated region (UTR) of PAM mRNA and named it the PAM mRNA-binding protein (PAM mRNA-BP) (16). Our study describes the isolation of the PAM mRNA-BP and demonstrates by several criteria that this protein is La antigen.
The La protein was first described as an autoreactive antigen in patients with the rheumatic diseases systemic lupus erythematosus and Sjogren's syndrome (4, 29, 42). Although first characterized in humans, La homologs have since been found in all eukaryotes including trypanosomes, yeast, and plants (27, 47). The La protein is a ubiquitous, abundant, monomeric phosphoprotein found in the nucleus of eukaryotic cells. It associates predominantly with a short poly(U) sequence (UUUOH) at the 3′ end of almost all nascent Pol III transcripts (27, 47). In yeast, the La protein (Lh1p1) associates with RNAs transcribed by Pol II that also contain 3′ poly(U) sequences (27, 47). The specific binding of La to precursor RNA molecules protects them from exonuclease digestion (13, 48) and thereby regulates downstream processing. For example, La helps to ensure the correct endonucleolytic digestion of the 5′ and the 3′ extensions of pre-tRNA (13, 48) and acts as a chaperone to promote the correct folding of these molecules (6). La also serves to retain precursor RNA molecules in the nucleus (5, 20, 24). Moreover, La assists the assembly of functional ribonucleoprotein particles (34), an activity that may be promoted by its association with RNA helicases (14).
In addition to its rather complex functional profile, La also has a role in translation (27, 47). For example, La can bind to the internal ribosome entry sites (IRES) of hepatitis C virus (HCV) (3) and the X-linked inhibitor of apoptosis protein (22), in both cases stimulating translation initiation. In the case of the HCV IRES, which lacks a 3′ UUUOH, La binds specifically to an internal sequence near the initiator AUG codon (3, 36, 37).
Like many RNA-binding proteins, La is modular. An N-terminal “La motif” is followed by either one or two RNA recognition motifs (RRMs; also referred to as RBDs or RNP domains) (46, 47). The size of La varies from about 32 kDa in yeasts, where La has only one RRM, to 50 kDa in humans, where La has two RRMs. Both the La motif and the central RRM are necessary for high-affinity RNA binding (7, 17), and it has been suggested that the role of the La motif is to provide specific recognition for UUUOH sequences (45, 27). The newly determined structures provide complementary data confirming that both the La motif and the central RRM synergize to form a functional RNA-binding domain and indicate that the La motif folds into a largely helical domain that seems to be an elaborated winged-helix module (2, 9). The C-terminal domain of the human La (hLa) exhibits considerable variation among eukaryotes. Recent structural work has revealed the presence in this domain of an unusual RRM encompassing residues 229 to 326, followed by a long, flexible polypeptide that contains a short basic motif, a regulatory phosphorylation site on Ser 366, and a nuclear localization signal (25). The C-terminal RRM also incorporates a functional nuclear retention element, which helps to ensure appropriate localization and processing of pre-tRNA molecules (23).
In this study, we report for the first time the following: (i) binding between the La protein and human PAM (hPAM) mRNAs occurs in vitro and in vivo; (ii) the La protein binds to the 3′ UTR of PAM mRNAs with a high affinity via an internal new binding motif of the 15-nucleotide (nt) sequence UUAAAAUCACUAACA; (iii) overexpression of La protein results in the nuclear retention of PAM mRNA followed by a down-regulation of endogenous PAM activity; (iv) the insertion of the PAM mRNA 3′ UTR in a luciferase reporter gene results in a considerable increase in the nuclear localization of the reporter chimeric mRNA accompanied with a decrease of luciferase activity, whereas (v) the deletion of the 15-nt sequence motif leads to a clear-cut cytoplasmic relocalization of the reporter mRNA. Furthermore, we demonstrate that PAM mRNA and reporter mRNA that contains the proposed La binding site are not retained in the nuclei by overexpression of La proteins that lack a conserved nuclear retention element. These results support the notion that the La protein contributes to intranuclear retention and chaperones PAM mRNAs.
MATERIALS AND METHODS
Cell culture.
The human glioblastoma U87 cell line was obtained from the American Type Culture Collection (Rockville, MD) and maintained in minimum essential medium; culture media were supplemented with 10% fetal calf serum (Invitrogen Life Technologies, Cergy Pontoise, France), penicillin (50 U/ml), streptomycin (50 μg/ml), glutamine (1 mg/ml), and other required factors. The cells were cultured at 37°C in a moist atmosphere of 95% air-5% CO2. The medium was replaced every 2 days.
Cell extracts and tissue preparation.
Cells from confluent cultures (75-cm2 flasks) were collected by centrifugation, washed once with Ca2+- and Mg2+-free phosphate-buffered saline (PBS), and homogenized in ice in lysis buffer A (10 mM HEPES, pH 7.5, 40 mM KCl, 3 mM MgCl2, 1 mM dithiothreitol [DTT)], 0.2% Nonidet P-40, 5% glycerol, 10 μg/ml leupeptin, and 0.5 mM phenylmethylsulfonyl fluoride [PMSF]) using a Teflon pestle. The homogenate was centrifuged at 800 × g for 10 min at 4°C to yield a pellet (P1) containing nuclei and a supernatant fraction (S1). The S1 fraction was centrifuged at 12,000 × g for 10 min, and cytoplasmic extracts were rapidly frozen and stored at −70°C. Pellet P1 was resuspended in 1 ml of TKM buffer (50 mM Tris-HCl, pH 7.5, 25 mM KCl, 5 mM MgCl2, and inhibitors) containing 0.25 M sucrose. The sucrose concentration of the sample was adjusted to approximately 1.62 M by the addition of 2 ml of TKM buffer containing 2.3 M sucrose; the material was layered onto 1 ml of TKM buffer containing 2.3 M sucrose and centrifuged at 100,000 × g for 30 min at 4°C (Beckman SW60 rotor). The resulting nuclear pellet was resuspended in 100 μl of buffer A, homogenized, and processed to prepare nuclear protein extracts. Rat tissues were homogenized in 5 volumes of ice-cold buffer A and treated as above to yield fractions P1 and S1. Sample protein concentrations were determined using a bicinchoninic acid protein assay reagent (Pierce Chemical Co., Interchim, Paris, France).
Purification of PAM mRNA binding protein.
The PAM 3′ UTR RNA binding protein was purified by RNA affinity chromatography using 500 mg of cytoplasmic extracts prepared from rat anterior pituitary gland as described by Fraboulet et al. (16). Briefly, 100 μg of the 5′ biotinylated PAM 3′ UTR RNA was coupled to μMACS streptavidin microbeads according to the manufacturer's instructions (Milteny Biotec, Auburn, Calif.). Cytoplasmic extracts containing the PAM mRNA binding protein was passed five times through the RNA affinity column equilibrated in binding buffer at a flow rate of 0.1 ml/min. After the flowthrough was collected, the column was washed with 5 volumes of binding buffer, and purified proteins were eluted with 200 μl of deionized water. The affinity-purified fractions were tested by UV cross-linking, gel-fractionated, and stained with Coomassie blue.
Protein identification.
Specific bands were cut from the gels and thoroughly washed with deionized water and acetonitrile. Cysteine residues in the gel-embedded proteins were then reduced with 50 mM DTT in 100 mM ammonium bicarbonate for 30 min at 56°C, and free cysteines were carbamidomethylated with 55 mM iodoacetamide for 30 min in the dark at room temperature. The reagents were removed by successive washes with 100% acetonitrile and 50 mM ammonium bicarbonate. Before digestion, gel pieces were shrunk with 100% acetonitrile that was then removed by suction. Trypsin (sequencing grade; Promega, Madison, Wis.) at a concentration of 12.5 ng/μl in 50 mM ammonium bicarbonate was absorbed by the gel pieces on ice. The samples were digested for 16 h at 37°C, and the supernatants were used for mass spectrometric analysis.
Mass spectrometry.
Automated nanoflow liquid chromatography-tandem mass spectrometry (MS/MS) was performed with a QTOF Micromass spectrometer (Waters/Micromass, Manchester, United Kingdom), using data-dependent analysis in positive ion mode. A nanoflow high-performance liquid chromatograph (UltiMate, Switchos2, FAMOS; LC Packings, Amsterdam, The Netherlands) was used for desalting and reverse-phase chromatographic separation of the samples, added from the autosampler. A fused silica precolumn (Zorbax SB-C18 with a particle size of 5 μm, an inner diameter of 75 μm, and a pore size of 300 Å) (Agilent, Wilmington, DE) was used as a desalting column before an 8-cm analytical column (Zorbax SB-C18 with a particle size of 3.5 μm, an inner diameter of 50 μm, and a pore size of 300 Å) (both columns from Agilent, Wilmington, DE). Flow into the mass spectrometer was 200 nl/min during the gradient from 5% buffer A (0% acetonitrile in 1% formic acid, 0.6% acetic acid, 0.005% heptafluorobutyric acid) to 40% buffer B (90% acetonitrile in 1% formic acid, 0.6% acetic acid, 0.005% heptafluorobutyric acid).
Database search and evaluation.
MS/MS peak lists were exported in Micromass pkl format and screened against protein sequences in the SWISS-PROT database using the MASCOT server (www.matrix-science.com). The spectra of all peptide hits stated as significant by MASCOT were verified by manually checking for the abundance of y- and b-ion signals and for m/z deviation patterns of the assigned peaks.
Protein overexpression and purification.
Escherichia coli BL21 cells harboring the plasmid pTrc-His-La for the full-length La (generously obtained from S. Schwartz, University of Uppsala, Sweden) were grown at 37°C in Luria broth supplemented with 50 μg/ml ampicillin. When the cells reached an optical density at 600 nm between 0.6 and 0.8, protein production was induced with 0.1 mM isopropyl β-d-thiogalactopyranoside. The cells were harvested 6 to 8 h after induction and stored at −80°C until further use. The protein purification was carried out entirely at 4°C.
Frozen cells were thawed at room temperature and resuspended in ice-cold PBS with a protease inhibitor (1× complete; Roche Applied Science) and lysed by pulse sonication, followed by incubation in 1% Triton X-100 on ice for 30 min. The lysate was cleared by centrifugation at 20,000 × g for 30 min, supplemented with 10 mM imidazole, and loaded onto NiS04-activated beads of chelating Sepharose Fast Flow (Amersham Biosciences) preequilibrated with buffer A (1 mM Na2HPO4, 1 mM NaH2PO4, pH 7.4 at room temperature, 50 mM NaCl, 10 mM imidazole). The resin-bound protein was washed with 10 volumes of buffer A. Imidazole at 400 mM was added to buffer A to release the protein from the resin. To further purify La protein, samples were then diluted fivefold in buffer B (20 mM HEPES, pH 7.6 at room temperature, 0.2 mM EDTA, 0.5 mM DTT, 2 mM MgCl2, 10% glycerol) and incubated with poly(U)-Sepharose 4B (Amersham Biosciences) for 2 h at 4°C. The resin-bound protein was washed five times with buffer B in the presence of 0.5 M KCl, and bound proteins were released with buffer B supplemented with 1 M KCl. After dialysis in buffer C (5 mM HEPES, pH 7.6, 25 mM KCl, 1 mM EDTA, 1 mM DTT, 10% glycerol, 1 mM PMSF), fractions were concentrated on a Centrikon filter.
Plasmid constructions.
The plasmid rPAM 3′ UTR was constructed as previously described (16). Briefly, the PAM 3′ UTR (nt 3614 to 3967) construct was obtained from PAM 3′ UTR plasmid digestion by BanI and EcoRI, rendered blunt ended, and subcloned into the EcoRV site of the pBluescript SK-II (pBS SK-II). The fragment was modified by PCR to produce a polyadenylated riboprobe after transcription with T3 RNA polymerase to produce a 429-nt transcript (S6; nt 3614 to 3967) including 76 nt of pBS SK-II, 353 bases of PAM 3′ UTR, and a 25-mer poly(A) tail. Plasmid hPAM 3′ UTR was constructed by subcloning the 3′ UTR sequence of the human PAM gene (bp 3304 to 3909) into the EcoRI site of pBS SK−II. The plasmid was rendered linear with EcoRV for transcription with T3 RNA polymerase to produce a 691-nt sense transcript including 86 nt of pBS SK-II and 605 bases of hPAM 3′ UTR (PAM 1). PCR was used to progressively generate smaller 3′ truncated human PAM cDNAs (see Fig. 6). The T3 RNA polymerase promoter sequence (upper primer) was paired with lower primers Sh6 (PAM cDNA bp 3752 to 3732; 5′-GCAGCTTTGTCGTCATG TAGC-3′), Sh5 (PAM cDNA bp 3863 to 3843; 5′-ATCCCAAAAACACACCAACTG-3′), and Sh8 (PAM cDNA bp 3783 to 3767; 5′-TGTTAGTGATTTTAAAA-3′) to produce PAM 2, PAM 3, and PAM 7 constructs, respectively. To generate PAM 4, PAM 5, and PAM 6 constructs, we used a 5′ primer that included a T7 RNA polymerase promoter sequence, which is underlined: T7 Sh1 (5′-AATACGACTCACTATAGGGTCTCCTTCTATTTTTTTAA-3′) and the 3′ primers Sh3 (PAM cDNA bp 3775 to 3796; 5′-CATTGCAATATAATGTTAGTGA-3′), Sh4 (PAM cDNA bp 3821 to 3800; 5′-TAAATAGAGACTTTTTTATTTC-3′), and Sh5 (PAM cDNA bp 3863 to 3843; 5′-ATCCCAAAAACACCAACTG-3′) were used. PCR-amplified DNA fragments were excised after electrophoresis in low-melting-point agarose, precipitated, and used for in vitro transcription (see Fig. 6).
FIG. 6.
La protein core binding site maps within the 15-nt segment of nt 3769 to 3783 of the PAM 3′ UTR RNA. (A) Summary of deletion analysis of the PAM 3′ UTR band shift complex. The striped bar is a representation of a complete 662-nt PAM 3′ UTR. RNA probes were prepared as described in Materials and Methods. The first and last nucleotides of each transcript are indicated. (B) UV cross-linking was performed with hLa protein (200 ng) and each described RNA probe. The same data were obtained with U87 cytoplasmic extracts (30 μg).
Preparation of RNA transcripts.
The in vitro transcription reaction mixture (20 μl), containing 40 mM Tris-HCl (pH 7.9), 6 mM MgCl2, 10 mM NaCl, 2 mM spermidine, 20 μM DTT, 500 μM rATP, 500 μM rGTP, 500 μM rCTP (Invitrogen Life Technologies), 10 μM UTP, [α-32P]UTP (50 μCi, 3,000 Ci/mmol; Amersham Biosciences), 20 U of T7 or T3 RNA polymerase (Invitrogen Life Technologies), 1 μg of DNA template, and 20 U of RNasin (Promega, Lyon, France), was incubated at 37°C for 1 h. After transcription, DNA templates were digested with 1 U of DNase/RNase-free RQ-1 (Promega). The radiolabeled RNA was precipitated and dissolved in 10 μl of loading buffer (7 M urea and 0.025% bromophenol blue) for electrophoresis on a 6% polyacrylamide gel containing 7 M urea. After autoradiography, the full-length radiolabeled RNA transcripts were identified and eluted from excised gel slices by incubating overnight at 37°C in 0.5 M ammonium acetate buffer containing 1 mM EDTA, 1 mM DTT, 10 μg tRNA, and 5 U RNasin. The radiolabeled probes were precipitated, reconstituted in water at a specific activity of 5 × 107 to 5 × 109 cpm/μl of RNA, and stored at −80°C. Unlabeled RNA transcripts were synthesized as described above with 10 mM rUTP, processed, quantified by absorbance at 260 nm, and stored at −80°C until use.
RNA UV cross-linking.
Total, nuclear, or cytoplasmic extracts (20 μg) were incubated (20 min at 30°C) with 2 × 105 cpm of radiolabeled transcript in 20 μl of binding buffer (15 mM HEPES, pH 8.0, 10 mM KCl, 10% glycerol, 1 mM DTT, 1 mM PMSF) containing 5 μg of yeast tRNA. After a 45-min incubation with RNase T1 (60 ng/μl) at 37°C, UV cross-linking studies were performed by exposing the reaction mixtures on ice for 10 min to 254-nm UV light in a Stratalinker (Stratagene, La Jolla, CA) on automatic settings. Subsequently, RNA-protein complexes were boiled for 5 min in 1× sodium dodecyl sulfate (SDS) gel loading buffer and resolved by 10% polyacrylamide-SDS gels, dried, and exposed to X-ray film for 6 to 15 h with an intensifying screen at −70°C. La-depleted U87 cytoplasmic extracts were obtained by immunodepletion using anti-La SW5 coupled to protein A Sepharose (Amersham Biosciences). Protein A-Sepharose Fast Flow (0.1 ml) suspended in 0.4 ml of 30 mM HEPES, pH 7.9, 3 mM MgCl2, and 140 mM KCl (buffer B) was conjugated gently with anti-hLa monoclonal antibody SW5 by rotation for at least 2 h at 4°C, washed three times with 1 ml of buffer B, pelleted, drained, and incubated with U87 cytoplasmic extract (0.4 ml) for 20 min at 4°C with rotation. This step was repeated two to five times, and the resulting sample was conserved as the La-immunodepleted fraction.
Supershift analyses.
Anti-La monoclonal antibody SW5 (20 to 400 ng) was added to the cytoplasmic extracts in binding buffer in the absence of RNA probe for 30 min at room temperature. RNA riboprobe was then added, and the incubation continued for an additional 30 min before gel analysis on a 10% native polyacrylamide gel in 0.5× Tris-borate-EDTA. The supershift control experiment was performed with increasing quantities of the anti-calcitonin receptor-like receptor (CRLR) polyclonal antibody. All the experiments were performed in triplicate with two different protein preparations.
Gel mobility shift assays.
The synthetic nonamer U was 5′ end labeled with T4 polynucleotide kinase (New England Biolabs, Ozyme, Paris) and [γ-32P]ATP (Amersham Pharmacia Biotech) and purified on a 6% polyacrylamide gel containing 7 M urea. For the gel mobility shift assays, 300 ng of RNA substrate was titrated with 50 to 1,000 nM 3′ UTR PAM 1 riboprobe and 10 to 200 nM U nonamer in binding buffer. The binding reactions were incubated on ice for 30 min in a total volume of 30 μl. After loading dye (40% sucrose, 0.01% [wt/vol] xylene cyanol) was added, the solutions were immediately resolved on a prerun 9% native polyacrylamide gel at room temperature in 0.5× TBE buffer (45 mM Tris-HCl, 45 mM boric acid, 0.1 mM EDTA). The gels were run for 1 h at 110 V and dried under vacuum, exposed to X-ray film, and quantified by autoradiographic densitometry using NIH Image 1.54 software (National Institutes of Health, Bethesda, MD).
Western blot analysis.
Total cell extracts were prepared from U87 cells transfected with 2, 5, 10, and 20 μg of pcDNA-hLa-wt or pcDNA3.1-LaΔ316-332 plasmids. Cell pellets were washed three times with ice-cold PBS, pH 7.2, and homogenized in ice lysis buffer (20 mM HEPES, pH 7.9, 10 mM NaCl, 1 mM MgCl2, 10% glycerol, 0.2 mM EDTA, 0.5 mM DTT, 0.2 mM EDTA, 10 μg/ml leupeptin, 0.5 mM PMSF, and 0.35% [vol/vol] Triton X-100). Nuclei and cell debris were removed by centrifugation at 12,000 × g for 10 min at 4°C. The supernatants were quickly frozen in liquid nitrogen and stored at −80°C. Protein content was assayed using a bicinchoninic acid protein assay kit with bovine serum albumin as a standard (Pierce, Perbio, Paris, France). Western Blot analysis was performed as previously described (12). Antibodies used were monoclonal anti-hLa SW5 (kind gift of G. Pruijn, The Netherlands), polyclonal anti-calnexin (Stressgen Biotechnologies Corp.), monoclonal anti-Histone H1 (Stressgen Biotechnologies Corp.), and polyclonal anti-β actin (Cytoskeleton, Inc.).
Reverse transcription-PCR (RT-PCR) of coimmunoprecipitated RNA.
U87 cells from a 75-cm2 flask were harvested in 1 ml of cold PBS and collected by centrifugation (10 min at 1,000 × g at 4°C). Cells were suspended in 100 μl of RNA binding buffer supplemented with 10 U of RNase inhibitor and homogenized, and cytoplasmic and nuclear fractions were prepared as described above. Immunoprecipitations (IPs) were performed using 0.5 μg of monoclonal anti-La antibody SW5 (a kind gift from G. Pruijn, University of Nijmegen, The Netherlands) or 50 μl of anti-CRLR polyclonal antibody or preimmune sera and 20 μl of protein A Sepharose (PAS) (Amersham Biosciences) as described (39). Briefly, after conjugation, the immunoglobulin G-PAS beads were washed five times with 1 ml of binding buffer and incubated with 50 μl of precleared extract in a final volume of 300 μl binding buffer supplemented with 10 U RNase inhibitor for 1 h at room temperature. The immunoglobulin G-PAS beads were spun out, and the supernatant was collected. The beads were then washed five times with 1 ml of cold binding buffer, and the RNA associated was isolated by repeated phenol-chloroform extraction. Total RNA was purified from equivalent amounts of the supernatants and input, as well as from all the immunoprecipitated material using guanidinium thiocyanate, phenol, and chloroform, followed by ethanol precipitation. RNA was reverse-transcribed into cDNA using 1 μg of hexamers (Amersham Biosciences) and Moloney murine leukemia virus reverse transcriptase as described by the manufacturer (Invitrogen Life Technologies). For the reactions containing heat-inactivated Moloney murine leukemia virus reverse transcriptase, the enzyme mix was heated to 96°C for 5 min before being added. PCR conditions using Taq polymerase (Invitrogen Life Technologies) were as follows: for PAM, 5 min at 95°C, followed by 30 cycles of 20 s at 94°C, 50 s at 62°C, and 1 min at 72°C; for GAPDH (glyceraldehyde-3-phosphate dehydrogenase), 25 cycles of 20 s at 94°C, 30 s at 60°C, and 30 s at 72°C; for mY1, 25 cycles of 20 s at 94°C, 30 s at 58°C, and 45 s at 72°C. Equal aliquots of the products were electrophoresed on a 1.6% agarose gel. The primers used were the following: for PAM, forward primer 5′-TACTGGGAAGATTTAGAGG-3′ and reverse primer 5′-CTTGGTTTTCAGGAGGAGGA-3′; for GAPDH, forward primer 5′-CAAATTCCATGGCACCGTC-3′ and reverse primer 5′-CCCATCT GATTTTGGAGGGA-3′; for hY1, forward primer 5′-AAAGACTAGTCAAGTGCAG TAGTAAGA-3′ and reverse primer 5′-CACTACCTTCGGACCAG-3′.
Slot blot analysis of RNA from nuclei and cytoplasm.
Nuclear fractions were prepared as described above. After gentle homogenization and centrifugation, 2 volumes of GTC solution (4 M guanidinium thiocyanate, 25 mM sodium citrate, pH 7.0, 0.1 M 2-mercaptoethanol) was added to the cytoplasmic fraction. The nuclear pellet was washed and resuspended directly in the GTC solution, and RNA was isolated as previously described (8). RNA was denatured by heating at 65°C for 15 min in 2.2 M formaldehyde, 6× SSC (1× SSC is 0.15 M NaCl, 0.015 M sodium citrate, pH 7.0) and cooled on ice before applying to a Hybond-N membrane (Amersham Biosciences) with the Schleicher and Schuell (Keene, NH) Minifold II Slot Blotter. After adding 1 × 106 cpm of the 32P-labeled probe and 1 ml of hybridization solution, the filters were hybridized as described (12). The blots were washed several times with 2× and 0.1× SSC containing 0.1% SDS at 55°C and exposed to autoradiographic film (Hyperfilm, Amersham Biosciences). To correct for the actual amount of RNA in each slot, blots were stripped and hybridized to human cDNA GAPDH and U6 RNA probes, respectively. The autoradiograms were analyzed by measuring absorbance (A) with a scanner-densitometer and NIH Image 1.54 Software (National Institutes of Health, Bethesda, MD). The results are expressed as absorbance (A) of PAM mRNA and luciferase RNA/A GAPDH RNA and A of intronic PAM RNA/A U6 RNA, with the panels representing the mean ± standard error of the mean (SEM).
Preparation of cell extracts and amidation assay.
U87 cells (3 × 105) were transfected with 2, 5, and 10 μg of hLa plasmid or pcDNA 3.1 vector with the Fugene 6 transfection reagent (Roche Applied Science). After 48 h, the cells were scraped into ice-cold PBS and collected by centrifugation at 2,000 × g for 5 min. The resulting cellular pellet was homogenized in 20 mM NaTES (N-tris [hydroxy-methyl] methyl-2-amino-ethane sulfonic acid), pH 7.4, 10 mM mannitol, containing 1% (vol/vol) Triton X-100, 2 μg/ml leupeptin, 16 μg/ml benzamidine, and 300 μg/ml PMSF, using a glass homogenizer at 4°C. The homogenates were frozen and thawed three times and then centrifuged at 2,000 × g for 10 min at 4°C. The supernatants were removed and assayed for protein content using a bicinchoninic acid protein assay kit with bovine serum albumin as a standard (Pierce, Perbio, Paris, France). Spent medium was removed from cells and cleared of cell debris by centrifugation at 400 × g for 5 min.
Enzyme assays were performed as described previously (35); samples (1.5 μg of cell extract protein or 15 μl of 48-h spent medium) were assayed in duplicate. Peptidylglycine-α-hydroxylating monooxygenase reactions were carried out in a final volume of 50 μl containing 150 mM sodium morpholineethanesulfonic acid (pH 5.0), 3.3 μM CuSO4, 0.18 mg/ml catalase, 0.5 mM ascorbate 0.5 μM α-N-acetyl-Tyr-Val-Gly, and 15,000 cpm of [mono-125I]-α-N-acetyl-Tyr-Val-Gly for 2 h. Dose-response studies indicated that 3.3 μM CuSO4 gave the maximum level of activity. The reaction was linear in time for up to 3 h and in the amount of protein up to 4 μg. Any α-N-acetyl-Tyr-Val-α-hydroxyglycine present at the end of the reaction was converted to α-N-acetyl-Tyr-Val-NH2 by the addition of 10 μl of 1 M NaOH; conversion was complete in less than 1 min. Substrate and α-amidated product were separated using ethyl acetate as described (35). Reaction velocities were expressed as pmol of product formed per mg protein per h (specific activity). The variation between duplicate samples was less than 5%. The reaction velocities reported are initial velocities, using a concentration of substrate about 10-fold below the Km of the enzyme for the peptide substrate. In general, no more than 10% of the substrate was converted into product.
Preparation of luciferase constructs.
All the mutants described in the study were generated by using a megapriming PCR method as described previously (38). The method utilizes three oligonucleotide primers to perform two rounds of PCR. The heterologous luciferase gene construct was generated by inserting the 605-bp whole 3′ UTR of the PAM gene into a unique XbaI site immediately downstream of the luciferase gene in pGL3 (Promega). Deletions within the putative La binding site were generated using a PCR approach with pBS-PAM as template using the following overlapping primers: for PAM 3′ UTRΔ3769-3783 sense primer, 5′-TCTATTTTTTTATATTGCAATG-3′; for PAM 3′ UTRΔ3756-3796 sense primer, 5′-GGCCATGGTC AAGCCTTGCCACCAGCTGGGCTTGGACTTC-3′. For the first round of PCR, the antisense primer corresponding to the 3′ end of the 3′ UTR was 5′-CCGGTACCCCCGGCTGCGAAGC GCAG-3′; to generate a megaprimer 20 cycles were used, with each cycle consisting of denaturation (94°C for 30 s), annealing (65°C for 30 s), and extension (72°C for 30 s) using Taq DNA polymerase (Invitrogen Life technologies). The megaprimers thus obtained were then used as 3′ primers in the second round of PCR along with the 5′-GGCCATGGTCAAGCCTTGCCACT-3′ primer corresponding to the 5′ end of the 3′ UTR. The PCRs were carried out for 25 cycles, with each cycle consisting of denaturation (94°C for 30 s), annealing (65°C for 30 s), and extension (72°C for 30 s) using Taq DNA polymerase. The underlined sequences correspond to XbaI sites included for subsequent subcloning. The products were inserted into a TOPO vector (Invitrogen life Technologies) for sequencing to verify deletions and recloned into the XbaI site of the pGL3 vector to generate PAM 3′ UTRΔ3769-3783/Luc and PAM 3′UTRΔ3756-3796/Luc.
Reporter assay using luciferase and quantification of reporter mRNA by slot blot analysis.
A dual luciferase reporter assay system was used. U87 cells (2 × 105 cells/ml) were transfected with a mixture of firefly luciferase reporter vector (200 ng), Renilla luciferase control vector pRL-TK (20 ng; Promega), and pBS SK+ vector (780 ng; total of 1 μg of vector), with the Fugene 6 transfection reagent (Roche Applied Science). After 24 h, the cells were lysed with assay buffer (Promega), and both firefly (Photinus pyralis) (reporter) and sea pansy (Renilla reniformis) (control) luciferase activities were measured sequentially using a Dual-Luciferase Assay Reporter System (Promega) with a Berthold Lumat LB9507 luminometer. In a second set of experiments, the luciferase reporter construct containing the PAM 3′ UTR (see Fig. 10A) was cotransfected with an increasing amount of either hLa-wt or LaΔ316-322 plasmids to assess the effect of the absence of nuclear retention element on luciferase activity. The ratio of reporter luciferase activity in relative light units was divided by the control Renilla luciferase activity to yield a normalized reporter luciferase value. The expression levels of luciferase/PAM 3′ UTR chimeric mRNA was assessed in both nuclei and the cytoplasm as described above.
FIG. 10.
Effect of the PAM 3′ UTR on the translational efficiency of chimeric RNA. (A) Schematic representation of luciferase reporter constructs containing the 3′ UTR of PAM (b) or the 3′ UTRΔ3769-3783 (c) and pLuc vector (a). All the constructs were under the simian virus 40 (SV40) promoter. (B) Luciferase reporter assay. U87 cells were transiently cotransfected with the indicated luciferase reporter constructs and the Renilla reporter construct (pRL-TK; see Materials and Methods) for 24 h. The results are presented as ratios of firefly luciferase reporter activity over sea pansy (Renilla) luciferase activity, the latter being used as a control. Each bar represents the mean ± SEM; asterisks indicate that the values are significantly different between a, b, and c (***, P < 0.0001). Similar data were obtained from three independent experiments. (C) U87 cells were transfected with increasing concentrations of b and c. Nuclear and cytosolic RNAs were prepared 24 h later. Slot blots containing 10 μg of each nuclear and cytosolic RNA sample were hybridized to probe corresponding to firefly luciferase cDNA and exposed to X-ray film at −70°C with an intensifying screen. The blot was reprobed with 32P-labeled GAPDH probe to normalize data for quantification. (D) Quantification analysis of the blots was performed as described in the legend of Fig. 7. Each bar represents the mean ± SEM of three independent experiments. For cytosolic (CYT) RNA, asterisks indicate that the values are significantly different between a and b versus c; for nuclear RNA (NUC), asterisks indicate that the values are significantly different between a and c versus b.
Statistical analyses.
All the results are expressed as the mean values ± SEM. Statistical analysis was performed by a one-way analysis of variance followed by Fisher's probable least squares difference test (Statview 512; Brain Power Inc., Calabasas, CA). Statistical significance was assessed at P < 0.05.
RESULTS
UV cross-linking analyses using a riboprobe corresponding to the PAM 3′ UTR (PAM 1) demonstrated the presence of protein(s) in nuclear and cytoplasmic extracts of the rat anterior pituitary (Fig. 1, lanes 3 and 5) and human U87 glioblastoma cell line (Fig. 1, lanes 4 and 6), which bind to this riboprobe to form a 60-kDa complex (16). The PAM RNA binding protein responsible for the complex was purified from rat anterior pituitary extracts by RNA affinity chromatography using a 5′ biotinylated PAM 3′ UTR RNA coupled to streptavidin microbeads. The purified protein, which exhibited efficient binding to 32P-PAM 3′ UTR RNA (Fig. 1, lane 7), was size-fractionated on an SDS-polyacrylamide gel followed by Coomassie blue staining (Fig. 2A). In addition to the expected 47- to 48-kDa polypeptide band, three additional polypeptide bands (44, 50, and 68 kDa) were also detected in experimental, but not control, experiments (Fig. 2A). These bands were cut from the gel (Fig. 2A) and digested with trypsin. The resulting peptides were separated by reverse-phase high-performance liquid chromatography and analyzed in line by electrospray ionization mass spectrometry and MS/MS by collision-induced dissociation. The resulting m/z values of the peptide ions and the corresponding collision-induced fragment ions were searched against theoretical masses of in silico trypsin-digested and -fragmented proteins from the SWISS-PROT database. Several proteins were identified. Among those, Rat La protein (accession no. P38656) was identified in band 2 by the tryptic peptide ions of m/z 418.2, 446.2, 473.2, 539.2, and 645.7 and their corresponding fragment ions. The peptide sequences could be read from abundant fragment ions and matched to the La protein sequence (Fig. 2B and C).
FIG. 1.
Characterization of the PAM mRNA-protein complex in rat anterior pituitary gland and U87 cells. UV cross-linking with the 32P-labeled PAM RNA 3′ UTR and nuclear (Nuc; lanes 2 and 3) or cytoplasmic (Cyto; lanes 5 and 6) extracts (30 μg) from rat anterior pituitary gland and U87 cells, and purified PAM mRNA-binding protein (50 ng) (lane 7). No RNA-protein complex was observed in the absence of protein extracts (lane 2). Lane 1 showed the PAM 1 probe in the absence of RNase T1. The RNA-protein complex at 60 kDa is indicated by an arrowhead.
FIG. 2.
Identification of the La protein as a potential PAM mRNA-binding protein. (A) Proteins from rat anterior pituitary extracts were affinity purified and fractionated by SDS-polyacrylamide gel electrophoresis. The gel lane that is obtained with the bands of 44, 47/48, 50, and 68 kDa is cut into several slices, which are then tryptic digested in gel. (B) Only the mass spectrum of the peptides that were generated from band 2 at 47/48 kDa in panel A are shown. The mass differences between the y-ion series indicate the amino acid series, which is shown above each spectrum. As this is an ion series, the sequence is written in the direction, from left to right, from the carboxy to the amino terminus. m/z, mass-to-charge ratio. (C) The peptide-sequencing data that are obtained from the mass spectra were searched against the protein databases (SWISS-PROT database). The positions of the four identified peptides are highlighted in bold in the La protein sequence.
La protein binds to PAM mRNA both in vitro and in vivo.
To determine whether the La antigen is the PAM mRNA-BP, bacterially expressed His-La protein was subjected to RNA UV cross-linking using the 32P-labeled PAM RNA 3′ UTR, and as expected, a complex at 60 kDa was detectable in U87 cells (Fig. 3A, lane 1). Importantly, RNA/protein complex was considerably reduced using La-immunodepleted U87 cytoplasmic extracts (compare lanes 1 and 2), confirming that La is required for complex formation. In contrast, the addition of 500 ng of the bacterially expressed His-La protein to the La-immunodepleted U87 cytoplasmic extracts resulted in the recovery of the 60-kDa single RNA-protein complex (Fig. 3A, compare lanes 2 and 3). To further confirm the specific binding between La and PAM RNA, a supershift analysis was carried out using the La-specific monoclonal antibody SW5 and U87 cytoplasmic extracts. As can be seen (Fig. 3B), the monoclonal antibody SW5 shifted the La-PAM RNA complex in a concentration-dependent manner (Fig. 3B, lanes 5 to 8), whereas incubation with increasing quantities of a nonspecific anti-CRLR antibody had no effect on the formation of the complex (Fig. 3B, lanes 10 to 12). Taken together, these results strongly suggest that La protein forms a specific RNA-binding protein complex with the PAM mRNA 3′ UTR in vitro.
FIG. 3.
La protein binds to the 3′ UTR of PAM RNA in vitro. (A) UV cross-linking of human PAM RNA 3′ UTR (PAM 1 probe) with nondepleted and La-immunodepleted U87 cytoplasmic extract (lanes 1 and 2) and bacterially produced His-La added to La-immunodepleted U87 cytoplasmic extract (lane 3). (B) Antibody to human La protein supershifts the PAM mRNA-BP/RNA complex. Gel mobility shift assay with PAM 1 probe in the absence (lanes 2 and 9) and presence (lanes 3 and 10) of U87 cytoplasmic extract. No complex can be observed when the PAM 1 probe is incubated only with anti-La (SW5) (lane 4). The presence of increasing amounts of anti-La (SW5) with U87 cytoplasmic extracts can supershift the complex (lanes 5 to 8). A complete supershift of the band at 60 kDa can be seen at a high concentration of anti-La protein (400 ng). However, no supershift is observed with anti-CRLR used as a negative control (lanes 11 and 12).
A specific physical association between La and PAM mRNA was also demonstrated in U87 cell lysate (cytoplasmic and nuclear extracts separately) by RT-PCR after IP with anti-La, but not preimmune serum, or anti-CRLR (Fig. 4).
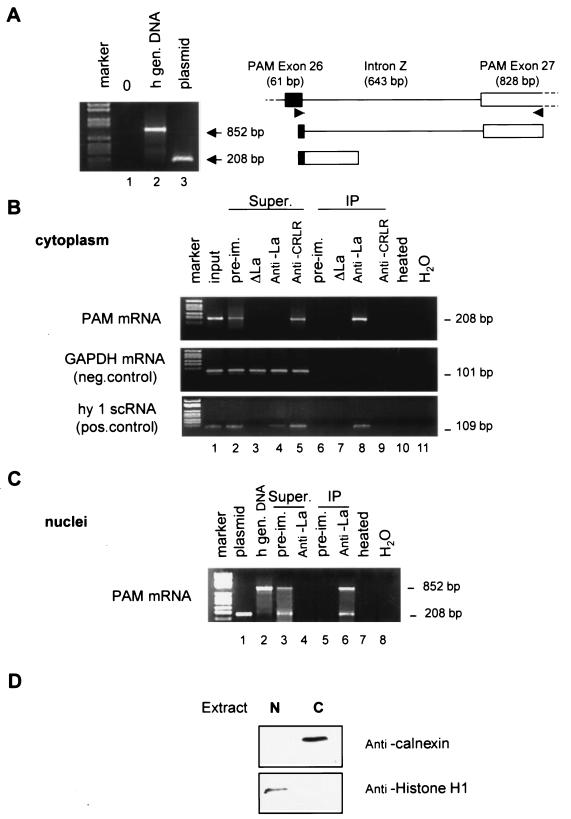
FIG. 4.
Association of La with PAM mRNA in vivo. (A) Structure of the PAM gene in the region of exons 26 and 27. Human genomic DNA (10 ng) was amplified (lane 2) using a sense oligonucleotide primer within exon 26 (bp 2917 to 2935) and antisense oligonucleotide within exon 27 (bp 3102 to 3121) of human PAM 1. Plasmid DNA (100 pg) was used as a control (lane 3). Amplified fragments were separated on a 1.6% agarose gel, and the ethidium bromide-stained gel is shown. Apparent molecular weights are shown to the side of the panel. (B) Equal aliquotsof purified U87 total RNA isolated from the input (lane 1), preimmune serum, or La-immunodepleted cytoplasmic extract or from anti-La or anti-CRLR immunoprecipitates (lanes 6 to 9) and from the supernatants (Super; lanes 2 to 5) of the immunoprecipitates were assayed by RT-PCR to detect PAM, GAPDH, and hY1 small cytoplasmic RNA (scRNA) transcripts. The reaction in lane 10 contained anti-La immunoprecipitated RNA, but the RT enzyme mix was heat inactivated prior to use; the reaction in lane 11 contained water instead of RNA. (C) Purified U87 nuclear RNA isolated from preimmune serum or anti-La immunoprecipitates (lanes 5 and 6) and from the supernatants (Super; lanes 3 and 4) of the immunoprecipitates were assayed by RT-PCR to detect PAM RNA transcripts. Lanes 1 and 2 were used as a control as described in panel A. Lanes 7 and 8 were as described for lanes 10 and 11 in panel B. (D) To assess the purity of the nuclear (N) and cytoplasmic (C) fractions, immunoblot analysis was done with anticalnexin or antihistone H1.
In the cytoplasmic extracts (Fig. 4B), mature PAM mRNA was readily detected in the anti-La IP (lane 8) but not in the preimmune IP (lane 6), in the La-immunodepleted IP (lane 7), and in the anti-CRLR IP (lane 9). As expected, PAM mRNA was also detected in total RNA prepared from the U87 cell extracts used for IPs (Input, lane 1). Moreover, preimmune serum and anti-CRLR did not deplete PAM mRNA from the extract (lanes 2 and 5), while anti-La did (lane 4). It is noteworthy that the pattern observed for PAM mRNA was similar to that for hY1 small cytoplasmic RNA (Fig. 4B), which is known to be stably associated with La (21). GAPDH mRNA, which is known not to be stably associated with La, served as a negative control (Fig. 4B).
Since the human La protein is predominantly nuclear phosphoprotein, we sought to determine whether La is associated with PAM primary transcripts and/or mature PAM mRNA in the nucleus (Fig. 4C). Tissue-specific alternative splicing of the single copy PAM gene can generate several forms of PAM mRNA in rat and human (10, 32, 33). Both the PAM sense and antisense primers are localized in exons 26 and 27, respectively, and the structure of this region of the PAM gene was investigated using the PCR (Fig. 4A). Exon 26 terminating with nt 2935 is separated from exon 27 by an approximately 643-nt intron.
RT-PCR after IP with anti-La of nuclear extracts demonstrated the presence of two specific products, one at 852 bp generated from PAM RNA primary transcript and the second at 208 bp that should be generated from mature PAM RNA (Fig. 4C, lane 6) as demonstrated for cytoplasmic extracts (Fig. 4B, lane 8). Moreover, preimmune serum did not deplete PAM mRNAs from the extracts (Fig. 4C, lane 3), while the anti-La did (Fig. 4C, lane 4). These results taken together suggest that the recovery of hPAM mRNAs requires the RNA-binding capacity of the La-protein and show that the La protein can interact with immature and mature PAM RNA in vivo. These results provide convincing evidence that PAM mRNA is specifically associated with La in U87 cells.
Characterization of La binding affinity with a 3′ UTR PAM mRNA ligand compared to a 9-nt poly(U) RNA ligand.
In order to determine the physiological importance of the La protein/3′ UTR PAM-mRNA interaction, affinity was determined in order to compare it to the known functional interactions of La. One of the most commonly admitted binding motifs of La is poly(U)OH, the terminal motif of nascent Pol III transcripts. For these reasons, a U nonamer, for which binding affinities to La had been reported as 25 nM (30) or 162 nM (25), was used as an internal standard. Equilibrium binding titration experiments were run to determine the apparent dissociation constants (KD) of wild-type hLa for different RNA substrates. The purified bacterially expressed La protein was used to calculate binding affinities. A gel shift analysis was first performed by incubating 300 ng of labeled PAM 3′ UTR RNA with different quantities of hLa protein. Increasing amounts of hLa protein were then added to the nonameric RNA substrate 9-nt U, with a polyuridylate sequence at the 3′ terminus to mimic the La-binding site on tRNA precursors (27). Binding titrations were carried out in identical conditions. As the protein concentration increased, the RNA probe was recruited into a single, slower migrating form, resulting from the formation of a specific complex (Fig. 5A and C). All KD values are apparent dissociation constants, with the assumption that the protein binds as a monomer since the state of oligomerization was not determined. Although gel shift bands were observed with 125 nM His-hLa for 3′ UTR PAM RNA, bands for hLa and the nonameric RNA interaction were observed starting with a 30 nM protein (Fig. 5A and C). A theoretical saturation binding curve was generated by plotting the percentage of bound 3′ UTR PAM RNA or nonamer RNA versus. hLa concentration. The relative KD for the interaction between PAM 3′ UTR RNA and hLa was estimated at about 155 nM (Fig. 5A), while that for the interaction between the nonamer RNA and the same protein was estimated at about 38 nM (Fig. 5C). The slope (n) of the line represents the ratio of hLa molecules to each RNA molecule in each complex (Fig. 5B and D, inserts). Similar KD and n values were obtained in three independent experiments. These data indicated a relatively high binding activity of hLa for the PAM 3′ UTR that was only about four times lower than that of the nonamer U RNA, i.e., of the same order of magnitude as the canonical 3′ end of Pol III transcripts.
FIG. 5.
Quantification of RNA-protein interactions. (A) Representative gel mobility shift assay with increasing amounts of the hLa protein and a constant amount of 3′ UTR PAM mRNA. Lane 1, free probe; lanes 2 to 11, probe plus His-hLa in the amounts indicated; M, multimer of the probe; fp, free probe. (B) The percent 32P-PAM 1 RNA bound was plotted against His-hLa concentration to generate a theoretical saturation binding curve (inset). The stoichiometry of the interaction of hLa with PAM 1 RNA, as determined by the slope of the line in the inset graph, was about 1:1. The dissociation constant was calculated using the following equation: log (percent bound/percent unbound) + 2 = n{log[His-hLa (nM)] + 1} − log KD. The KD was estimated to be 155 nM for hLa-PAM 1 RNA. (C) Representative gel mobility shift assay with increasing amount of His-hLa protein and a constant amount of oligo(U). Lane 1, free probe; lanes 2 to 9, probe plus hLa in the amounts indicated. (D) The percent 32P-oligo(U) bound was plotted against the concentration of His-hLa to generate a theoretical saturation binding curve. The data from the saturation binding curve were transformed (inset). The KD was estimated to be 38 nM.
Mapping the La-PAM mRNA binding site.
We previously identified a 20-nt cis-element, 5′-CACUAACAUUAUAUUGCAAU-3′, in the 3′ UTR of rat PAM mRNA that interacted with PAM mRNA-BP (16). To further investigate the required sequence for the La protein-RNA interaction, RNA UV cross-linking was carried out using the bacterially expressed La protein and deletion variants of the human PAM 3′ UTR transcripts. In vitro transcribed PAM transcripts and deletions thereof (PAM 1 to PAM 9) were assessed for their binding capacity to La protein (Fig. 6). While the various 3′ UTR PAM transcripts (PAM 1, PAM 3, PAM 4, and PAM 5 to PAM 6) (Fig. 6B) had the same high binding capacities, binding to the PAM 7 transcript was very low (Fig. 6B, lane 7), and PAM 2 transcripts failed to reveal any binding activity at all (Fig. 6B, lane 2). These data show that the La protein interacts with a region common to full-length 3′ UTR PAM RNA and PAM 6 corresponding to the 3′ UTR RNA sequence 3756 to 3796. Interestingly, deletions of sequences 3756 to 3796 or 3769 to 3783 to generate PAM 8 and PAM 9 transcripts, respectively, were sufficient to completely abolish the formation of the RNA-La protein complex (Fig. 6B, lanes 8 and 9). According to these data, La protein did not recognize the RNA-designated PAM 2 that lacked the putative binding site. In addition, sequences adjacent to the 15-nt binding site were apparently not directly involved in the interaction, since no binding was observed with PAM 8. These results clearly demonstrate that La protein binds to 3′ UTR PAM RNA and that the PAM cDNA sequence of bp 3769 to 3783 presents the major La protein binding recognition site.
Regulation of PAM expression by La protein: endogenous PAM expression and reporter assay.
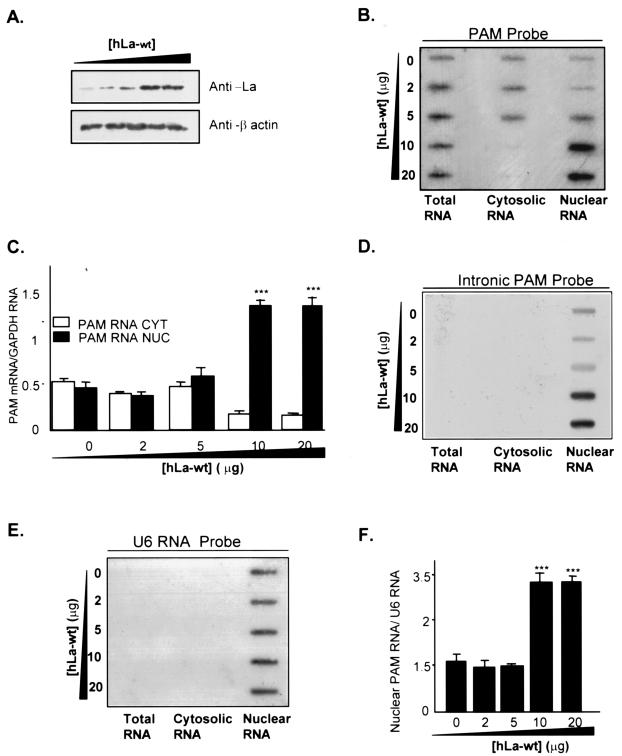
In order to assign a function to the PAM-La protein interaction, we examined the effect of La protein on the expression of endogenous PAM mRNA (Fig. 7). U87 cells were transiently transfected with hLa-wt plasmid and showed that La protein accumulates in the cell at increasingly larger amounts as the amount of transfected hLa-wt plasmid increases (Fig. 7A). Total, cytoplasmic, and nuclear RNAs prepared from U87 cells were analyzed by slot blotting initially hybridized to hPAM cDNA spanning the full-length of PAM mRNA (Fig. 7B). The amount of PAM mRNA in each sample was then normalized to the amount of GAPDH mRNA used as a control. A three- to fourfold increase in the levels of PAM mRNA was detected in the nuclear fraction compared to total and cytoplasmic fractions when high transfectant concentrations were used (Fig. 7C). In parallel, the PAM mRNA levels in cytoplasm showed a three- to fourfold decrease (Fig. 7C). Since the PAM cDNA probe cannot exclude hybridization with intron-containing pre-mRNA, nuclear slot blots were hybridized with a PAM intronic (I-PAM) probe (Fig. 7D), derived from genomic clone G33 (15) to assess primary (unspliced) PAM RNA transcript levels. We had previously shown that I-PAM contains no repetitive sequences or PAM exon sequences (15). The amount of unspliced immature PAM mRNA was quantified and normalized to the amount of small nuclear U6 RNA (Fig. 7E), which was also used to reveal the appropriate subcellular distribution. A two- to threefold increase in the levels of unspliced PAM RNA was obtained, along with increased La protein expression (Fig. 7F). Although mature PAM mRNA might be present in the nuclei as demonstrated by IP studies (Fig. 4C), the data in Fig. 7 indicate that increased expression of La protein caused an accumulation of unspliced nuclear PAM RNA, suggesting that unspliced mRNA constitutes the major source of the signal in Fig. 7. The same results were obtained using other intronic fragments derived from PAM genomic clones (data not shown). Based on these results, La protein is likely to be involved in the nuclear retention of PAM mRNA.
FIG. 7.
Potential regulation of PAM gene expression by La protein. (A) U87 cells were transfected with increasing amounts (0, 2, 5, 10, and 20 μg) of hLa plasmid. The Western blot analysis demonstrates that La protein accumulates in the cell at increasingly larger amounts as the amount of transfected La plasmid increases. β-Actin levels were monitored as controls for loading. (B to F) Effect of La protein overexpression on PAM RNA levels in nuclear and cytosolic fractions. Nuclear (NUC), cytosolic (CYT), and total RNA were prepared from U87 cells at 24 or 48 h posttransfection, and slot blots containing 10 μg of each RNA were prehybridized to probes corresponding to hPAM cDNA (B), and the I-PAM probe (D), respectively. The slot was subsequently stripped and reprobed with a cDNA corresponding to the GAPDH probe (not shown) and U6 RNA probe (E) to normalize data for quantification. The autoradiograms were densitometrically analyzed, and the levels of PAM RNA in panel B were normalized to levels of GAPDH mRNA on the same blot (C). The levels of nuclear PAM RNA in panel D were quantified and normalized to the levels of U6 RNA in panel E (F). Each bar represents the mean ± SEM of two independent experiments. The asterisks indicate that the values for samples 4 and 5 are significantly different from samples 1, 2, and 3 (***, P < 0.0001).
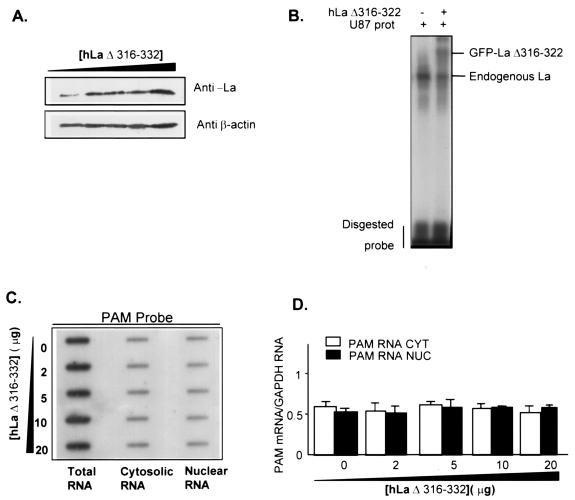
To further demonstrate that La is exerting its influence on PAM mRNA via a direct interaction, we examined the effect of La antigen on PAM mRNA subcellular localization by using the truncated La (amino acids 316 to 332) protein EGFP-hLaΔ316-332 (where EGFP is enhanced green fluorescent protein; a generous gift of R. V. Intine and R. J. Maraia) that has been characterized as a useful tool to bind RNA but fails to be retained in the nucleus (23). Residues deleted in hLaΔ316-332 were previously shown to be required for retention of La in microinjected frog oocyte nuclei (5, 20). U87 cells were transiently transfected with pcDNA3.1-LaΔ316-332 plasmid, and immunoblots of total extracts revealed that LaΔ316-332 protein accumulates in the cell at increasingly larger amounts as the amount of transfected LaΔ316-332 plasmid increases (Fig. 8A). Band shift assays demonstrate that GFP-hLaΔ316-332 protein forms a complex with PAM 1 probe (Fig. 8B), suggesting that the nuclear retention element-deficient hLaΔ316-332 protein might interact with PAM mRNA in vivo. Therefore, we examined the subcellular localization of PAM mRNA after the transfection of increasing amounts of either pcDNA3.1-hLaΔ316-332 or GFP-hLaΔ316-332 plasmids, and the data demonstrated that in contrast to hLa-wt (Fig. 7B and D), hLaΔ316-332 failed to retain PAM mRNA in the nuclei (Fig. 8C to E), as expected. This result strongly suggests a direct association between PAM mRNA and hLaΔ316-332 to form a complex that might be exported from nuclei.
FIG. 8.
Effect of high expression of hLaΔ316-332 on subcellular localization of PAM mRNA. U87 cells were transfected with increasing amounts of the hLaΔ316-332 plasmid (0, 2, 5, 10, and 20 μg), and 24 or 48 h later, cells were homogenized to prepare RNA and protein extracts, respectively. (A) hLaΔ316-332 protein levels in total extract were detected by Western blotting. β-Actin levels were monitored as controls for loading. (B) Representative gel mobility shift assay with U87 cytoplasmic extracts transfected with 20 μg of either empty vector (lane 1) or GFP-hLaΔ316-332 plasmid (lane 2). (C) PAM mRNA levels in total, cytosolic, and nuclear RNA were analyzed by slot blot and quantified as described in the legend of Fig. 7. (D) Levels of PAM mRNA were normalized to GAPDH mRNA levels. (E) Levels of nuclear PAM RNA detected with intronic PAM probe were normalized to levels of nuclear U6 RNA. CYT, cytosolic RNA; NUC, nuclear RNA.
Regulation of PAM activity by La protein.
Since the overexpression of La protein had a dramatic effect on the subcellular localization of PAM mRNA (Fig. 7), the effect of increased hLa protein expression on PAM specific activity was also assessed (Fig. 9). hLa protein overexpression decreased the level of the endogenous PAM activity in both cell extracts and medium approximately to 56% and 36%, respectively, of the level in control cells transfected with the empty vector (Fig. 9A and B). The decrease of PAM activity observed in the medium mirrored the decrease of PAM activity found in the cell extracts. The change in PAM activity after La protein overexpression was in the same direction as the change in levels of PAM mRNA in the cytoplasm. Based on these results, La protein is likely to be involved in the regulation of PAM protein expression by modulating the PAM mRNA levels into the cell.
FIG. 9.
Effect of La protein expression on PAM specific activity in U87 cells. U87 cells were transfected with indicated amounts of hLa plasmid or pcDNA 3.1 vector for 48 h. PAM-specific activity in cell extracts (A) and medium (B) was measured as described in Materials and Methods. Data from three experiments (n = 3 for each plasmid concentration in three experiments) were used to calculate the mean specific activity; each sample was assayed in duplicate. Data are presented as mean ± SEM. The asterisks indicate that the values are significantly different between both groups of cells (**, P < 0.01; ***, P < 0.001).
Following this, we used a reporter assay system containing heterologous luciferase gene constructs to investigate how the La protein modulates the expression of its putative targets in vivo. The firefly luciferase reporter plasmid was transiently transfected in U87 cells, in which La protein is expressed endogenously. The luciferase reporter gene combined with the entire 0.605-kb 3′ UTR of the PAM mRNA was placed under the control of the simian virus 40 promoter (Fig. 10A). The expression level of the reporter gene was quantified by assaying luciferase luminescence. Control cells transformed with vector alone showed high luciferase enzymatic activity, whereas the presence of the full-length PAM mRNA 3′ UTR resulted in a clear decrease of luciferase activity (P < 0.0001) (Fig. 10 B). In contrast, the enzymatic activity was recovered when the luciferase reporter gene with the truncated PAM mRNA 3′ UTRΔnt 3769-3783 was used to eliminate the La protein-binding site (Fig. 10 B). Slot blot analysis with a luciferase firefly-specific probe revealed that while luciferase RNA mainly accumulated in the cytoplasm, chimeric RNA with the 3′ UTR was significantly more concentrated in the nuclei compared to the cytoplasm (Fig. 10C and D). The observed decrease of the luciferase activity might be due to the nuclear retention of chimeric RNA with the full-length 3′ UTR. In contrast to full-length PAM 3′ UTR, the chimeric RNA with 3′ UTRΔnt 3769-3783 was clearly concentrated in the cytoplasm (Fig. 10 C and D), suggesting that the deleted sequence plays a key role in the nuclear retention of the chimeric RNA, probably via direct association with the La protein.
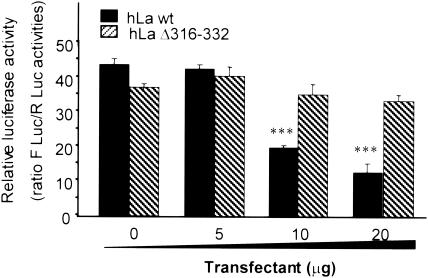
To further support this hypothesis, cells were cotransfected with a luciferase firefly 3′ UTR construct and increasing amounts of hLa-wt or hLaΔ316-332. The high expression of hLa-wt led to a dramatic decrease of luciferase activity (Fig. 11); in contrast, a high expression of hLaΔ316-332 failed to induce any decrease of the luciferase activity (Fig. 11). This demonstrated that chimeric RNA interacts with the hLaΔ316-332 but that the whole complex might be exported from nuclei. Based on the data shown in Fig. 7, 8, and 10, we assume that hLa-wt retained the chimeric RNA in the nucleus and hLaΔ316-332 did not.
FIG. 11.
Effect of high expression of hLaΔ316-332 on luciferase activity. U87 cells were transiently cotransfected with the luciferase reporter containing the 3′ UTR of PAM (see construct b in Fig. 10 A) and increasing amounts of either hLa-wt or hLaΔ316-332 as shown. After 48 h the cells were lysed, and the results are presented as ratios of firefly luciferase activity and sea pansy (Renilla) luciferase activity, used as a control. Data from three experiments (n = 3 for each plasmid concentration in three experiments) were used to calculate the mean luciferase activity. Data are presented as mean ± SEM. The asterisks indicate that the values are significantly different (P < 0.001).
Taken together, these observations indicate that PAM mRNA is likely one of the targets of La protein in vivo, providing convincing evidence that the La protein appears to retain PAM mRNAs via a direct association with the sequence nt 3769 to 3783 contained in the 3′ UTR.
DISCUSSION
The 3′ UTRs of the PAM mRNAs are conserved in size but are widely divergent in sequence except for a short region that is highly conserved among species and is located at roughly the same distance from the stop codon, suggesting that this region has a specific function. The importance of this conserved region was revealed by its requirement for binding protein both in vitro and in vivo. RNA probes and UV cross-linking identified a 47-kDa RNA binding protein present in mouse and human cells as well as in the rat tissues that interacts specifically with the cis-element in the 3′ UTR of PAM mRNA. Since the results suggest that the identified RNA binding protein might have an important role in the regulation of PAM expression, this protein was purified on the basis of its ability to interact with the conserved sequence tract in PAM mRNA and was identified as the La antigen (27, 45, 47). Indeed, PAM mRNA appears to be stably associated with La in U87 cells, since La immunodepletion abolished UV cross-linking (Fig. 3) and PAM mRNA recovery from the anti-La immunoprecipitate (Fig. 4).
Concerning the mechanism of action, the common feature of the interactions between La and the 3′ UTR of hPAM mRNA and between La and HCV-5′UTR-IRES is that La binds to an internal sequence. This internal sequence is located about 479 bp downstream from the stop codon and does not correspond to any known RNA binding motif of La protein. Mapping of La binding sites using several deletion mutants (reference 15 and the present work) indicates that binding requires the entire UUAAAAUCACUAACA sequence (nt 3769 to 3783). Interestingly, it is localized in a specific and conserved region in the 3′ UTR of different species (16) and presents a palindromic AAUCACUAA motif not yet described as a potential ligand of the La protein that might be the determinant involved in the interaction. Nevertheless, if we admit that certain parts of this sequence are used to position La on its binding site, it remains possible that, in the current state of our work, other motifs, whether or not localized in the vicinity of this sequence, contribute to optimal binding.
Concerning the functional aspect, there are common features in the La protein and 3′ UTR PAM mRNAs and La-pre-tRNA interactions in that they contribute to the retention of immature transcripts. The present results indicate that overexpression of hLa-wt protein induces a four- to fivefold decrease of PAM-mRNA levels in the cytoplasm (Fig. 7B and C), with an increase of these transcripts in the nucleus, suggesting that PAM-mRNA may be retained by La protein via direct interaction with the 3′ UTR. This process may repress the expression of endogenous PAM protein, as demonstrated by the decrease in PAM activity and suggested by the repressed expression of luciferase enzymatic activity when a luciferase reporter contained the 3′ UTR of PAM mRNA, in comparison to the corresponding wild-type messenger or the messenger lacking the La protein binding site (Fig. 10). Rat and human PAM are encoded by a complex gene consisting of 27 exons that encompasses more than 160 kilobases of genomic DNA (33). Interestingly, the analysis of nuclear RNAs clearly demonstrated a three- to fourfold increase in the levels of intron-containing RNA compared to levels obtained with lower La protein levels (Fig. 7D and F). These data suggest that La protein can bind the primary transcript at least at the transcriptional termination step since the binding site of La is localized in exon 27 encoding the 3′ UTR (33). They indicate that La may serve as a molecular link between Pol II termination and posttranscriptional processing. Alternative splicing of primary transcript generates numerous PAM mRNA transcripts (11, 33). It is tempting to hypothesize that La protein may act as a chaperone that protects and stabilizes primary transcripts of PAM at the precursor stage, maintaining them through a critical maturation process, after which time La retains a mature PAM mRNA in a stable form until it receives a signal to release it, such as 3′ UTR structural modifications and translocation into the cytoplasm occurs. Thus, as described for Pol III transcripts (18, 19, 28), La may act to stabilize the primary transcripts of PAM at the precursor stage and ensure their intranuclear retention for the processing by RNA-modifying enzymes and other factors. Regardless of the mechanism of action of La protein in addition to its role of chaperone for PAM mRNA transcripts, our findings implicate La in a process essential for producing a bioactive amidated peptide by regulating the synthesis of PAM activity through modulation of the metabolism of PAM mRNAs. It seems that having a pool of PAM mRNA that interacts with La could warrant a rapid production of PAM activity, rather than going through a de novo mRNA synthesis.
Recently, an interesting study in which Adilakshmi et al. (1) demonstrate that p53 along with two other identified proteins, MTF-1 and La, posttranscriptionally regulate the synthesis of the S25 protein by controlling the nuclear export of the stress-induced S25 mRNA. IP experiments performed on the nuclear extracts and subsequent immunoblot assays indicated that p53, MTF-1, and the La antigen form or are included in a complex associated specifically with the nuclear extracts in amino acid-starved but not fed Fao cells (1). In contrast to PAM mRNA for which we demonstrate a direct association with La, it is not clear from the author's study whether S25 mRNA has a direct interaction with La or via MTF-1 and p53 proteins.
Although the La antigen is mostly nuclear, it is also present in the cytoplasm and increases there under certain conditions (13, 47). For example, in poliovirus-infected cells, La is redirected to the cytoplasm, where it is believed to interact with the 3′ UTR of poliovirus mRNA to positively influence its translation (27, 47). In addition to multiple virus-derived mRNAs (27, 47), La has been reported to interact with cellular mRNAs. La antigen was identified as part of a multiprotein complex interacting with the IRES region of XIAP mRNA that is required for its translation in vitro and in vivo (22). Recently, the La antigen was reported to activate mdm2 translation upon binding to its mRNA (44). Here we cannot rule out that La antigen might be involved in translation of PAM mRNA since IP experiments data demonstrate that hLa is associated to PAM mRNA in the cytoplasm. Further studies, such as cloning and characterization of the 3′ UTR of hPAM mRNA that is in progress may help resolve this issue.
Taken together, the data described in this report indicate that La protein contributes to the modulation of expression by the following methods: (i) recognition of a specific conserved 3′ UTR internal sequence in PAM mRNA that is required for nuclear retention, possibly to help for a correct folding and efficient pre-PAM RNA processing; (ii) retention in the nucleus that is dependent on increased levels of the RNA binding protein La and its direct interaction with a short segment of PAM mRNA 3′ UTR; and (iii) overexpression of La protein that induced an increase of PAM mRNA levels in the nucleus and consequently resulted in the down-regulation of PAM activity.
Our findings for the first time implicate La protein in a biosynthesis process to produce a bioactive amidated peptide via modulation of the expression of PAM RNAs coding for a key posttranslational processing enzyme. Thus, the La protein-PAM mRNA association would facilitate the production of sufficient PAM activity from a preexisting mature PAM mRNA pool in nuclei and/or cytoplasm more efficiently than by regulation of de novo mRNA synthesis.
Acknowledgments
We are very to grateful to R. V. Intine and R. J. Maraia (Laboratory of Molecular Growth Regulation, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD) for providing the GFP-hLaΔ316-322 construct. We thank Ger J. M. Pruijn (University of Nijmegen, Nijmengen, The Netherlands) for generously providing monoclonal antibody SW5 to the La antigen. We thank S. Schwartz (Uppsala University, Uppsala, Sweden) for generously providing the pTrc-His-La vector. We also thank P. M. Martin (Laboratoire de Transfert d'oncologie biologique, AP-HM) for his encouragement during the completion of these studies. We thank Véronique Gagna for her excellent secretarial assistance.
This work was supported by institutional funds of the CNRS and Inserm as well as by the Danish Biotechnology Instrument Centre (DABIC). F.B. was financed by the Association pour la recherche contre le cancer (ARC). J.B. was financed by Biacore AB (Uppsala, Sweden).
REFERENCES
- 1.Adilakshmi, T., and R. O. Laine. 2002. Ribosomal protein S25 mRNA partners with MTF-1 and La to provide a p53-mediated mechanism for survival or death. J. Biol. Chem. 277:4147-4151. [DOI] [PubMed] [Google Scholar]
- 2.Alfano, C., D. Sanfelice, J. Babon, G. Kelly, A. Jacks, S. Curry, and M. R. Conte. 2004. Structural analysis of cooperative RNA binding by the La motif and central RRM domain of human La protein. Nat. Struct. Mol. Biol. 11:323-329. [DOI] [PubMed] [Google Scholar]
- 3.Ali, N., G. J. Pruijn, D. J. Kenan, J. D. Keene, and A. Siddiqui. 2000. Human La antigen is required for the hepatitis C virus internal ribosome entry site-mediated translation. J. Biol. Chem. 275:27531-27540. [DOI] [PubMed] [Google Scholar]
- 4.Alspaugh, M. A., and E. M. Tan. 1975. Antibodies to cellular antigens in Sjogren's syndrome. J. Clin. Invest. 55:1067-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boelens, W. C., I. Palacios, and I. W. Mattaj. 1995. Nuclear retention of RNA as a mechanism for localization. RNA 1:273-283. [PMC free article] [PubMed] [Google Scholar]
- 6.Chakshusmathi, G., S. D. Kim, D. A. Rubinson, and S. L. Wolin. 2003. A La protein requirement for efficient pre-tRNA folding. EMBO J. 22:6562-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, Y. N., D. J. Kenan, J. D. Keene, A. Gatignol, and K. T. Jeang. 1994. Direct interactions between autoantigen La and human immunodeficiency virus leader RNA. J. Virol. 68:7008-7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 9.Dong, G., G. Chakshusmathi, S. L. Wolin, and K. M. Reinisch. 2004. Structure of the La motif: a winged helix domain mediates RNA binding via a conserved aromatic patch. EMBO J. 23:1000-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eipper, B. A., C. B. Green, T. A. Campbell, D. A. Stoffers, H. T. Keutmann, R. E. Mains, and L. Ouafik. 1992. Alternative splicing and endoproteolytic processing generate tissue-specific forms of pituitary peptidylglycine alpha-amidating monooxygenase (PAM). J. Biol. Chem. 267:4008-4015. [PubMed] [Google Scholar]
- 11.Eipper, B. A., D. A. Stoffers, and R. E. Mains. 1992. The biosynthesis of neuropeptides: peptide alpha-amidation. Annu. Rev. Neurosci. 15:57-85. [DOI] [PubMed] [Google Scholar]
- 12.El Meskini, R., F. Boudouresque, and L. Ouafik. 1997. Estrogen regulation of peptidylglycine alpha-amidating monooxygenase messenger ribonucleic acid levels by a nuclear posttranscriptional event. Endocrinology 138:5256-5265. [DOI] [PubMed] [Google Scholar]
- 13.Fan, H., J. L. Goodier, J. R. Chamberlain, D. R. Engelke, and R. J. Maraia. 1998. 5′ Processing of tRNA precursors can be modulated by the human La antigen phosphoprotein. Mol. Cell. Biol. 18:3201-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fouraux, M. A., M. J. Kolkman, A. Van der Heijden, A. S. De Jong, W. J. Van Venrooij, and G. J. Pruijn. 2002. The human La (SS-B) autoantigen interacts with DDX15/hPrp43, a putative DEAH-box RNA helicase. RNA 8:1428-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraboulet, S., F. Boudouresque, C. Delfino, F. Fina, C. Oliver, and L. Ouafik. 1996. Effect of thyroid hormones on peptidylglycine alpha-amidating monooxygenase gene expression in anterior pituitary gland: transcriptional studies and messenger ribonucleic acid stability. Endocrinology 137:5493-5501. [DOI] [PubMed] [Google Scholar]
- 16.Fraboulet, S., F. Boudouresque, C. Delfino, and L. Ouafik. 1998. Identification of a novel cis-element in the 3′-untranslated region of mammalian peptidylglycine alpha-amidating monooxygenase messenger ribonucleic acid. Endocrinology 139:894-904. [DOI] [PubMed] [Google Scholar]
- 17.Goodier, J. L., H. Fan, and R. J. Maraia. 1997. A carboxy-terminal basic region controls RNA polymerase III transcription factor activity of human La protein. Mol. Cell. Biol. 17:5823-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottlieb, E., and J. A. Steitz. 1989. Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J. 8:851-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlieb, E., and J. A. Steitz. 1989. The RNA binding protein La influences both the accuracy and the efficiency of RNA polymerase III transcription in vitro. EMBO J. 8:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimm, C., E. Lund, and J. E. Dahlberg. 1997. In vivo selection of RNAs that localize in the nucleus. EMBO J. 16:793-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendrick, J. P., S. L. Wolin, J. Rinke, M. R. Lerner, and J. A. Steitz. 1981. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol. Cell. Biol. 1:1138-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holcik, M., and R. G. Korneluk. 2000. Functional characterization of the X-linked inhibitor of apoptosis (XIAP) internal ribosome entry site element: role of La autoantigen in XIAP translation. Mol. Cell. Biol. 20:4648-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Intine, R. V., M. Dundr, T. Misteli, and R. J. Maraia. 2002. Aberrant nuclear trafficking of La protein leads to disordered processing of associated precursor tRNAs. Mol. Cell 9:1113-1123. [DOI] [PubMed] [Google Scholar]
- 24.Intine, R. V., A. L. Sakulich, S. B. Koduru, Y. Huang, E. Pierstorff, J. L. Goodier, L. Phan, and R. J. Maraia. 2000. Control of transfer RNA maturation by phosphorylation of the human La antigen on serine 366. Mol. Cell 6:339-348. [DOI] [PubMed] [Google Scholar]
- 25.Jacks, A., J. Babon, G. Kelly, I. Manolaridis, P. D. Cary, S. Curry, and M. R. Conte. 2003. Structure of the C-terminal domain of human La protein reveals a novel RNA recognition motif coupled to a helical nuclear retention element. Structure (Cambridge) 11:833-843. [DOI] [PubMed] [Google Scholar]
- 26.Mains R.E., I. M. D., V. May, D. A. Stoffers, S. N. Perkins, L. Ouafik, E. J. Husten, and B. A. Eipper. 1990. Cellular and molecular aspects of peptide hormone biosynthesis. Front. Neuroendocrinol. 11:52-89. [Google Scholar]
- 27.Maraia, R. J., and R. V. Intine. 2001. Recognition of nascent RNA by the human La antigen: conserved and divergent features of structure and function. Mol. Cell. Biol. 21:367-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maraia, R. J., D. J. Kenan, and J. D. Keene. 1994. Eukaryotic transcription termination factor La mediates transcript release and facilitates reinitiation by RNA polymerase III. Mol. Cell. Biol. 14:2147-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattioli, M., and M. Reichlin. 1974. Heterogeneity of RNA protein antigens reactive with sera of patients with systemic lupus erythematosus. Description of a cytoplasmic nonribosomal antigen. Arthritis Rheum. 17:421-429. [DOI] [PubMed] [Google Scholar]
- 30.Ohndorf, U. M., C. Steegborn, R. Knijff, and P. Sondermann. 2001. Contributions of the individual domains in human La protein to its RNA 3′-end binding activity. J. Biol. Chem. 276:27188-27196. [DOI] [PubMed] [Google Scholar]
- 31.Ouafik, L., V. May, D. W. Saffen, and B. A. Eipper. 1990. Thyroid hormone regulation of peptidylglycine alpha-amidating monooxygenase expression in anterior pituitary gland. Mol. Endocrinol. 4:1497-1505. [DOI] [PubMed] [Google Scholar]
- 32.Ouafik, L. H., M. G. Mattei, P. Giraud, C. Oliver, B. A. Eipper, and R. E. Mains. 1993. Localization of the gene encoding peptidylglycine alpha-amidating monooxygenase (PAM) to human chromosome 5q14-5q21. Genomics 18:319-321. [DOI] [PubMed] [Google Scholar]
- 33.Ouafik, L. H., D. A. Stoffers, T. A. Campbell, R. C. Johnson, B. T. Bloomquist, R. E. Mains, and B. A. Eipper. 1992. The multifunctional peptidylglycine alpha-amidating monooxygenase gene: exon/intron organization of catalytic, processing, and routing domains. Mol. Endocrinol. 6:1571-1584. [DOI] [PubMed] [Google Scholar]
- 34.Pannone, B. K., S. D. Kim, D. A. Noe, and S. L. Wolin. 2001. Multiple functional interactions between components of the Lsm2-Lsm8 complex, U6 snRNA, and the yeast La protein. Genetics 158:187-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perkins, S. N., E. J. Husten, R. E. Mains, and B. A. Eipper. 1990. pH-dependent stimulation of peptidylglycine alpha-amidating monooxygenase activity by a granule-associated factor. Endocrinology 127:2771-2778. [DOI] [PubMed] [Google Scholar]
- 36.Pruijn, G. J., J. P. Thijssen, P. R. Smith, D. G. Williams, and W. J. Van Venrooij. 1995. Anti-La monoclonal antibodies recognizing epitopes within the RNA-binding domain of the La protein show differential capacities to immunoprecipitate RNA-associated La protein. Eur. J. Biochem. 232:611-619. [DOI] [PubMed] [Google Scholar]
- 37.Pudi, R., S. Abhiman, N. Srinivasan, and S. Das. 2003. Hepatitis C virus internal ribosome entry site-mediated translation is stimulated by specific interaction of independent regions of human La autoantigen. J. Biol. Chem. 278:12231-12240. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar, G., and S. S. Sommer. 1990. The “megaprimer” method of site-directed mutagenesis. BioTechniques 8:404-407. [PubMed] [Google Scholar]
- 39.Steitz, J. A. 1989. Immunoprecipitation of ribonucleoproteins using autoantibodies. Methods Enzymol. 180:468-481. [DOI] [PubMed] [Google Scholar]
- 40.Stoffers, D. A., C. B. Green, and B. A. Eipper. 1989. Alternative mRNA splicing generates multiple forms of peptidyl-glycine alpha-amidating monooxygenase in rat atrium. Proc. Natl. Acad. Sci. USA 86:735-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tajima, M., T. Iida, S. Yoshida, K. Komatsu, R. Namba, M. Yanagi, M. Noguchi, and H. Okamoto. 1990. The reaction product of peptidylglycine alpha-amidating enzyme is a hydroxyl derivative at alpha-carbon of the carboxyl-terminal glycine. J. Biol. Chem. 265:9602-9605. [PubMed] [Google Scholar]
- 42.Tan, E. M. 1989. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv. Immunol. 44:93-151. [DOI] [PubMed] [Google Scholar]
- 43.Thiele, E. A., K. L. Marek, and B. A. Eipper. 1989. Tissue-specific regulation of peptidyl-glycine alpha-amidating monooxygenase expression. Endocrinology 125:2279-2288. [DOI] [PubMed] [Google Scholar]
- 44.Trotta, R., T. Vignudelli, O. Candini, R. V. Intine, L. Pecorari, C. Guerzoni, G. Santilli, M. W. Byrom, S. Goldoni, L. P. Ford, M. A. Caligiuri, R. J. Maraia, D. Perrotti, and B. Calabretta. 2003. BCR/ABL activates mdm2 mRNA translation via the La antigen. Cancer Cell 3:145-160. [DOI] [PubMed] [Google Scholar]
- 45.van Venrooij, W. J., R. L. Slobbe, and G. J. Pruijn. 1993. Structure and function of La and Ro RNPs. Mol. Biol. Rep. 18:113-119. [DOI] [PubMed] [Google Scholar]
- 46.Varani, G., and K. Nagai. 1998. RNA recognition by RNP proteins during RNA processing. Annu. Rev. Biophys. Biomol. Struct. 27:407-445. [DOI] [PubMed] [Google Scholar]
- 47.Wolin, S. L., and T. Cedervall. 2002. The La protein. Annu. Rev. Biochem. 71:375-403. [DOI] [PubMed] [Google Scholar]
- 48.Yoo, C. J., and S. L. Wolin. 1997. The yeast La protein is required for the 3′ endonucleolytic cleavage that matures tRNA precursors. Cell 89:393-402. [DOI] [PubMed] [Google Scholar]