Abstract
Background:
Although depression is a leading cause of disability worldwide, the pathophysiological mechanisms underlying this disorder—particularly those involving the gut microbiome—are poorly understood.
Method:
To investigate, we conducted a community-based observational study to explore complex associations between changes in the gut microbiome, cytokine levels, and depression symptoms in 51 participants (Mage = 49.56, SD = 13.31) receiving an immersive psychosocial intervention. A total of 142 multi-omics samples were collected from participants before, during, and three months after the nine-day inquiry-based stress reduction program.
Results:
Results revealed that depression was associated with both an increased presence of putatively pathogenic bacteria and reduced microbial beta-diversity. Following the intervention, we observed reductions in neuro-inflammatory cytokines and improvements in several mental health indicators. Interestingly, participants with a Prevotella-dominant microbiome showed milder symptoms when depressed, along with a more resilient microbiome and more favorable inflammatory cytokine profile, including reduced levels of CXCL-1.
Conclusions:
These findings reveal a potentially protective link between the Prevotella-dominant microbiome and depression, as evidenced by a reduced pro-inflammatory environment and fewer depressive symptoms. These insights, coupled with observed improvements in neuroinflammatory markers and mental health from the intervention, may highlight potential avenues for microbiome-targeted therapies for managing depression.
Keywords: Gut microbiome, Psychosocial intervention, Neuroinflammation, CXCL-1
1. Introduction
Depression is a highly prevalent and economically burdensome psychiatric disorder that is associated with a variety of serious physical health conditions (Slavich and Irwin, 2014). Moreover, this burden has been exacerbated by the recent COVID-19 pandemic (Collaborators, 2021; Gruber et al., 2021; Kupcova et al., 2023; Nishimi et al., 2022; Slavich, 2022). Depression affects a wide range of individuals across ages, genders, and geographical locations (Collaborators, 2022; Friedrich, 2017; Kessler and Bromet, 2013). Despite its substantial societal impact, however, much remains unknown about the underlying biology of depression.
This gap in knowledge stems in part from the inherent molecular complexity of the human brain and behavior (Bassett and Gazzaniga, 2011; Fries et al., 2023; Krishnan and Nestler, 2008), coupled with the challenges of replicating psychiatric disorders in animal models (Krishnan and Nestler, 2011; Monteggia et al., 2014; Planchez et al., 2019), and the reliance on self-reported diagnostic tools like the Beck Depression Inventory-II (BDI-II) (Faro and Pereira, 2020) and the Patient Health Questionnaire (PHQ-9) (Schutt et al., 2016), which may introduces biases. Furthermore, our insights into depression have been constrained by the limited application of longitudinal multi-omics profiling studies, which are crucial for unraveling the complex molecular and cellular mechanisms underlying mental health disorders (Mengelkoch et al., 2023; Moriarity and Slavich, 2023). These collective challenges have hindered our understanding of the underlying molecular and cellular mechanisms of depression and have consequently impeded the advancement of novel pharmacological and psychotherapeutic interventions for major depressive disorder (Marwaha et al., 2023).
Recent studies have highlighted a significant bi-directional association between depression and inflammation, shedding light on the frequent co-occurrence of depression with various inflammatory disorders (Fung et al., 2017; Kim et al., 2007). This association is often viewed through frameworks such as the Social Signal Transduction Theory of Depression, which suggests that psychosocial stressors can trigger an inflammatory response, elevating depression risk in susceptible individuals (Slavich and Irwin, 2014). The interaction between depression and inflammation is complex and reciprocal: inflammation can precipitate depressive symptoms (Borovikova et al., 2000; Wang et al., 2003) and, in addition, depression can intensify inflammation through behavioral and physiological pathways (Moriarity et al., 2023b; Schneider et al., 2023). This reciprocal relationship highlights the intricate connection between mental and physical health. Importantly, psychosocial interventions have emerged as effective in bolstering immune function, presenting a viable alternative to traditional antide-pressants in managing depression-associated inflammation (Black and Slavich, 2016; Chandran et al., 2021; Shields et al., 2020).
Recognizing the dynamic between depression, inflammation, and immunity necessitates examining the gut microbiome’s impact on mental health. The gut microbiome, a key regulator of the immune system, significantly affects human behavior via the gut-brain axis (Chang et al., 2022; Martin et al., 2018; Rogers et al., 2016; Shoubridge et al., 2022). Research indicates that psychiatric and behavioral disorders, including addiction (Binh Tran et al., 2023; Leclercq et al., 2014; Tran et al., 2023), depression (Bosch et al., 2022; Jiang et al., 2015; McGuinness et al., 2022; Zhao et al., 2022), aggression (Choi et al., 2017), and impaired social cognition (Carbia et al., 2023; Sarkar et al., 2018) correlate with notable microbiome alterations. These changes are deeply intertwined with brain function and behavior by regulation of key metabolites such as serotonin (5-HT) (Erspamer, 1954; Yano et al., 2015), tryptophan (Diaz Heijtz et al., 2011; Reus et al., 2015; Tran et al., 2023; Valles-Colomer et al., 2019), and γ-Aminobutyric acid (GABA) (Strandwitz et al., 2019), pivotal for neurotransmission, mood, cognition, and stress response. Serotonin, targeted by many antidepressants (Artigas, 2013; Khan, 2022), influences a wide range of psychological and physiological functions. Tryptophan is a precursor for serotonin synthesis, thus influencing serotonin levels and, consequently, mood and emotional states (Correia and Vale, 2022; Kikuchi et al., 2021).
In turn, GABA in the primary inhibitory neurotransmitter in the brain (Allan and Johnston, 1983; McCormick, 1989) and plays a key role in reducing neuronal excitability throughout the nervous system (Bussi et al., 2023; Duman et al., 2019; Guyon, 2014; Liu et al., 2023b), impacting processes like anxiety regulation and stress response. The microbiome extends its influence to the immune system, notably in cytokine regulation (Lynch et al., 2023; Reus et al., 2015; Schachter et al., 2018; Zhao et al., 2023), crucial for neuroinflammation and neural-immune interactions (Bairamian et al., 2022; Carlessi et al., 2021). Furthermore, certain cytokines and chemokines, influenced by the microbiome, play a pivotal role in signal transduction processes (Cox and Weiner, 2018; Guyon, 2014; Legler and Thelen, 2018; Sowa and Tokarski, 2021). These observations underscore the multifaceted impact of the gut microbiome on brain function and behavior, highlighting its role in both metabolic regulation and direct immune modulation.
Recent research has shown that the gut microbiome may play a causal role in depression, as evidenced by experiments that involve transferring microbiomes from depressed patients to mice, leading to depressive-like behaviors and altered metabolism (Luo et al., 2018; Pu et al., 2021; Zheng et al., 2016). Specifically, bacteria such as Escherichia have been linked to promoting depressive symptoms (Luo et al., 2018; Sudo et al., 2004). These effects may be mediated through the modulation of the hypothalamic–pituitary–adrenal (HPA) axis and cytokine production (Sudo et al., 2004), and significantly influence cytokine production, including Brain-Derived Neurotrophic Factor (BDNF) (Bercik et al., 2011) and Interleukin-6 (IL-6) (Sudo et al., 2004). Furthermore, the microbiome’s influence on the metabolism and efficacy of antidepressants has emerged as a significant area of interest, highlighting its potential to shape psychotherapeutic treatment outcomes (Lukic et al., 2019; Valles-Colomer et al., 2019; Vasileva et al., 2024; Wang et al., 2023).
The longitudinal interplay between the microbiome and immune system in the context of depression, and in response to an immersive psychosocial intervention, remains unexplored, largely due to differences in human and animal immune and mental health systems (Medetgul-Ernar and Davis, 2022), and the impracticality of applying psychotherapeutic strategies such as self-inquiry and meditation in animal models. Research indicates that microbiome compositions, summarized by enterotypes like Bacteroidetes, Firmicutes, or Prevotella, are crucial for nutrient processing, inflammatory responses, and drug metabolism (Scher et al., 2020; Spanogiannopoulos et al., 2022; Weersma et al., 2020; Zhong et al., 2019; Zhou et al., 2024a). Specifically, Firmicutes (Park et al., 2023) and Prevotella (Lee et al., 2020) enterotypes have been related to mental health, with the latter associated with increased positive emotions, though further research is required.
To address this knowledge gap, we conducted a longitudinal study involving participants undergoing a highly immersive, inquiry-based stress reduction program. Our primary aim was to investigate whether individuals with different gut microbiome enterotypes exhibit distinct molecular and immunological responses during the depression reduction program. Based on the research summarized above, we hypothesized that specific enterotypes may influence the effectiveness of psychosocial interventions in alleviating depressive symptoms through their interaction with the host immune system. By integrating gut microbiome analyses, plasma cytokine profiling, and comprehensive mental health assessments before and after the program, we sought to explore the potential role of gut microbiome enterotypes in modulating inflammation and mental health outcomes over time. This approach thus provides novel insights into microbiome-host dynamics during an intervention with known therapeutic benefits.
2. Method
2.1. Participant recruitment and program overview
This study was pre-approved by the Stanford Institutional Review Board (IRB protocol #48982). We recruited individuals aged 18 years and older to participate in a longitudinal study examining the effects of an Inquiry-Based Stress Reduction (IBSR) program on depression and associated biological markers. Participants were recruited via email and in-person interactions from among individuals enrolled in the IBSR program. Exclusion criteria included a current diagnosis of cancer, recent use of steroid medications, and being a staff member of the IBSR program. IBSR is a standardized, nine-day intensive program involving self-inquiry, meditation, and other stress reduction activities designed to restructure maladaptive beliefs and promote psychological well-being. The program was conducted at the Ojai Valley Inn in California, where participants stayed for the duration of the intervention. Diet and living accommodations were provided by the hotel, with no specific dietary instructions given to participants. No fasting was required prior to sample collection, and the collection time during the day was at participants’ free will. IBSR has been previously demonstrated to improve beliefs related to social safety and depression without the use of pharmaceutical agents (Feldman et al., 2021; Landau et al., 2021; Schnaider-Levi et al., 2020).
Baseline characteristics such as age, biological sex, and body mass index (BMI) were collected; however, not all participants consented to share this information, resulting in some missing data (i.e., three participants without BMI values and eleven participants without age values). Participants’ initial depression status was classified as either “depressed” or “non-depressed.” This categorization was based on their total BDI-II (Beck Depression Inventory-II) score, using a score of 14 and above as the threshold for being depressed, as per standard convention (Faro and Pereira, 2020). Due to the small sample size and potential impact on statistical power, participants with missing data were not excluded from analyses. Instead, we assessed whether age, sex, and BMI differed significantly across microbiome enterotype groups to evaluate potential confounding effects. We conducted one-way ANOVA tests to compare age and BMI across the three enterotype clusters and chisquared tests to assess the association between sex and enterotype clusters. The results indicated no significant differences: Age: F(2, 130) = 0.031, p = 0.969; BMI: F(2, 127) = 2.047, p = 0.133; Sex: χ2 = 1.8462, df = 1, p = 0.174.
2.2. Psychological profiling
All participants were profiled at baseline, daily throughout the retreat, one month later, and three months later (note: the six-month follow-up is ongoing). Participants completed psychometric surveys through REDCap evaluating mental health and well-being. For the characterization of social threat-related beliefs, the Dysfunctional Attitudes Scale (DAS-17), short-form, was used to assess social-threat-related beliefs. A subset of the Primal World Beliefs Index (PI-18), which measures underlying beliefs about the world (e.g. “The world is safe,” vs. “the world is dangerous”), was used to assess a broader subset of underlying beliefs and subsequent belief change. Additionally, the Beck Depression Inventory-II (BDI-II) was used to assess depression1, the GAD-7 was used to assess anxiety, and the Perceived Stress Scale was used to assess stress levels (PSS-10). Additional surveys included: PERMA profiler, Big Five Personality Index (BFI-10), Satisfaction with Life Survey (SLWS), Close Relationships Questionnaire (CRQ-36), Adult Hope Scale (AHS), Adverse Childhood Experiences (ACEs) Questionnaire, the Acceptance and Action Questionnaire (AAQ), and the Gratitude Survey (GS). The total BDI-II scores were interpreted following established guidelines (Beck et al., 1996): scores ranging from 0 to 13 indicated no-to-minimal depression, 14 to 19 indicated mild depression, 20 to 28 indicated moderate depression, and 29 to 63 indicated severe depression.
2.3. Blood collection
Blood samples were obtained at four distinct time intervals to facilitate comprehensive biological profiling. Initial collections were performed on-site at the beginning (Day 1, T1) and conclusion (Day 9, T2) of the retreat. Subsequent collections at one-month (T3) and three-month (T4) intervals were facilitated through a collaboration with Phlebotek, utilizing their in-home mobile phlebotomy services to accommodate participants nationwide. Each session involved the collection of one 10-ml red top tube and one 10-ml lavender top tube for the separation and preservation of plasma, cells, and serum. These samples were immediately shipped overnight on dry ice to the Snyder Laboratory at Stanford, where they were preserved at —80 °C pending further experimental analysis.
2.4. Cytokines Luminex assay
The cytokine assay employed a 76-plex kit (EMD Millipore H76), executed by the Human Immune Monitoring Center at Stanford University as part of our Integrated Personal Omics Profiling techniques (Zhou et al., 2024a). The assay kits, sourced from EMD Millipore Corporation, Burlington, MA, were used in accordance with the manufacturer’s guidelines, with specific modifications as delineated below. The H76 kits comprise three distinct panels: Panel 1. consists of Milliplex HCYTMAG60PMX41BK, supplemented with IL-18 and IL-22 to create a 43-plex. Panel 2. incorporates Milliplex HCP2MAG62KPX23BK, with the addition of MIG/CXCL9, forming a 24-plex. Panel 3. features Milliplex HSP1MAG-63 K, which is augmented with Resistin, Leptin, and HGF to yield a 9-plex. Samples were combined with antibody-coupled magnetic beads in a 96-well plate and incubated overnight at 4 °C with shaking. Both cold and room-temperature incubation steps were conducted on an orbital shaker at speeds ranging from 500 to 600 rpm. The plates were then washed twice using a wash buffer in a Biotek ELx405 washer. Subsequently, a biotinylated detection antibody was added and incubated at room temperature for an hour, followed by a 30-minute incubation with streptavidin-PE, while shaking. After a final washing step, PBS was introduced into the wells, and readings were obtained using the Luminex FlexMap3D Instrument, with a lower limit of 50 beads per sample per cytokine. Custom Assay CHEX control beads (Radix Biosolutions Inc. Georgetown, Texas) were incorporated into all wells. Three technique replicates were performed as a standard protocol from the core facility.
2.5. Microbiome data analysis
Microbiome samples were collected using UBiome kits (UBiome, San Francisco, CA), and sequencing was conducted using 150 bp paired-end sequencing. After 30 min of bead-beating lysis, stool samples were processed using a silica-guanidinium thiocyanate-based nucleic acid isolation protocol on a liquid-handling robot. The 16S rRNA V4 region was amplified via 35 cycles of PCR with primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). DNA from each sample was barcoded and pooled to create a sequencing library, which was purified using columns and microfluidic DNA fractionation to reduce unwanted fragments. DNA concentration was quantified using the Bio-Rad MyiQ with the Kapa iCycler qPCR kit (Bio-Rad Laboratories, Hercules, CA, USA). Sequencing was performed on the Illumina NextSeq 500 platform (Illumina, San Diego, CA, USA) with a 2 × 150 bp paired-end sequencing protocol. Raw sequencing data from stool and nasal samples were obtained from our previous publication, and the same cutoff was used for skin and oral sequencing data during demultiplexing. Reads with Q-scores < 35 and ambiguous bases (Ns) were trimmed before further analysis.
Data analysis was performed using DADA2 (version 1.20.0) in R (version 4.1.1), using forward reads due to insufficient overlap between paired-end sequences. Quality filtering parameters included: maximum ‘Ns’ set to zero, maximum expected errors set to two, truncation length of 150, and truncation quality of two. Taxonomic units were assigned using DADA2′s assignTaxonomy and addSpecies functions based on 16S sequences that met quality criteria, using the Ribosomal Database Project database (version 18) as the reference. Relative abundances were determined by normalizing read counts to total reads per time point. Rarefaction to 10,000 reads per sample (sequencing depth cutoff) led to the exclusion of three samples (X224325473, X467325054, X559299082) and 803 ASVs from all further analyses.
2.6. Enterotype analysis
The enterotypes of the microbiome samples were determined following previously established methods. Initially, sample counts were normalized to their relative abundance, and noise was filtered by retaining only features with a relative abundance exceeding 1 %. Statistical dissimilarity between microbial communities was quantified using Jensen-Shannon divergence (JSD) and Kullback-Leibler divergence (KLD). A distance matrix was subsequently generated from these metrics. Partitioning Around Medoids (PAM) clustering was employed on the distance matrix to categorize the samples into distinct clusters. The optimal number of clusters (k) was determined by evaluating the Calinski-Harabasz index (CH index) for k values ranging from 1 to 20. A k value of 3 was selected based on the CH index and prior literature. Each cluster exhibited distinct microbial signatures: Cluster 1 was predominantly characterized by the genus Bacteroidetes; Cluster 2 mainly featured the family Ruminococcaceae; and Cluster 3 was primarily composed of the genus Prevotella. To validate the robustness of this clustering, silhouette width was calculated, offering a measure of the similarity between each sample and others within its respective cluster relative to other clusters. Additionally, Between-Class Analysis (BCA), informed by Principal Coordinate Analysis (PCoA) scores, was performed to visualize the separation between the established enterotypes. Finally, the samples were annotated with their respective enterotype classifications for subsequent analyses.
2.7. Change in depression (BDI-II) scores
Each participant’s change in depression (i.e., BDI-II score) over time (i.e., delta BDI) was calculated by comparing their baseline score (T1) with their follow-up score at the later time points (T3 or T4). Specifically, BDI-II scores at T1 were subtracted from the scores at T3 if available, or from T4 if T3 was missing. The formula for delta BDI was defined as:
where T3 and T4 are the BDI-II scores at time points 3 and 4, respectively. This approach ensured that the maximum available follow-up score was used for each participant.
2.8. Permutational multivariate analysis of variance (PERMANOVA)
Two types of PERMANOVA analyses were conducted to explore the influence of various factors on the gut microbiome and cytokine profiles. The analyses were performed using the adonis2 function from the R package vegan.
Gut Microbiome PERMANOVA: The distance matrix for the gut microbiome was computed using the Bray-Curtis distance measure on the phyloseq object physeq_PERMANOVA. The PERMANOVA model was constructed to evaluate the effects of time points (Time) and depression status (depressed) on the microbial community Bray-Curtis distance matrix. A total of 9,999 permutations were executed for this analysis.
Cytokine Profile PERMANOVA: For the cytokine profiles, a distance matrix was calculated using the Euclidean distance measurements on a selected set of cytokines. The PERMANOVA model in this case was formulated to include time (Time), enterotype cluster (Enterotype), and depression status (depressed) as explanatory variables. The analysis was run with 9,999 permutations.
2.9. Bayesian mixed-effects model for microbial taxa and cytokine interactions
To search for microbe-cytokine interactions that were altered under depression and impacted by patient enterotype, we constructed Gaussian-family longitudinal Bayesian mixed-effects models with 2000 iterations, 4 chains, and a 1000-iteration burn-in. The models were constructed in the form:
where is the cytokine of interest for each participant, , is a microbe of interest across each microbe, , is the depression status, is the enterotype, and is the treatment time point. We fit a random intercept for each participant, , to accommodate different baseline cytokine values.
Normalized and transformed data were used for 80 cytokines and 364 microbes. These models were constructed using the “brms” (Bürkner, 2021) and “tidybayes”(Kay, 2020) packages in R version 4.1.0. All models were evaluated by (a) an expected log predicted density above the null, Ci ~ 1, using leave-one-out cross-validation comparison, (b) each term converged with an Ȓ < 1.5, and (c) where the 95 % credible interval of the distribution of posterior draws did not cross zero. Only models and terms that passed these three criteria were further evaluated.
2.10. Network analysis of microbiome family co-occurrence
To investigate the co-occurrence patterns of gut microbiome families in relation to depression, we constructed two separate networks based on individuals’ stages of depression. These analyses were performed using the R package “phylosmith” (Version 1.0.7) (Smith, 2019). Initially, gut microbiome data were normalized and aggregated at the family level. This step aims to simplify the complexity of the network by reducing the number of nodes, thereby facilitating visualization. Subsequently, pairwise Spearman correlation coefficients (rho) were computed to assess the strength and direction of associations between different microbial families. To determine an effective cutoff for rho that signifies meaningful associations in our data, we generated a null distribution of rho values through 10,000 permutations, following the guidelines provided by the authors. Based on this analysis, we identified cutoff values at the extreme tails (0.0001 significance level), which corresponded to rho values of 0.49 (positive association) and —0.42 (negative association). Further examination revealed that the maximum Benjamini-Hochberg (BH) adjusted p-value corresponding to these rho cutoffs was 0.00113. Given this finding, we opted for this more stringent criterion (Padj ≤ 0.00113) to define significant associations in our subsequent network analysis. The co-occurrence networks were constructed and visualized using the default settings in “phylosmith”. These networks illustrate the patterns of microbial family co-occurrence, providing insights into the microbial interactions that may be associated with different stages of depression.
2.11. Two-part zero-inflated beta regression model with random effects
To investigate taxonomic differences between individuals classified as depressed and non-depressed in our longitudinal study, a two-part Zero-Inflated Beta Regression model with random effects (ZIBR) (Chen and Li, 2016) was used. Initially, data were categorized into three temporal groups: pre-treatment (T1), immediate post-treatment (T2), and extended post-treatment (T3 and T4). Data cleaning procedures involved the elimination of columns devoid of microbial counts. Additionally, taxa with fewer than four zero counts, thereby lacking a zero-inflated nature, were excluded from subsequent analyses. Following these filtering measures, the dataset was narrowed down to 20 individuals for whom data across all three temporal categories were available. This subset consisted of 11 individuals classified as depressed and 9 as non-depressed, yielding a total of 60 samples for analysis. The zibr function from the ZIBR package was employed to perform analysis on 289 distinct microbial taxa. Both the logistic and beta regression components of the ZIBR model were adjusted for depression status through the inclusion of a covariate. Hypothesis testing was conducted to assess the statistical significance of the association between individual microbial taxa and depression status, considering the zero-inflated nature of the dataset. Joint p-values were computed and subsequently adjusted using the Benjamini-Hochberg (BH) method to control for the False Discovery Rate (FDR). To further mitigate the risk of false positives, taxa appearing only once in each group (singletons) were excluded from the results.
2.12. Mediation analysis
Data processing was performed before running the mediation analysis to explore associations between gut microbiome (X), plasma cytokine levels (M), and psychometric parameters (Y). Specifically, only variables with a mean of non-zero values greater than 10 % were retained. The gut microbiome data were then transformed using the centered log-ratio (CLR) transformation, and a prevalence filter was applied to include variables with more than 20 % prevalence. Preliminary linear regression analyses were conducted to evaluate the associations between the gut microbiome and both plasma cytokine levels and psychometric parameters. Only associations meeting a P-value threshold of 0.2 were retained for subsequent analyses. Mediation analyses were then carried out using the “mediation” package in R, deploying Generalized Linear Models (GLMs) with a Gaussian distribution. A bootstrap method with 500 simulations was employed based on our previous work (Moriarity et al., 2023a) to estimate the Average Causal Mediation Effects (ACME) and Average Direct Effects (ADE). To assess the validity of the meditative pathways, pairs demonstrating significant ACME (P-values < 0.05) were considered to represent the indirect effects of the gut microbiome on psychometric parameters, mediated through plasma cytokine levels. Statistical comparisons between different biological conditions were also conducted to evaluate the influence of the meditative effect on each mediated pathway. Besides the traditional cutoff recommended for reporting mediation effects as the ACME P-value < 0.05, significant mediation effects were reported only when passing an additional threshold. The mediation effect was considered significant only if both the P-value for the total effect in the mediation model and the p-value from the linear model evaluating the direct effect of the gut microbiome (X) on psychometric parameters (Y) were less than 0.1. This dual-threshold method aims to add a layer of stringency to the analysis, reducing the likelihood of Type I errors. Although each test could traditionally be evaluated at P < 0.05, this conservative approach requires both to be below P < 0.1 to strike a balance between stringency and sensitivity in the analysis.
3. Results
3.1. Study design overview, depression progression, and microbiome composition analysis
Of the 57 individuals who signed up for the program (9 males and 48 females), 6 females did not complete any biological sample collection (blood or stool). Fifty-one participants completed the 9-day inquiry-based stress reduction program. Baseline characteristics such as age, biological sex, and body mass index (BMI) were collected. Participants ranged in age from 24 to 76 years old (mean ± standard deviation: 49.56 ± 13.31) and their BMI ranged from 13.1 to 30.0 (22.55 ± 3.00). Samples were collected upon arrival (T1), after the stay (T2), one (T3) and three months (T4) post-retreat, totaling 141 stool and 123 plasma samples (see Method Fig. 1A, Fig. S1A). Mental health assessments were also conducted at these points, focusing on depression, anxiety, perceived stress, and well-being.
Fig. 1. Study Design, Depression Trajectories, and Microbiome Composition Analysis.
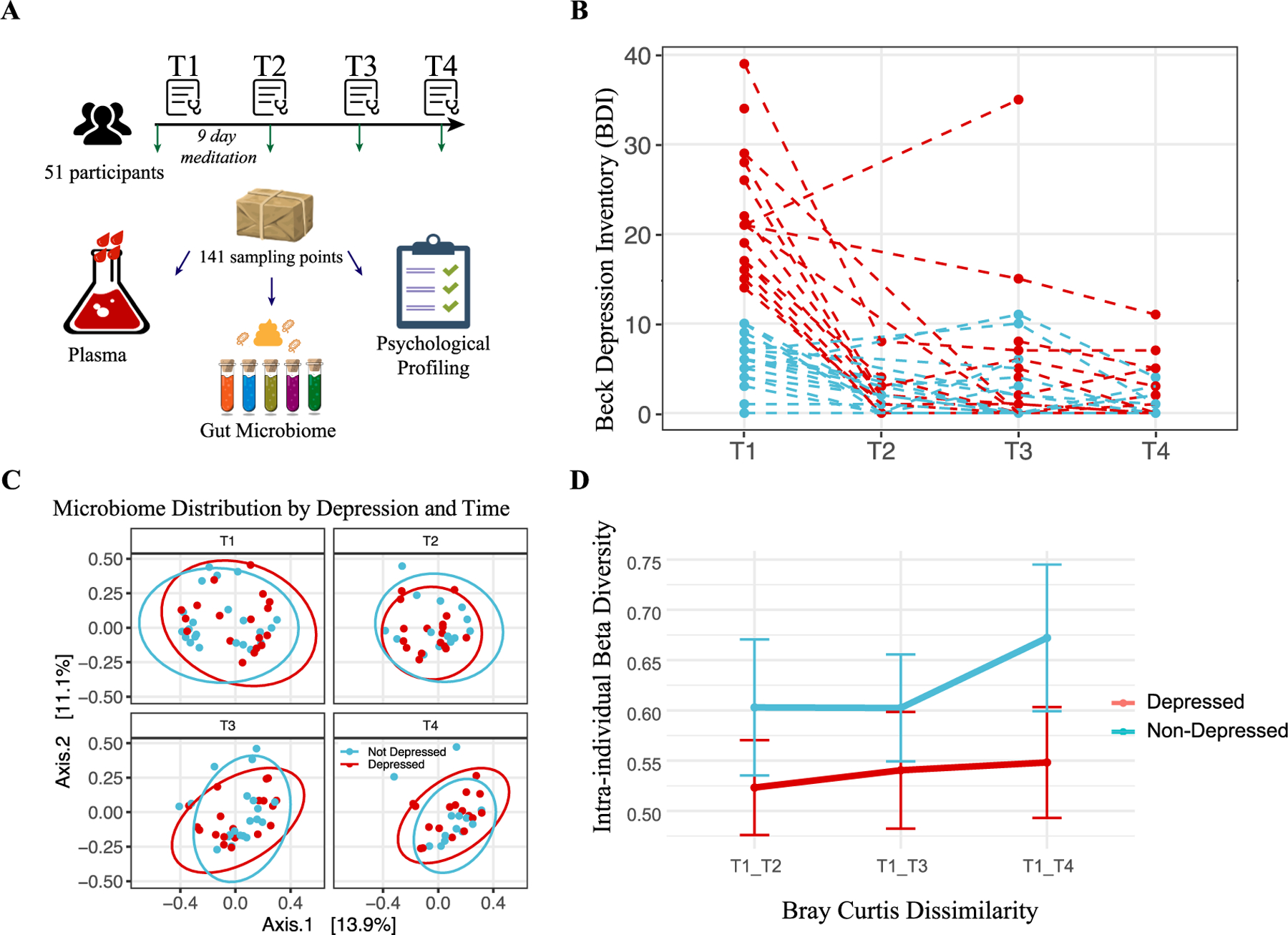
(A) Cartoon representation of the study’s methodology (Image adapted from Pixabay). (B) Beck Depression Inventory-II (BDI-II) scores of participants across four time points, with each dashed line corresponding to one individual. The color coding indicates whether the individual was depressed (red) or non-depressed (blue) at baseline. (C) Principal Coordinates Analysis (PCoA) of microbiome composition at the four time points. The first two axes are plotted, with the variance captured annotated along each axis. Red and blue colors denote the initial depression status of the participants, with red indicating depressed and blue indicating non-depressed individuals. (D) Intra-individual dissimilarity between T1 and the rest of the program.
At baseline (T1), 24 participants were identified as depressed based on their BDI-II scores. Of these 24 participants, 23 (95.83 %) exhibited a significant decrease in BDI-II scores post-program (T2), and this reduction was sustained over subsequent assessments at one month (T3) and three months (T4) post-retreat. In contrast, the non-depressed group showed minimal changes in BDI-II scores over the same period. Analyses revealed a significant difference in the degree of change in BDI-II scores between the depressed (n = 24) and non-depressed (n = 27) groups (W = 294, p = 2.776 × 10−05). The corresponding Cliff’s Delta estimate is 0.82, reflecting a large effect size. For the duration of the study, those classified as depressed at T1 remained in the “depressed” group for analysis purposes, regardless of any changes in depression status at later time points (Fig. 1B). This consistent grouping enabled us to assess the longitudinal effects of the intervention based on initial depression status and to examine the differential changes in BDI-II scores (delta response) between the two groups, as initial depression status was strongly correlated with the magnitude of change in depressive symptoms over time.
To further explore associations between changes in depressive symptoms and the gut microbiome, we examined correlations between the change in BDI-II scores (delta BDI-II from T1 to later points) and the relative abundances of the top 20 most abundant microbial taxa. This analysis revealed a significant negative correlation between the mean abundance of Subdoligranulum and participants’ decrease in BDI-II scores (rho = −0.677, padj = 0.00011), and partial analysis suggested that higher levels of Subdoligranulum were associated with greater reductions in depressive symptoms following the intervention (Fig. S1B). Additionally, a partial correlation analysis controlling for initial BDI-II scores showed a moderate negative correlation (Spearman rho = −0.406, p = 0.016), reinforcing that the gut microbiome’s composition, particularly genus Subdoligranulum abundance, was associated with the degree of improvement in depressive symptoms that participants exhibited following the intervention.
A Permutational Multivariate Analysis of Variance (PERMANOVA) revealed that the initial depression status accounted for a small-yet-statistically significant proportion of the variation in gut microbiome composition (R2 = 1.4 %, Pr (>F) = 0.004). Yet, this did not significantly overshadow intrapersonal variations (2.3 % variance, p = 0.21), which were not consistent across participants (Fig. 1C, S1C). For the non-depressed group, participation in the program (i.e., meditation) was associated with a substantial increase in microbial richness. This effect was measured using the Chao1 index, which showed a rise from the start to the end of the program (T1-T3: beta = 35.06, p = 0.099), as well as one month later (T1-T4: beta = 84.84, p = 0.0016). Additionally, we observed a trend toward increased intraindividual beta-diversity (p = 0.078) (Fig. 1D), which is consistent with findings from the largest meta-analysis on the subject (McGuinness et al., 2022). This finding suggests that the immersive intervention program contributed to a mild, and potentially beneficial, increase in microbial diversity (Bosch et al., 2022), a change not observed for those in the depressed group (Fig. S1D).
To specifically address the longitudinal design and zero-inflated nature of our microbiome data, a separate analytical strategy was employed using a two-part Zero-Inflated Beta Regression model with Random effects (ZIBR) (Chen and Li, 2016). This approach identified three genera—Solobacterium, Anaerofilum, and Escherichia/Shigella—that exhibited differential distribution based on depression status across time (Table S1). Notably, while Solobacterium has previously been reported to increase among academic-related chronic stress among young students (Nani et al., 2017), our data revealed a significant increase in the gut microbiome of depressed individuals. Such findings suggest translocation of pathogens from one body site to another during disease stage. Escherichia/Shigella (Barandouzi et al., 2020; Jiang et al., 2015; Ling et al., 2022; Simpson et al., 2021) and Anaerofilum (Barandouzi et al., 2020; Kelly et al., 2016) have also been previously associated with depression and stress, thus providing an external validation for the model. This finding is consistent with our broader understanding that the gut microbiome’s association with mental depression appears to be characterized by the small-to-modest increase in pathogens, which, although impactful, represent only a minor fraction of the total microbiome, rather than systematic shifts in the core community.
3.2. Enterotype-based analysis suggests a protective role of prevotella in depression
Although our differential abundance analysis did not reveal significant pathogen overgrowth in individuals with depressive symptoms, we investigated whether enterotypes—distinct microbial configurations defined by specific bacterial genera dominance(Arumugam et al., 2011)—were related to changes in depression status over time. Enterotypes are known to significantly influence nutrient metabolism (Ridaura et al., 2013; Turnbaugh et al., 2006; Wu et al., 2011), the immune environment (Schirmer et al., 2016; Song et al., 2023; Wu et al., 2024; Zhou et al., 2024a), and disease onset and treatment (Schussler-Fiorenza Rose et al., 2019; von Schwartzenberg et al., 2021; Zhou et al., 2019; Zhou et al., 2020), suggesting their potential role in depression dynamics. Combined with prior knowledge (Arumugam et al., 2011; Costea et al., 2018), we grouped our cohort into high Bacteroides, high Prevotella and a Firmicutes enriched cluster that are low for the formal mentioned two genera but high for Ruminococcus (Fig. 2A, S2A), leading to the classification into Bacteroides (Ba), Ruminococcus (Ru), and Prevotella (Pr) enterotypes. Our results show that these enterotypes remained stable throughout the study, indicating that short-term interventions like meditation may not significantly alter these established microbial communities (Fig. S2B).
Fig. 2. Enterotype Analysis Reveals Potential Beneficial Role of Prevotella in Depression.
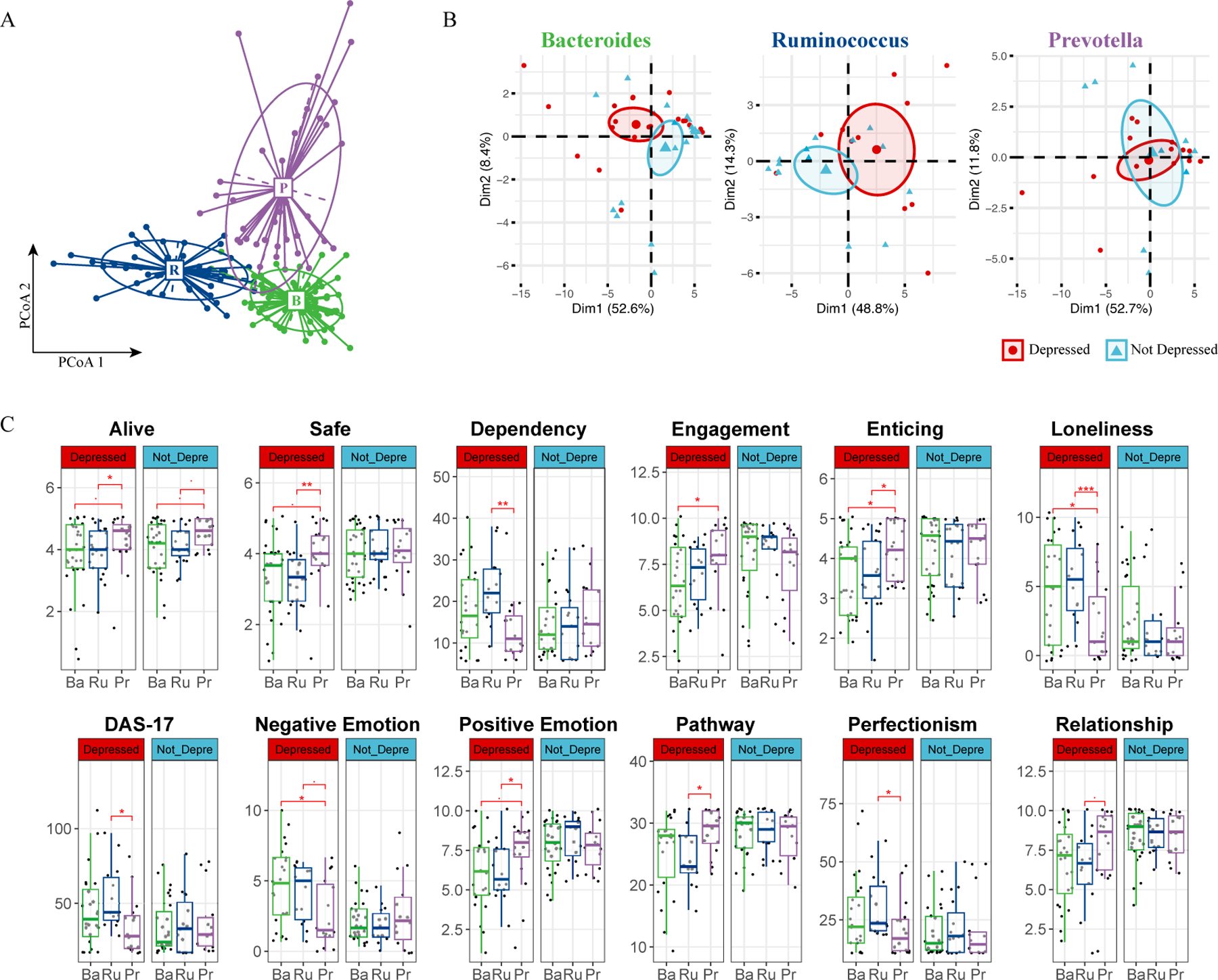
(A) Enterotype Classification of Microbiome Samples. Samples were clustered into three enterotypes. Each cluster is represented by a predominant genus, detailed in Supplementary Fig. S2A. Colors represent different enterotypes: Ruminococcus (R, blue), Bacteroidetes (B, green), and Prevotella (P, purple). (B) PCA of Psychometric Parameters Based on Depression Status. Samples are colored based on each individual’s initial depression status (red for depressed, blue for non-depressed). The first two principal components (PCs) are plotted, with the variance explained by each PC annotated. (C) Pairwise Comparison of Psychometric Data by Enterotype and Depression Status. Comparison across Ruminococcus (Ru, blue), Bacteroidetes (Ba, green), and Prevotella (Pe, purple) enterotypes. A two-sided Student’s T-test was used for each comparison. Significance levels are indicated as follows: p < 0.1 (.), p < 0.05 (*), p < 0.01 (**), p < 0.005 (***).
In turn, our psychometric data analysis revealed marked differences between depressed and non-depressed participants within the Bacteroides and Ruminococcus enterotypes, as shown by distinct clustering in Principal Component Analysis (PCA); such differentiation was absent in the Prevotella group (Fig. 2B). The similar variance explained by the first two principal components suggests that individuals within the Prevotella enterotype, regardless of depression status, exhibited comparable psychometric profiles. Further analysis revealed a unique trend within the Prevotella group: individuals scored higher on feeling “alive,” a pattern maintained across depression statuses (Fig. 2C). Additionally, depressed individuals with the Prevotella enterotype reported a greater sense of safety, enticement, and positive emotions. They also reported higher levels of engagement and relationship satisfaction, coupled with lower tendencies towards dependency, loneliness, and perfectionism. Their scores on the Dysfunctional Attitudes Scale (DAS17) were also consistently lower (Fig. 2C). The observations specific to the depressed individuals within the Prevotella group are not due to an overrepresentation of Prevotella in either the depressed or non-depressed groups (Fig. S2B), nor did we find a statistically different average BDI-II score at the beginning of the program or throughout its entirety for the Prevotella group. In addition, we did not identify any significant association of enterotype with participants’ Adverse Childhood Experiences (ACEs) score (Fig. S2C); in fact, none of the above-mentioned psychometric parameters were hierarchically clustered with ACEs (Fig. S2D).
3.3. Prevotella enterotype is linked to lower inflammation and enhanced microbiome stability
Acknowledging the positive psychometric outcomes linked to the Prevotella enterotype, we next investigated possible associations between enterotypes, baseline immune responses, and participants’ depressive symptoms. To understand the immune profile associated with depression further, we examined cytokines, chemokines, and growth factors in date-matched plasma samples using an 80-plex Luminex assay. Using a PERMANOVA test that analyzed variance in cytokine data by timepoints, enterotype, and depression status across 9,999 permutations, we identified a significant association between systemic inflammation, as reflected in cytokine levels, and depression status (R2 = 1.91 %, Pr > F = 0.005). Intriguingly, the enterotype also contributed to a minor, yet statistically significant, variation in cytokine levels (R2 = 3.84 %, Pr > F = 0.001).
Observing that cytokine levels vary with both enterotype and depression status, we performed hierarchical clustering of cytokine data averaged across these factors. We aimed to assess whether depressed individuals with the Prevotella enterotype have cytokine profiles similar to non-depressed groups. Our analysis revealed that they indeed clustered with non-depressed groups across all enterotypes, indicating a similar inflammatory profile (Fig.3A).
Fig. 3. Association of Prevotella Enterotype with Reduced Inflammation and Increased Gut Microbiome Stability.
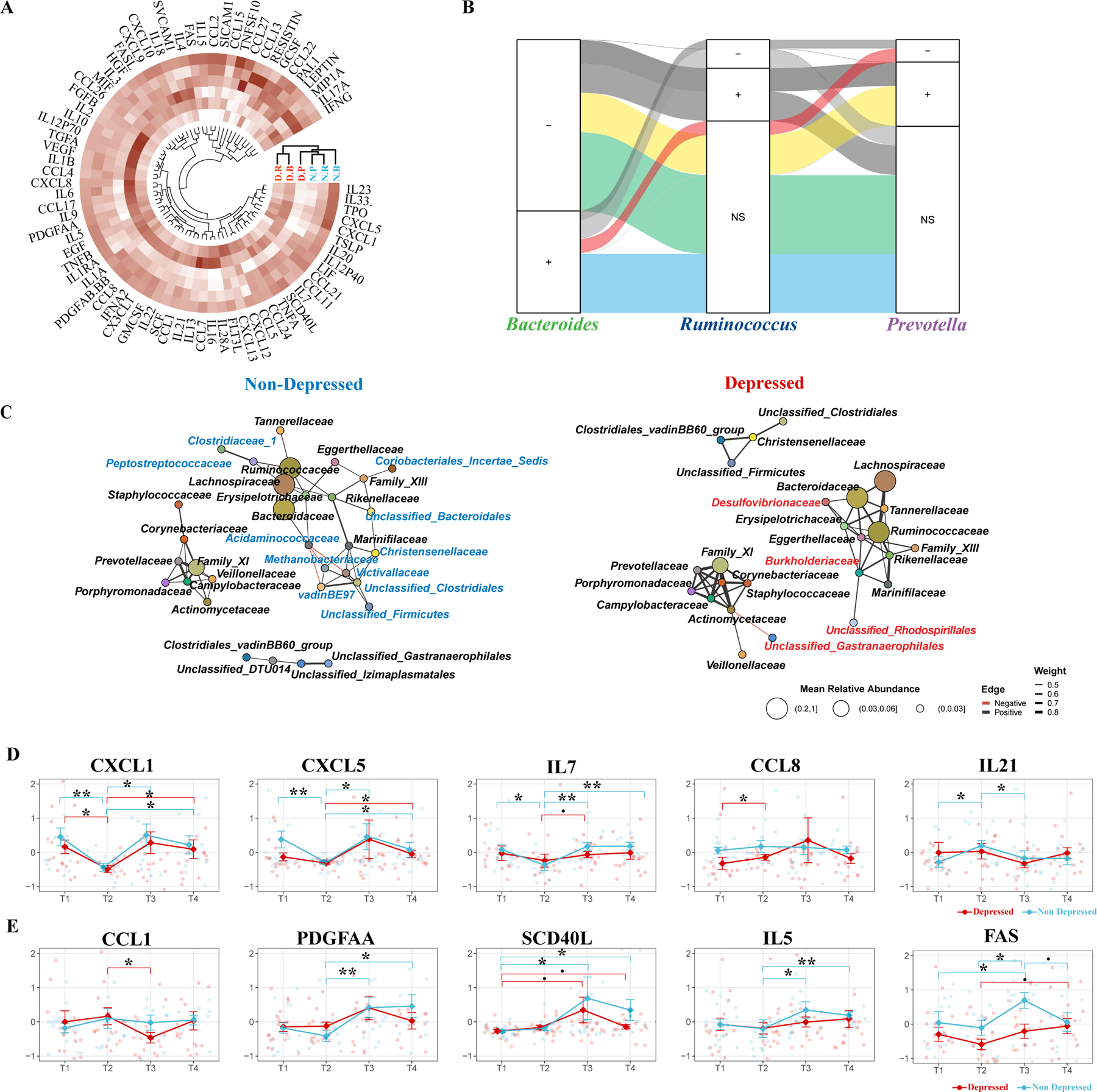
(A) Hierarchical Clustering of Cytokine Values. Clustering based on enterotype and depression status, illustrating the cytokine profiles across different groups. (B) Bayesian mixed-effects model identifying significant correlations between microbial genera and cytokine levels across three enterotypes (Bacteroides, Ruminococcus, and Prevotella). The left column represents significant cytokine-microbe associations identified in the Bacteroides enterotype. The middle and right columns show how these associations are either maintained or altered in the Ruminococcus and Prevotella enterotypes, respectively. A positive or negative sign indicates the direction of the correlation (positive or negative) in the Bacteroides enterotype and how this relationship changes in the Ruminococcus and Prevotella enterotypes. “Ns” denotes no significant change in the given enterotype. The color-coded flows (green, blue, red, and yellow) represent the consistency or divergence of these associations across the enterotypes. (C) Co-occurrence Network of Microbial Families. Network representations comparing depressed and non-depressed individuals. The left network represents the non-depressed group, and the right network represents the depressed group. Unique microbial families to each network are color-coded (blue for non-depressed, red for depressed). (D) Immediate Changes in Cytokines Post-Intervention. Significant Cytokine Changes Between T1 and T2. Identification of cytokines that showed significant differences between T1 and T2 in at least one group (depressed or non-depressed). (E) Delayed Changes in Cytokines Post-Intervention Significant Cytokine Changes Between T2 and T3: Identification of cytokines that showed significant differences between T2 and T3 in at least one group (depressed or non-depressed). A pairwise two-sided Student’s T-test was used for comparing cytokine levels between different time points. Significance levels are indicated as follows: BH-adjusted p < 0.1 (.), BH-adjusted p < 0.05 (*), BH-adjusted p < 0.01 (**), BH-adjusted p < 0.005 (***).
Using a customized longitudinal Bayesian mixed-effects modeling (Zhou et al., 2024a), we identified 978 cytokine-microbe associations affected by depression with over 95 % credibility. Most associations (493, 50.4 %) showed consistent associations across all three enterotypes, with balanced positive and negative correlations between cytokine levels and microbial abundances (Fig. 3B, green and blue flows). The next most-frequent category (191 associations, 19.5 %) exhibited consistent patterns in enterotypes Bacteroides and Ruminococcus but differed in enterotype Prevotella (Fig. 3B, red and yellow flows). For example, the association between IL and 22 levels and Ruminococcus abundance was negative in enterotype Bacteroides, unchanged in enterotype Ruminococcus, but shifted to positive in enterotype Prevotella (Fig. S3A). Similarly, MIP-1A (CCL-3) levels showed a strong positive association with Blautia abundance in enterotype Bacteroides, were unaffected in enterotype Ruminococcus, but significantly altered in enterotype Prevotella (Fig. S3B). These patterns support and explain the cytokine expression clustering among enterotypes under depression (Fig. 3A), suggesting that enterotype Prevotella exhibits unique cytokine-microbe interactions in depressed individuals.
Since cytokines and chemokines, which surge during inflammation, regulate gut microbiome stability (Zhou et al., 2020), the differing cytokine profiles may reflect variations in microbiome ecology. To explore this, we constructed family-level co-occurrence (Goodrich et al., 2014; Guo et al., 2023; Wu et al., 2021) networks of the gut microbiome for both depressed and non-depressed groups. Consistent with earlier findings (Fig. 2A, S2A), the families of Bacteroides (Bacteroidaceae, Enterotype Ba) and Ruminococcus (Ruminococcaceae, Enterotype Ru) formed a major module, while Prevotella (Prevotellaceae, Enterotype Pr) formed a distinct module (Fig. 3C). Our network analysis revealed eleven bacterial families uniquely associated with the Bacteroidaceae-Ruminococcaceae module in non-depressed individuals and three families unique to depressed individuals within the same cluster. The Prevotellaceae module showed similar patterns in both groups. These shifts in microbiome interdependencies suggest variations in stability, especially under Bacteroides or Ruminococcus dominance versus Prevotella. Interestingly, such altered co-occurrence patterns have been linked to diminished responses to antidepressants (Wang et al., 2023) and may predict generally a lower well-being in depression.
The interdependency of the gut microbiome between individuals initially classified as depressed and those who were not reveals potential significant biological implications. In our study, the core microbiome clusters in non-depressed individuals predominantly included families like Peptostreptococcus and Clostridiaceae. These clusters are known for their short-chain fatty acid (SCFA) production (Bangsgaard Bendtsen et al., 2012; Louis and Flint, 2017), which are beneficial for gut health and anti-inflammatory responses. Conversely, the microbiome of depressed individuals exhibited a significant presence of opportunistic pathogens, notably the family Desulfovibrionaceae (Fig. 3C). The genus Desulfovibrio within this family, implicated in diseases such as inflammatory bowel disease (IBD) (Fite et al., 2013), depression (Humbel et al., 2020; Simpson et al., 2021), and obesity (Petersen et al., 2019), may contribute to these conditions through its production of hydrogen sulfide (Singh et al., 2023; Singh and Lin, 2015) and immunogenic lipopolysaccharides (LPS) (Kapral et al., 2010; Weglarz et al., 2003). These substances are known to inflict inflammation-induced damage to the blood–brain barrier (Li et al., 2020; Ortega et al., 2022) and enhance intestinal permeability (Kiecolt-Glaser et al., 2018; Kronsten et al., 2022; Maes et al., 2008), illustrating a possible pathway by which alterations in the gut microbiome may influence systemic inflammation and, consequently, mental health.
Despite our PERMANOVA not showing a significant variance in cytokine levels over time overall, we pursued the identification of specific cytokines with significant temporal shifts. Using a two-sided Student’s T-test, we identified five cytokines that showed significant alterations at T2 and/or T3, indicative of short and/or long-term effects associated with the program. Notably, CXCL-1 (GROA), demonstrated a consistent decrease at T2 across both groups (Fig.3D). CXCL-1 has been directly associated with the development of depression, as evidenced in both animal models (Chai et al., 2019; Saika et al., 2018; Song et al., 2020) and human clinical studies (Bot et al., 2015; Camacho-Arroyo et al., 2021; Fanelli et al., 2019). Following the nine-day immersive psychosocial intervention program, the cytokines CXCL-5, IL-21, IL-7, and CCL-8 exhibited immediate perturbations. In contrast, PDGF-AA, SCD-40L, IL-5, CCL-1, and FAS exhibited perturbations two weeks post-program, indicating a delayed response (Fig.3E). Furthermore, the interaction between microbiome composition and cytokines CXCL-1, CXCL-5, and FAS was significantly altered by the stage of depression, as revealed by our Bayesian mixed-effects model. These results further link the altered inflammatory status of depressed individuals with their gut microbiome composition (Fig. S3C, Table S2). Intriguingly, the Prevotella enterotype group exhibited significantly lower levels of seven out of ten mentioned cytokines compared to the Bacteroidetes or Ruminococcus enterotypes, a pattern predominantly observed within the depressed cohort, except for CXCL1 (Fig. S3D). This finding points to a potential microbial influence on the cytokine environment, further complicating the association between gut microbiota, immune response, and mental health.
3.4. Mediation analysis reveals links between microbiome, cytokines, and mental health outcomes
Our analysis, incorporating a mediation model to examine the interplay between gut microbiome composition (X), mental health outcomes (Y), and plasma cytokine levels (M) uncovered 179 significant mediating effects (Table S3), suggesting intricate relations under assumed (Cryan and Dinan, 2012; Kronsten et al., 2022; Moriarity et al., 2023a) causal frameworks. In this model, we pinpointed several bacterial genera potentially linked to depression-like symptoms, highlighting the top five genera with the strongest signals (Fig. 4A). Specifically, we identified Sellimonas and Bifidobacterium as key bacterial genera associated with depression symptoms, corroborating their roles as biomarkers identified in comparative studies (Bosch et al., 2022; Wang et al., 2014) of microbiomes in healthy individuals and those with Major Depressive Disorder (MDD). The data also indicated Erysipelatoclostridium’s potential mediating role in exacerbating negative emotions via its effects on IL-33 (P = 0.016), Leptin (P = 0.028), and PAI-1 (P = 0.008) levels (Fig.4A, Table S3). This finding is consistent with prior studies proposing Erysipelatoclostridium as a depression marker (Kraaij et al., 2023; Zorkina et al., 2022) and its positive association with anorexia nervosa (Fan et al., 2023) and radiation-induced intestinal injury (Cai et al., 2022).
Fig. 4. Mediation Linkage between Microbiome, Cytokine and Mental Health.
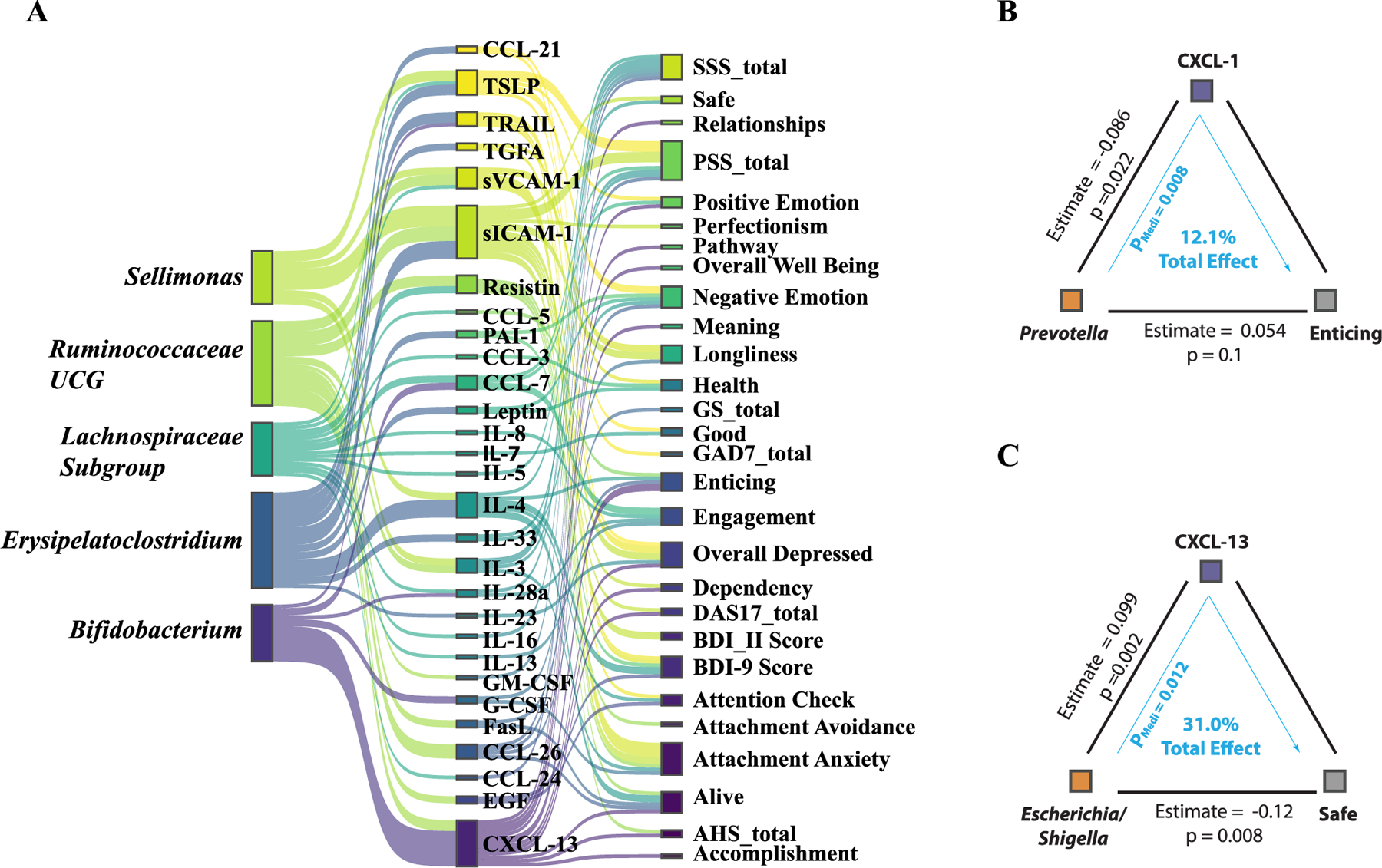
(A) The top five bacterial genera identified through mediation analysis for their influence on mental health outcomes, mediated by cytokine levels. (B) The mediation association involving the psychometric parameter ‘Enticing,’ the genus Prevotella, and the cytokine CXCL1. (C) The mediation association involving the psychometric parameter ‘Safe,’ the genus Escherichia/Shigella, and the cytokine CXCL13.
These data shed light on the potentially beneficial effects of the Prevotella genus, notably in modulating CXCL-1 expression and its significant role in enhancing perceptions of the world as enticing (Fig. 4B). CXCL-13′s mediation of feelings associated with enticement (P = 0.016) and safety (P = 0.012), along with its negative correlation with the Escherichia/Shigella genus—more prevalent in depressed individuals (Simpson et al., 2021)—underscores the complexity of microbiome-influenced emotional responses (Fig. 4B).
Given these observations, we explored the dietary factors that might influence Prevotella abundance to provide better understanding to the context of its potential links to psychosocial outcomes. Our prior research (Lancaster et al., 2022) demonstrated that consuming mixed dietary fibers Arabinoxylan and long-chain inulin (LCinulin) significantly increases Prevotella levels (Fig. S4A), suggesting a dietary pathway to enhance psychosocial intervention effects (Jiang et al., 2022; Kovatcheva-Datchary et al., 2015). These observations do not conclusively define Prevotella as a psych-biome marker but do provide evidence that dietary interventions may potentially modulate neuroinflammatory cytokines and help individuals manage stress, highlighting the intricate interplay between diet, gut microbiota, and mental health (Liu et al., 2023a).
4. Discussion
To date, associations between gut microbiome dysbiosis and various mental health disorders, especially depression, have been predominantly explored in cross-sectional studies (Jiang et al., 2015; Radjabzadeh et al., 2022; Valles-Colomer et al., 2019). The present investigation advances this research by longitudinally examining the microbiome’s role in mental wellness. By moving beyond simple longitudinal or cross-sectional analyses that compare depressed individuals with healthy controls, the present data yield an interesting possibility: the existence of a Prevotella-dominant enterotype may contribute to a more benign inflammatory environment, specifically in relation to depression-related symptoms. This proposition is supported by our detailed examination of cytokine and chemokine profiles, including CXCL-1, which suggests a potential for mitigating inflammatory responses. Additionally, our co-occurrence analysis challenges the prevailing notion that an individual’s depression status directly influences their gut microbiome composition. Instead, we observed that the stability of the microbiome, particularly among those with Prevotella dominance, appears less perturbed by depressive states compared to the microbiomes of individuals with Bacteroidetes or Ruminococcus enterotypes. This distinction points to a potentially critical role of microbiome composition in moderating baseline inflammation and maintaining gut epithelial integrity under the strain of depressive conditions—a concept that underscores the importance of microbial diversity in mental health and may open up new avenues for therapeutic interventions aimed at reducing depression risk (Ait-Belgnaoui et al., 2012).
Although the primary effect of the depression-reduction program may not be attributed to alterations in the microbiome, our findings suggest an interesting dynamic. In particular, whereas participants who recovered from depression did not show extensive microbiome remodeling post-intervention, non-depressed participants exhibited pronounced microbiome remodeling during the 9-day immersive intervention program. This finding suggests that individuals with depression at baseline may possess more static or unresponsive gut microbiomes. Such “unresponsive gut microbiomes,” often characterized by low diversity, have been linked to various inflammatory conditions, including insulin resistance (Zhou et al., 2019; Zhou et al., 2020), viral infections (Chen et al., 2022; Upadhyay et al., 2023; Yeoh et al., 2021), and cognitive decline associated with liver transplantation (Bajaj et al., 2017; Kriss et al., 2018). We believe this “unresponsive gut microbiome” may be a phenotype of depression-related dysbiosis, a consequence rather than a cause of depression-like symptoms.
Psychological research has long grappled with the question of whether ill-being and well-being are opposite or distinct entities. Our findings provide possible biological support that ill-being and well-being represent separate dimensions, each potentially influenced differently by the gut microbiome’s composition. This is particularly evident in the context of enterotypes, where Prevotella-dominant profiles are associated with positive emotional states (Lee et al., 2020). Prevotella, known for its metabolic activity (Betancur-Murillo et al., 2022), plays a role in producing neuroactive signaling molecules (Miri et al., 2023; Ortega et al., 2022), vitamins (Rudzki et al., 2021), and other mood-influencing compounds (Ke et al., 2023; van der Spek et al., 2023).This leads to an intriguing question: does Prevotella contribute to well-being through elevated production of these active signaling molecules? Our prior research (Lancaster et al., 2022) demonstrated that Prevotella levels increase with mixed fiber intake. In addition, the negative correlation between Subdoligranulum, a known butyrate-producing bacterium (Van Hul et al., 2020), and reductions in depressive symptoms suggests that butyrate may play a key role in mental health improvement. This finding is consistent with research linking dietary fiber intake to lower depression risk (Chen et al., 2023; Fatahi et al., 2021; Kim et al., 2020), which may be mediated by short-chain fatty acids (SCFAs) produced by fiber-digesting microbiota. SCFAs are known to regulate serotonin production (Reigstad et al., 2015) and potentially other neuroregulatory molecules. This association thus underscores the growing interest in ‘psychobiotics’ – probiotics that improve mental health through SCFA production and other mechanisms (Bokoliya et al., 2023; van de Wouw et al., 2018).
Taxonomic comparisons of microbiomes between depressed and non-depressed individuals often reveal inconsistent signals (Simpson et al., 2021), which is partly attributable to the highly personalized nature of both the microbiome and mental health. Nonetheless, certain trends and mechanisms have emerged as relatively consistent across research. For example, Sellimonas, proposed as a depression biomarker (Radjabzadeh et al., 2022) and noted for its antibiotic resistance (Munoz et al., 2020), was highlighted in our mediation analysis. Our findings on Bifidobacterium also require attention; while much psychobiome research has focused on Lactobacillus and Bifidobacterium (Chen et al., 2023; Lee et al., 2021; Steenbergen et al., 2015; Venkataraman et al., 2021), our analysis and prior studies suggest that Bifidobacterium’s role in psychiatric disorders may be more complex than previously thought (Chung et al., 2019; Knudsen et al., 2021; Wang et al., 2014). This highlights the need for mechanistic studies to elucidate the roles of potential probiotic strains in mental health. Our mediation analysis underscores bacteria previously associated with depression, suggesting a possible link between depressive symptoms and dysbiotic gut microbiome changes.
Our findings indicate that gut microbiome may influence depression through mechanisms involving immune system modulation, particularly inflammation, which has been strongly linked to depression (Slavich et al., 2020; Slavich and Irwin, 2014; Slavich et al., 2010). Despite observing significant psychometric improvements in depressed individuals, their microbiome and cytokine profiles showed remarkable stability post-intervention. This persistence, even amid depression recovery, implies that these biological markers might not directly drive depressive states. Although a comprehensive analysis of inflammation’s role and its cellular underpinnings remains to be fully explored, the present data highlight subtle yet noteworthy shifts in inflammatory cytokines and chemokines following a psychosocial intervention that were observable both immediately and over time. These alterations, though modest and not as pronounced as those seen in studies of respiratory viral infections (Zhou et al., 2024b), suggest the presence of a low-grade inflammatory state rather than an acute immune reaction (Furman et al., 2019).
Our study highlights several cytokines that are of particular interest in mental health research (Polacchini et al., 2018). Notably, the immersive psychosocial intervention that we tested significantly reduced CXCL-1 levels, a neuroinflammatory cytokine, across all participants, including those not diagnosed with depression. CXCL-1, implicated in various neuropsychiatric disorders and typically elevated in depression (Camacho-Arroyo et al., 2021; Lee et al., 2009), has been identified as a potential therapeutic target via the CXCL1-GSK3β pathway in animal studies (Chai et al., 2019; Saika et al., 2018). Other cytokines such as CCL-17 and CXCL-5 also showed reductions and are associated with depressive states in the literature (Freff et al., 2022; Li et al., 2017). Our mediation analysis sheds light on the role of cytokines like soluble VCAM-1 and ICAM-1 in bridging the microbiome-brain axis, crucial for maintaining the integrity of blood–brain and gut epithelial barriers (Kronsten et al., 2022; Slyepchenko et al., 2017). Furthermore, cytokines including CXCL-13 and IL-4, known for their neuroprotective functions (Trolese et al., 2020) and ability to counter IL-1β-induced depressive-like behavior (Park et al., 2015), displayed significant mediative effects in our analysis. These findings highlight a nuanced role of inflammation in mental health, suggesting its potential to modulate neuroinflammatory conditions rather than exacerbating them (Maes et al., 2008). Notably, depressed individuals with a Prevotella-dominant enterotype exhibited lower baseline levels of these cytokines, indicating a microbiome-immune system interaction that might favor psychosocial treatment effectiveness. This effect points to a significant interplay between specific gut microbiome compositions, immune responses, and mental health outcomes, suggesting the integration of microbiome considerations into psychosocial intervention strategies.
4.1. Limitations
These findings should be interpreted considering several limitations. Firstly, depression was measured using self-report instruments, which, despite their common use and validity, should ideally be supplemented with clinician-rated assessments in future studies to enhance diagnostic accuracy. Secondly, the study’s scope lacks the statistical power necessary for the definitive identification of microbiome or cytokine biomarkers for depression. This issue is compounded by a limited sample size, which constrains the reliability of our findings, especially concerning changes—or the lack thereof—among prevalent bacterial genera. In addition, we had some missing data on key baseline characteristics, including age, sex, and BMI, which restricted our ability to fully control for these potential confounders in multivariate analyses without further reducing the sample size. Although additional analyses indicated no significant differences in age, sex, or BMI across enterotype clusters, future studies with larger, more complete datasets are needed to confirm these findings and allow for more robust statistical adjustments.
Thirdly, our mediation analysis, while offering valuable insights, rests on assumed causal links that have not been statistically verified beyond existing theoretical frameworks (Moriarity et al., 2023a). The potential for stress-induced microbiome alterations via cytokine pathways or the influence of other, unidentified confounding factors cannot be overlooked. Moreover, our analysis focuses on identifying potential agents of causation within the microbiome community concerning cytokine levels but stops short of categorically determining the microbiome’s impact as either beneficial or detrimental. This ambiguity stems from the personalized nature of microbiome and cytokine interactions and the absence of conclusive evidence to underpin such claims based on correlation alone. For example, the association with the Prevotella enterotype may be more indicative of dietary preferences, such as high fiber consumption, rather than a direct mood-enhancing property of Prevotella. Consequently, the associations identified herein should primarily be considered indicative of concurrent occurrences rather than direct biological mechanisms. To establish definitive mechanisms, additional research using more rigorously designed experimental studies is necessary. Indeed, this study was designed not as a clinical trial but as a community-based observational study to explore associations between psychosocial intervention, microbiome changes, cytokine levels, and depressive symptoms in high resolution. Therefore, causality and directionality cannot be assumed, and experimental studies and randomized controlled trials are needed to further interrogate the present findings and advance this body of work.
4.2. Conclusion
Despite these limitations, our findings catalyze an intriguing hypothesis: individuals with depression might benefit from fostering a microbiome composition that is ‘healthier’ in the context of depression-related inflammation. This specific microbial configuration could pre-dispose individuals to a more favorable inflammatory baseline, which, in turn, might enhance or correlate with the effectiveness of psychosocial therapeutic interventions tailored to depression. This hypothesis is novel in its suggestion that the gut microbiome may play a significant role in either augmenting or correlating with the outcomes of such interventions in humans, and invites further exploration into the nuanced interplay between gut microbiome dynamics and inflammation in depression. This study may also help inform future research focused on elucidating the potential of microbiome-focused strategies to complement traditional mental health treatments, emphasizing the need for more targeted research into how microbial compositions influence depression-specific inflammatory processes and psychosocial wellbeing.
Supplementary Material
Acknowledgements
We thank all of the individuals who participated in this study. We also thank Mrs. Ada Yee Ki Chen and Mrs. Lisa Stainton for their administrative and research support. Our appreciation extends to the Stanford Immune Monitoring Core for their contribution to the cytokine Luminex Multiplex Assay.
Funding
The research was supported by NIH grants R01-MH116529 and R25-HG010857. X.Z. was supported by NIA fellowship Resource Centers for Minority Aging Research grant P30 AG059307, D.J.S. was supported by NIA grant K01 AG070310, and J.S.J. was supported by the Kennedy Trust for Rheumatology Research. G.M.S. was supported by grant #OPR21101 from the California Governor’s Office of Planning and Research/California Initiative to Advance Precision Medicine. We are also sincerely grateful for the generous support from Leona M. and Harry B. Helmsley Charitable Trust (grant no. G-2004-03820), the Innovative Medicines Accelerator (grant no. IMA-1051) grant, and the RAMBAM-Stanford International Collaboration grant at Stanford University. The findings and conclusions in this article are those of the authors and do not necessarily represent the views or opinions of these organizations, which had no role in designing or planning this study; in collecting, analyzing, or interpreting the data; in writing the article; or in deciding to submit this article for publication.
Footnotes
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the authors used ChatGPT in order to improve the quality of the scientific writing. After using this tool/service, the authors reviewed and edited the content as needed and took full responsibility for the content of the publication.
CRediT authorship contribution statement
Xin Zhou: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis, Data curation, Conceptualization. Ariel B. Ganz: Conceptualization, Data curation, Writing – review & editing, Project administration. Andre Rayner: Formal analysis, Data curation. Tess Yan Cheng: Formal analysis, Methodology. Haley Oba: Methodology, Formal analysis. Benjamin Rolnik: Investigation, Funding acquisition. Samuel Lancaster: Formal analysis, Data curation. Xinrui Lu: Formal analysis. Yizhou Li: Supervision, Formal analysis. Jethro S. Johnson: Methodology, Formal analysis, Data curation. Rebecca Hoyd: Methodology, Formal analysis. Daniel J. Spakowicz: Methodology, Formal analysis. George M. Slavich: Writing – review & editing, Conceptualization. Michael P. Snyder: Writing – review & editing, Supervision, Resources, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: M.P.S. is a co-founder and scientific advisor for Crosshair Therapeutics, Exposomics, Filtricine, Fodsel, Iollo, InVu Health, January AI, Marble Therapeutics, Mirvie, Next Thought AI, Orange Street Ventures, Personalis, Protos Biologics, Qbio, RTHM, SensOmics. M.P.S. serves as a scientific advisor for Abbratech, Applied Cognition, Enovone, Jupiter Therapeutics, M3 Helium, Mitrix, Neuvivo, Onza, Sigil Biosciences, TranscribeGlass, WndrHLTH, Yuvan Research. M.P.S. is a co-founder of NiMo Therapeutics. M.P.S. is an investor and scientific advisor of R42 and Swaza. M.P.S. is an investor in Repair Biotechnologies. A.B.G. is a co-founder of Arben Ventures and Xthena Partners. B.R. is a co-founder of Arben Ventures, Enovone, and an advisor to Bexson and Northstar Care. After data collection was complete, as well as after most data analysis was completed, A.B.G. and B.R. co-founded what was formerly InquiryRx with Byron Katie, but ultimately decided not to continue the venture. Trudy Green was the 100 % owner of InquiryRx. Byron Katie lectured in Stanford BIOS 237, a class co-taught by A.B.G. and M.P.S. and spoke at the Stanford Mental Healthcare Innovations Summit. At the time of the initial publication of this article, the Academic Incubator Fund, managed by Arben Ventures, of which A.B.G. and B.R. are co-founders and managing partners, is an investor in 36 companies—namely, Bloch Quantum Imaging Solutions, Elemind Technologies, North-StarCare, Mercy BioAnalytics, Othership, Superbio.ai, Empo Health, Kelso Health, Acta Pharmaceuticals, Enovone, Superhumn, Parallel Health, Mitrix Bio, Bexson Biomedical, Allia Health, Glyphic Biotechnologies, RTHM, QBio, NextThought AI, ReMinded, Lynx Tech, Firefly VR-P, Amira Health, Imago Systems, SeeMedX, Vertility Health, OnFirm, ModoScript, Outro Health, Concha Labs, The Natural Nipple, Navan Technologies, TranscribeGlass, Dognosis, MoveJoy, and Kangaroo Bio–and an advisor to Bloch Quantum Imaging Solutions, Elemind Technologies, Allia Health, and SeeMedX. The remaining authors do not have any conflicts of interest with respect to this work.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2024.12.027.
Data availability
All data generated and analyzed during this study are available for scientific research upon request to the corresponding author. The analysis code is publicly accessible at https://github.com/xzhou7/mentalmicrobiome.
References
- Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V, 2012. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 37, 1885–1895. [DOI] [PubMed] [Google Scholar]
- Allan RD, Johnston GA, 1983. Synthetic analogs for the study of GABA as a neurotransmitter. Med. Res. Rev 3, 91–118. [DOI] [PubMed] [Google Scholar]
- Artigas F, 2013. Serotonin receptors involved in antidepressant effects. Pharmacol. Ther 137, 119–131. [DOI] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Dore J, Meta HITC, Antolin M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Merieux A, Melo Minardi R, M’Rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P, 2011. Enterotypes of the human gut microbiome. Nature 473, 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairamian D, Sha S, Rolhion N, Sokol H, Dorothee G, Lemere CA, Krantic S, 2022. Microbiota in neuroinflammation and synaptic dysfunction: a focus on Alzheimer’s disease. Mol. Neurodegener 17, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj JS, Fagan A, Sikaroodi M, White MB, Sterling RK, Gilles H, Heuman D, Stravitz RT, Matherly SC, Siddiqui MS, Puri P, Sanyal AJ, Luketic V, John B, Fuchs M, Ahluwalia V, Gillevet PM, 2017. Liver transplant modulates gut microbial dysbiosis and cognitive function in cirrhosis. Liver Transpl 23, 907–914. [DOI] [PubMed] [Google Scholar]
- Bangsgaard Bendtsen KM, Krych L, Sorensen DB, Pang W, Nielsen DS, Josefsen K, Hansen LH, Sorensen SJ, Hansen AK, 2012. Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PLoS One 7, e46231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barandouzi ZA, Starkweather AR, Henderson WA, Gyamfi A, Cong XS, 2020. Altered composition of gut microbiota in depression: a systematic review. Front. Psych 11, 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Gazzaniga MS, 2011. Understanding complexity in the human brain. Trends Cogn. Sci 15, 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W, 1996. Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J. Pers. Assess 67, 588–597. [DOI] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM, 2011. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141, 599–609, 609 e591–593. [DOI] [PubMed] [Google Scholar]
- Betancur-Murillo CL, Aguilar-Marin SB, Jovel J, 2022. Prevotella: a key player in ruminal metabolism. Microorganisms 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binh Tran TD, Nguyen H, Sodergren E, Addiction C, Dickson PE, Wright SN, Philip VM, Weinstock GM, Chesler EJ, Zhou Y, Bubier JA, 2023. Microbial glutamate metabolism predicts intravenous cocaine self-administration in diversity outbred mice. Neuropharmacology 226, 109409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DS, Slavich GM, 2016. Mindfulness meditation and the immune system: a systematic review of randomized controlled trials. Ann. N. Y. Acad. Sci 1373, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoliya SC, Russell J, Dorsett Y, Panier H, Singh V, Daddi L, Yuan H, Dedon LR, Liu Z, Barson JR, Covault J, Bubier JA, Zhou Y, 2023. Short-chain-fatty acid valerate reduces voluntary alcohol intake in male mice. Res Sq. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ, 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462. [DOI] [PubMed] [Google Scholar]
- Bosch JA, Nieuwdorp M, Zwinderman AH, Deschasaux M, Radjabzadeh D, Kraaij R, Davids M, de Rooij SR, Lok A, 2022. The gut microbiota and depressive symptoms across ethnic groups. Nat. Commun 13, 7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bot M, Chan MK, Jansen R, Lamers F, Vogelzangs N, Steiner J, Leweke FM, Rothermundt M, Cooper J, Bahn S, Penninx BW, 2015. Serum proteomic profiling of major depressive disorder. Transl. Psychiatry 5, e599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürkner P-C, 2021. Bayesian item response modeling in R with brms and Stan. J. Stat. Softw 100, 1–54. [Google Scholar]
- Bussi IL, Neitz AF, Sanchez REA, Casiraghi LP, Moldavan M, Kunda D, Allen CN, Evans JA, de la Iglesia HO, 2023. Expression of the vesicular GABA transporter within neuromedin S(+) neurons sustains behavioral circadian rhythms. PNAS 120, e2314857120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Yang Y, Kong Y, Guo Q, Xu Y, Xing P, Sun Y, Qian J, Xu R, Xie L, Hu Y, Wang M, Li M, Tian Y, Mao W, 2022. Gut bacteria erysipelatoclostridium and its related metabolite Ptilosteroid A could predict radiation-induced intestinal injury. Front. Public Health 10, 862598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Arroyo I, Flores-Ramos M, Mancilla-Herrera I, Cruz FMC, Hernandez-Ruiz J, Diaz GP, Labonne BF, Del Pilar Meza-Rodriguez M, Gelman PL, 2021. Chemokine profile in women with moderate to severe anxiety and depression during pregnancy. BMC Pregnancy Childbirth 21, 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbia C, Bastiaanssen TFS, Iannone LF, Garcia-Cabrerizo R, Boscaini S, Berding K, Strain CR, Clarke G, Stanton C, Dinan TG, Cryan JF, 2023. The Microbiome-Gut-Brain axis regulates social cognition & craving in young binge drinkers. EBioMedicine 89, 104442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlessi AS, Borba LA, Zugno AI, Quevedo J, Reus GZ, 2021. Gut microbiota-brain axis in depression: the role of neuroinflammation. Eur. J. Neurosci 53, 222–235. [DOI] [PubMed] [Google Scholar]
- Chai HH, Fu XC, Ma L, Sun HT, Chen GZ, Song MY, Chen WX, Chen YS, Tan MX, Guo YW, Li SP, 2019. The chemokine CXCL1 and its receptor CXCR2 contribute to chronic stress-induced depression in mice. FASEB J. 33, 8853–8864. [DOI] [PubMed] [Google Scholar]
- Chandran V, Bermudez ML, Koka M, Chandran B, Pawale D, Vishnubhotla R, Alankar S, Maturi R, Subramaniam B, Sadhasivam S, 2021. Large-scale genomic study reveals robust activation of the immune system following advanced Inner Engineering meditation retreat. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Wei Y, Hashimoto K, 2022. Brain-gut-microbiota axis in depression: A historical overview and future directions. Brain Res. Bull 182, 44–56. [DOI] [PubMed] [Google Scholar]
- Chen Y, Gu S, Chen Y, Lu H, Shi D, Guo J, Wu WR, Yang Y, Li Y, Xu KJ, Ding C, Luo R, Huang C, Yu L, Xu M, Yi P, Liu J, Tao JJ, Zhang H, Lv L, Wang B, Sheng J, Li L, 2022. Six-month follow-up of gut microbiota richness in patients with COVID-19. Gut 71, 222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EZ, Li H, 2016. A two-part mixed-effects model for analyzing longitudinal microbiome compositional data. Bioinformatics 32, 2611–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liu B, Ren L, Du H, Fei C, Qian C, Li B, Zhang R, Liu H, Li Z, Ma Z, 2023. High-fiber diet ameliorates gut microbiota, serum metabolism and emotional mood in type 2 diabetes patients. Front. Cell. Infect. Microbiol 13, 1069954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Park MN, Kim CS, Lee YK, Choi EY, Chun WY, Shin DM, 2017. Long-term consumption of sugar-sweetened beverage during the growth period promotes social aggression in adult mice with proinflammatory responses in the brain. Sci. Rep 7, 45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YE, Chen HC, Chou HL, Chen IM, Lee MS, Chuang LC, Liu YW, Lu ML, Chen CH, Wu CS, Huang MC, Liao SC, Ni YH, Lai MS, Shih WL, Kuo PH, 2019. Exploration of microbiota targets for major depressive disorder and mood related traits. J. Psychiatr. Res 111, 74–82. [DOI] [PubMed] [Google Scholar]
- Collaborators C-M-D, 2021. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 398, 1700–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators GBDMD, 2022. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 9, 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia AS, Vale N, 2022. Tryptophan metabolism in depression: a narrative review with a focus on serotonin and kynurenine pathways. Int. J. Mol. Sci 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costea PI, Hildebrand F, Arumugam M, Backhed F, Blaser MJ, Bushman FD, de Vos WM, Ehrlich SD, Fraser CM, Hattori M, Huttenhower C, Jeffery IB, Knights D, Lewis JD, Ley RE, Ochman H, O’Toole PW, Quince C, Relman DA, Shanahan F, Sunagawa S, Wang J, Weinstock GM, Wu GD, Zeller G, Zhao L, Raes J, Knight R, Bork P, 2018. Enterotypes in the landscape of gut microbial community composition. Nat. Microbiol 3, 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LM, Weiner HL, 2018. Microbiota signaling pathways that influence neurologic disease. Neurotherapeutics 15, 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG, 2012. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci 13, 701–712. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S, 2011. Normal gut microbiota modulates brain development and behavior. PNAS 108, 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Sanacora G, Krystal JH, 2019. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron 102, 75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erspamer V, 1954. Pharmacology of indole-alkylamines. Pharmacol. Rev 6, 425–487. [PubMed] [Google Scholar]
- Fan Y, Stoving RK, Berreira Ibraim S, Hyotylainen T, Thirion F, Arora T, Lyu L, Stankevic E, Hansen TH, Dechelotte P, Sinioja T, Ragnarsdottir O, Pons N, Galleron N, Quinquis B, Levenez F, Roume H, Falony G, Vieira-Silva S, Raes J, Clausen L, Telleus GK, Backhed F, Oresic M, Ehrlich SD, Pedersen O, 2023. The gut microbiota contributes to the pathogenesis of anorexia nervosa in humans and mice. Nat. Microbiol 8, 787–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanelli G, Benedetti F, Wang SM, Lee SJ, Jun TY, Masand PS, Patkar AA, Han C, Serretti A, Pae CU, Fabbri C, 2019. Reduced CXCL1/GRO chemokine plasma levels are a possible biomarker of elderly depression. J. Affect. Disord 249, 410–417. [DOI] [PubMed] [Google Scholar]
- Faro A, Pereira CR, 2020. Factor structure and gender invariance of the Beck Depression Inventory - second edition (BDI-II) in a community-dwelling sample of adults. Health Psychol Behav Med 8, 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatahi S, Matin SS, Sohouli MH, Gaman MA, Raee P, Olang B, Kathirgamathamby V, Santos HO, Guimaraes NS, Shidfar F, 2021. Association of dietary fiber and depression symptom: a systematic review and meta-analysis of observational studies. Complement. Ther. Med 56, 102621. [DOI] [PubMed] [Google Scholar]
- Feldman O, Goldstien E, Rolnik B, Ganz AB, Lev-Ari S, 2021. Inquiry based stress reduction (IBSR) improves overall stuttering experience among adults who stutter: a randomized controlled trial. J. Clin. Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fite A, Macfarlane S, Furrie E, Bahrami B, Cummings JH, Steinke DT, Macfarlane GT, 2013. Longitudinal analyses of gut mucosal microbiotas in ulcerative colitis in relation to patient age and disease severity and duration. J. Clin. Microbiol 51, 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freff J, Beins EC, Broker L, Schwarte K, Leite Dantas R, Maj C, Arolt V, Dannlowski U, Nothen MM, Baune BT, Forstner AJ, Alferink J, 2022. Chemokine receptor 4 expression on blood T lymphocytes predicts severity of major depressive disorder. J. Affect. Disord 310, 343–353. [DOI] [PubMed] [Google Scholar]
- Friedrich MJ, 2017. Depression is the leading cause of disability around the world. J. Am. Med. Assoc 317, 1517. [DOI] [PubMed] [Google Scholar]
- Fries GR, Saldana VA, Finnstein J, Rein T, 2023. Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 28, 284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung TC, Olson CA, Hsiao EY, 2017. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci 20, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM, 2019. Chronic inflammation in the etiology of disease across the life span. Nat. Med 25, 1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, Ley RE, 2014. Human genetics shape the gut microbiome. Cell 159, 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Prinstein MJ, Clark LA, Rottenberg J, Abramowitz JS, Albano AM, Aldao A, Borelli JL, Chung T, Davila J, Forbes EE, Gee DG, Hall GCN, Hallion LS, Hinshaw SP, Hofmann SG, Hollon SD, Joormann J, Kazdin AE, Klein DN, La Greca AM, Levenson RW, MacDonald AW, McKay D, McLaughlin KA, Mendle J, Miller AB, Neblett EW, Nock M, Olatunji BO, Persons JB, Rozek DC, Schleider JL, Slavich GM, Teachman BA, Vine V, Weinstock LM, 2021. Mental health and clinical psychological science in the time of COVID-19: Challenges, opportunities, and a call to action. Am. Psychol 76, 409–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Wu G, Tan Y, Li Y, Jin X, Qi W, Guo X, Zhang C, Zhu Z, Zhao L, 2023. Guild-level microbiome signature associated with COVID-19 severity and prognosis. mBio 14, e0351922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A, 2014. CXCL12 chemokine and GABA neurotransmitter systems crosstalk and their putative roles. Front. Cell. Neurosci 5, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbel F, Rieder JH, Franc Y, Juillerat P, Scharl M, Misselwitz B, Schreiner P, Begre S, Rogler G, von Kanel R, Yilmaz B, Biedermann L, Swiss IBD Cohort Study Group, 2020. Association of alterations in intestinal microbiota with impaired psychological function in patients with inflammatory bowel diseases in remission. Clin. Gastroenterol. Hepatol. 18 (2019–2029), e2011. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B, 2015. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun 48, 186–194. [DOI] [PubMed] [Google Scholar]
- Jiang L, Shang M, Yu S, Liu Y, Zhang H, Zhou Y, Wang M, Wang T, Li H, Liu Z, Zhang X, 2022. A high-fiber diet synergizes with Prevotella copri and exacerbates rheumatoid arthritis. Cell. Mol. Immunol 19, 1414–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapral M, Weglarz L, Parfiniewicz B, Lodowska J, Jaworska-Kik M, 2010. Quantitative evaluation of transcriptional activation of NF-kappaB p65 and p50 subunits and IkappaBalpha encoding genes in colon cancer cells by Desulfovibrio desulfuricans endotoxin. Folia Microbiol (Praha) 55, 657–661. [DOI] [PubMed] [Google Scholar]
- Kay M, 2020. tidybayes: Tidy Data and Geoms for Bayesian Models (R package version 3.0. 2). [Google Scholar]
- Ke S, Wang X-W, Ratanatharathorn A, Huang T, Roberts AL, Grodstein F, Kubzansky LD, Koenen KC, Liu Y-Y, 2023. Association of probable post-traumatic stress disorder with dietary pattern and gut microbiome in a cohort of women. Nat. Ment. Health 1, 900–913. [Google Scholar]
- Kelly JR, Borre Y, C, O.B., Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG, 2016. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 82, 109–118. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Bromet EJ, 2013. The epidemiology of depression across cultures. Annu. Rev. Public Health 34, 119–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, 2022. The serotonin theory of depression and why we use antidepressants. Br. J. Gen. Pract 72, 536–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Wilson SJ, Bailey ML, Andridge R, Peng J, Jaremka LM, Fagundes CP, Malarkey WB, Laskowski B, Belury MA, 2018. Marital distress, depression, and a leaky gut: Translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology 98, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi AM, Tanabe A, Iwahori Y, 2021. A systematic review of the effect of L-tryptophan supplementation on mood and emotional functioning. J. Diet Suppl 18, 316–333. [DOI] [PubMed] [Google Scholar]
- Kim Y, Hong M, Kim S, Shin WY, Kim JH, 2020. Inverse association between dietary fiber intake and depression in premenopausal women: a nationwide population-based survey. Menopause 28, 150–156. [DOI] [PubMed] [Google Scholar]
- Kim YK, Na KS, Shin KH, Jung HY, Choi SH, Kim JB, 2007. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 31, 1044–1053. [DOI] [PubMed] [Google Scholar]
- Knudsen JK, Bundgaard-Nielsen C, Hjerrild S, Nielsen RE, Leutscher P, Sorensen S, 2021. Gut microbiota variations in patients diagnosed with major depressive disorder-a systematic review. Brain Behav 11, e02177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Bjorck I, Backhed F, 2015. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab 22, 971–982. [DOI] [PubMed] [Google Scholar]
- Kraaij R, Schuurmans IK, Radjabzadeh D, Tiemeier H, Dinan TG, Uitterlinden AG, Hillegers M, Jaddoe VWV, Duijts L, Moll H, Rivadeneira F, Medina-Gomez C, Jansen PW, Cecil CAM, 2023. The gut microbiome and child mental health: a population-based study. Brain Behav. Immun 108, 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ, 2008. The molecular neurobiology of depression. Nature 455, 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ, 2011. Animal models of depression: molecular perspectives. Curr. Top. Behav. Neurosci 7, 121–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriss M, Hazleton KZ, Nusbacher NM, Martin CG, Lozupone CA, 2018. Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery. Curr. Opin. Microbiol 44, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronsten VT, Tranah TH, Pariante C, Shawcross DL, 2022. Gut-derived systemic inflammation as a driver of depression in chronic liver disease. J. Hepatol 76, 665–680. [DOI] [PubMed] [Google Scholar]
- Kupcova I, Danisovic L, Klein M, Harsanyi S, 2023. Effects of the COVID-19 pandemic on mental health, anxiety, and depression. BMC Psychol 11, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster SM, Lee-McMullen B, Abbott CW, Quijada JV, Hornburg D, Park H, Perelman D, Peterson DJ, Tang M, Robinson A, Ahadi S, Contrepois K, Hung CJ, Ashland M, McLaughlin T, Boonyanit A, Horning A, Sonnenburg JL, Snyder MP, 2022. Global, distinctive, and personal changes in molecular and microbial profiles by specific fibers in humans. Cell Host Microbe 30 (848–862), e847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau C, Novak AM, Ganz AB, Rolnik B, Friedman E, Lev-Ari S, 2021. Effect of inquiry-based stress reduction on well-being and views on risk-reducing surgery among women with BRCA variants in Israel: a randomized clinical trial. JAMA Netw. Open 4, e2139670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Starkel P, Windey K, Tremaroli V, Backhed F, Verbeke K, de Timary P, Delzenne NM, 2014. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. PNAS 111, E4485–E4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Chung JH, Lee KH, Shin MJ, Oh BH, Lee SH, Hong CH, 2009. Simultaneous measurement of 23 plasma cytokines in late-life depression. Neurol. Sci 30, 435–438. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Hong JK, Kim JK, Kim DH, Jang SW, Han SW, Yoon IY, 2021. Effects of probiotic NVP-1704 on mental health and sleep in healthy adults: an 8-week randomized, double-blind, placebo-controlled trial. Nutrients 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Yoon SH, Jung Y, Kim N, Min U, Chun J, Choi I, 2020. Emotional well-being and gut microbiome profiles by enterotype. Sci. Rep 10, 20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legler DF, Thelen M, 2018. New insights in chemokine signaling. F1000Res 7, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang Z, Zhang C, Chen J, Su Y, Huang J, Yi Z, Yuan C, Hong W, Wang Y, Wu Z, Hu Y, Cao L, Peng D, Guan Y, Zou Y, Yu S, Cui D, Fang Y, 2017. Reduced ENA78 levels as novel biomarker for major depressive disorder and venlafaxine efficiency: result from a prospective longitudinal study. Psychoneuroendocrinology 81, 113–121. [DOI] [PubMed] [Google Scholar]
- Li T, Zheng LN, Han XH, 2020. Fenretinide attenuates lipopolysaccharide (LPS)-induced blood-brain barrier (BBB) and depressive-like behavior in mice by targeting Nrf-2 signaling. Biomed. Pharmacother 125, 109680. [DOI] [PubMed] [Google Scholar]
- Ling Z, Cheng Y, Chen F, Yan X, Liu X, Shao L, Jin G, Zhou D, Jiang G, Li H, Zhao L, Song Q, 2022. Changes in fecal microbiota composition and the cytokine expression profile in school-aged children with depression: a case-control study. Front. Immunol 13, 964910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, van Beek N, Cepic A, Andreani NA, Chung CJ, Hermes BM, Yilmaz K, Benoit S, Drenovska K, Gerdes S, Glaser R, Goebeler M, Gunther C, von Georg A, Hammers CM, Holtsche MM, Hubner F, Kiritsi D, Schauer F, Linnenmann B, Huilaja L, Tasanen-Maatta K, Vassileva S, Zillikens D, Sadik CD, Schmidt E, Ibrahim S, Baines JF, 2023b. The gut microbiome in bullous pemphigoid: implications of the gut-skin axis for disease susceptibility. Front. Immunol 14, 1212551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wang H, Chen X, Zhang Y, Zhang H, Xie P, 2023a. Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine 90, 104527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P, Flint HJ, 2017. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol 19, 29–41. [DOI] [PubMed] [Google Scholar]
- Lukic I, Getselter D, Ziv O, Oron O, Reuveni E, Koren O, Elliott E, 2019. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl. Psychiatry 9, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Zeng B, Zeng L, Du X, Li B, Huo R, Liu L, Wang H, Dong M, Pan J, Zheng P, Zhou C, Wei H, Xie P, 2018. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Transl. Psychiatry 8, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CMK, Cowan CSM, Bastiaanssen TFS, Moloney GM, Theune N, van de Wouw M, Florensa Zanuy E, Ventura-Silva AP, Codagnone MG, Villalobos-Manriquez F, Segalla M, Koc F, Stanton C, Ross P, Dinan TG, Clarke G, Cryan JF, 2023. Critical windows of early-life microbiota disruption on behaviour, neuroimmune function, and neurodevelopment. Brain Behav. Immun 108, 309–327. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC, 2008. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol. Lett 29, 117–124. [PubMed] [Google Scholar]
- Martin CR, Osadchiy V, Kalani A, Mayer EA, 2018. The brain-gut-microbiome axis. Cell. Mol. Gastroenterol. Hepatol 6, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwaha S, Palmer E, Suppes T, Cons E, Young AH, Upthegrove R, 2023. Novel and emerging treatments for major depression. Lancet 401, 141–153. [DOI] [PubMed] [Google Scholar]
- McCormick DA, 1989. GABA as an inhibitory neurotransmitter in human cerebral cortex. J. Neurophysiol 62, 1018–1027. [DOI] [PubMed] [Google Scholar]
- McGuinness AJ, Davis JA, Dawson SL, Loughman A, Collier F, O’Hely M, Simpson CA, Green J, Marx W, Hair C, Guest G, Mohebbi M, Berk M, Stupart D, Watters D, Jacka FN, 2022. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol. Psychiatry 27, 1920–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medetgul-Ernar K, Davis MM, 2022. Standing on the shoulders of mice. Immunity 55, 1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengelkoch S, Miryam Schussler-Fiorenza Rose S, Lautman Z, Alley JC, Roos LG, Ehlert B, Moriarity DP, Lancaster S, Snyder MP, Slavich GM, 2023. Multi-omics approaches in psychoneuroimmunology and health research: Conceptual considerations and methodological recommendations. Brain Behav Immun 114, 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miri S, Yeo J, Abubaker S, Hammami R, 2023. Neuromicrobiology, an emerging neurometabolic facet of the gut microbiome? Front. Microbiol 14, 1098412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Malenka RC, Deisseroth K, 2014. Depression: the best way forward. Nature 515, 200–201. [DOI] [PubMed] [Google Scholar]
- Moriarity DP, Mengelkoch S, Slavich GM, 2023a. Incorporating causal inference perspectives into psychoneuroimmunology: A simulation study highlighting concerns about controlling for adiposity in immunopsychiatry. Brain Behav. Immun 113, 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity DP, Slavich GM, 2023. The future is dynamic: a call for intensive longitudinal data in immunopsychiatry. Brain Behav. Immun 112, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity DP, Slavich GM, Alloy LB, Olino TM, 2023b. Hierarchical inflammatory phenotypes of depression: a novel approach across five independent samples and 27,730 adults. Biol. Psychiatry 93, 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz M, Guerrero-Araya E, Cortes-Tapia C, Plaza-Garrido A, Lawley TD, Paredes-Sabja D, 2020. Comprehensive genome analyses of Sellimonas intestinalis, a potential biomarker of homeostasis gut recovery. Microb. Genom 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nani BD, Lima PO, Marcondes FK, Groppo FC, Rolim GS, Moraes AB, Cogo-Muller K, Franz-Montan M, 2017. Changes in salivary microbiota increase volatile sulfur compounds production in healthy male subjects with academic-related chronic stress. PLoS One 12, e0173686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimi K, Neylan TC, Bertenthal D, Dolsen EA, Seal KH, O’Donovan A, 2022. Post-traumatic stress disorder and risk for hospitalization and death following COVID-19 infection. Transl. Psychiatry 12, 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega MA, Alvarez-Mon MA, Garcia-Montero C, Fraile-Martinez O, Guijarro LG, Lahera G, Monserrat J, Valls P, Mora F, Rodriguez-Jimenez R, Quintero J, Alvarez-Mon M, 2022. Gut microbiota metabolites in major depressive disorder-deep insights into their pathophysiological role and potential translational applications. Metabolites 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Li C, Wu X, Zhang T, 2023. Gut microbiota alterations and their functional differences in depression according to enterotypes in asian individuals. Int. J. Mol. Sci 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Shim HS, An K, Starkweather A, Kim KS, Shim I, 2015. IL-4 inhibits IL-1beta-induced depressive-like behavior and central neurotransmitter alterations. Mediators Inflamm. 2015, 941413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C, Bell R, Klag KA, Lee SH, Soto R, Ghazaryan A, Buhrke K, Ekiz HA, Ost KS, Boudina S, O’Connell RM, Cox JE, Villanueva CJ, Stephens WZ, Round JL, 2019. T cell-mediated regulation of the microbiota protects against obesity. Science 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchez B, Surget A, Belzung C, 2019. Animal models of major depression: drawbacks and challenges. J. Neural Transm. (Vienna) 126, 1383–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacchini A, Girardi D, Falco A, Zanotta N, Comar M, De Carlo NA, Tongiorgi E, 2018. Distinct CCL2, CCL5, CCL11, CCL27, IL-17, IL-6, BDNF serum profiles correlate to different job-stress outcomes. Neurobiol. Stress 8, 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y, Tan Y, Qu Y, Chang L, Wang S, Wei Y, Wang X, Hashimoto K, 2021. A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain Behav. Immun 94, 318–326. [DOI] [PubMed] [Google Scholar]
- Radjabzadeh D, Bosch JA, Uitterlinden AG, Zwinderman AH, Ikram MA, van Meurs JBJ, Luik AI, Nieuwdorp M, Lok A, van Duijn CM, Kraaij R, Amin N, 2022. Gut microbiome-wide association study of depressive symptoms. Nat. Commun 13, 7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC, 2015. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 29, 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reus GZ, Jansen K, Titus S, Carvalho AF, Gabbay V, Quevedo J, 2015. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: evidences from animal and human studies. J. Psychiatr. Res 68, 316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI, 2013. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S, 2016. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol. Psychiatry 21, 738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzki L, Stone TW, Maes M, Misiak B, Samochowiec J, Szulc A, 2021. Gut microbiota-derived vitamins - underrated powers of a multipotent ally in psychiatric health and disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 107, 110240. [DOI] [PubMed] [Google Scholar]
- Saika F, Matsuzaki S, Kobayashi D, Kiguchi N, Kishioka S, 2018. Chemokine CXCL1 is responsible for cocaine-induced reward in mice. Neuropsychopharmacol Rep 38, 145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Harty S, Lehto SM, Moeller AH, Dinan TG, Dunbar RIM, Cryan JF, Burnet PWJ, 2018. The microbiome in psychology and cognitive neuroscience. Trends Cogn. Sci 22, 611–636. [DOI] [PubMed] [Google Scholar]
- Schachter J, Martel J, Lin CS, Chang CJ, Wu TR, Lu CC, Ko YF, Lai HC, Ojcius DM, Young JD, 2018. Effects of obesity on depression: a role for inflammation and the gut microbiota. Brain Behav. Immun 69, 1–8. [DOI] [PubMed] [Google Scholar]
- Scher JU, Nayak RR, Ubeda C, Turnbaugh PJ, Abramson SB, 2020. Pharmacomicrobiomics in inflammatory arthritis: gut microbiome as modulator of therapeutic response. Nat. Rev. Rheumatol 16, 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, Ter Horst R, Jansen T, Jacobs L, Bonder MJ, Kurilshikov A, Fu J, Joosten LAB, Zhernakova A, Huttenhower C, Wijmenga C, Netea MG, Xavier RJ, 2016. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 167 (1125–1136), e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaider-Levi L, Ganz AB, Zafrani K, Goldman Z, Mitnik I, Rolnik B, Lev-Ari S, 2020. The effect of inquiry-based stress reduction on teacher burnout: a controlled trial. Brain Sci 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KM, Blank N, Alvarez Y, Thum K, Lundgren P, Litichevskiy L, Sleeman M, Bahnsen K, Kim J, Kardo S, Patel S, Dohnalova L, Uhr GT, Descamps HC, Kircher S, McSween AM, Ardabili AR, Nemec KM, Jimenez MT, Glotfelty LG, Eisenberg JD, Furth EE, Henao-Mejia J, Bennett FC, Pierik MJ, Romberg-Camps M, Mujagic Z, Prinz M, Schneider CV, Wherry EJ, Bewtra M, Heuckeroth RO, Levy M, Thaiss CA, 2023. The enteric nervous system relays psychological stress to intestinal inflammation. Cell 186 (2823–2838), e2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schussler-Fiorenza Rose SM, Contrepois K, Moneghetti KJ, Zhou W, Mishra T, Mataraso S, Dagan-Rosenfeld O, Ganz AB, Dunn J, Hornburg D, Rego S, Perelman D, Ahadi S, Sailani MR, Zhou Y, Leopold SR, Chen J, Ashland M, Christle JW, Avina M, Limcaoco P, Ruiz C, Tan M, Butte AJ, Weinstock GM, Slavich GM, Sodergren E, McLaughlin TL, Haddad F, Snyder MP, 2019. A longitudinal big data approach for precision health. Nat. Med 25, 792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutt PE, Kung S, Clark MM, Koball AM, Grothe KB, 2016. Comparing the beck depression inventory-II (BDI-II) and patient health questionnaire (PHQ-9) depression measures in an outpatient bariatric clinic. Obes. Surg 26, 1274–1278. [DOI] [PubMed] [Google Scholar]
- Shields GS, Spahr CM, Slavich GM, 2020. Psychosocial interventions and immune system function: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiat 77, 1031–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoubridge AP, Choo JM, Martin AM, Keating DJ, Wong ML, Licinio J, Rogers GB, 2022. The gut microbiome and mental health: advances in research and emerging priorities. Mol. Psychiatry 27, 1908–1919. [DOI] [PubMed] [Google Scholar]
- Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CSM, 2021. The gut microbiota in anxiety and depression - A systematic review. Clin. Psychol. Rev 83, 101943. [DOI] [PubMed] [Google Scholar]
- Singh SB, Carroll-Portillo A, Lin HC, 2023. Desulfovibrio in the Gut: The Enemy within? Microorganisms 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SB, Lin HC, 2015. Hydrogen sulfide in physiology and diseases of the digestive tract. Microorganisms 3, 866–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, 2022. Social safety theory: understanding social stress, disease risk, resilience, and behavior during the COVID-19 pandemic and beyond. Curr. Opin. Psychol 45, 101299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Giletta M, Helms SW, Hastings PD, Rudolph KD, Nock MK, Prinstein MJ, 2020. Interpersonal life stress, inflammation, and depression in adolescence: testing Social Signal Transduction Theory of Depression. Depress. Anxiety 37, 179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR, 2014. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull 140, 774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, O’Donovan A, Epel ES, Kemeny ME, 2010. Black sheep get the blues: a psychobiological model of social rejection and depression. Neurosci. Biobehav. Rev 35, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slyepchenko A, Maes M, Jacka FN, Kohler CA, Barichello T, McIntyre RS, Berk M, Grande I, Foster JA, Vieta E, Carvalho AF, 2017. Gut microbiota, bacterial translocation, and interactions with diet: pathophysiological links between major depressive disorder and non-communicable medical comorbidities. Psychother. Psychosom 86, 31–46. [DOI] [PubMed] [Google Scholar]
- Smith S, 2019. phylosmith: an R-package for reproducible and efficient microbiome analysis with phyloseq-objects. Journal of Open Source Software 4. [Google Scholar]
- Song AQ, Gao B, Fan JJ, Zhu YJ, Zhou J, Wang YL, Xu LZ, Wu WN, 2020. NLRP1 inflammasome contributes to chronic stress-induced depressive-like behaviors in mice. J. Neuroinflammation 17, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Zhang H, Zhang Y, Goh B, Bao B, Mello SS, Sun X, Zheng W, Gazzaniga FS, Wu M, Qu F, Yin Q, Gilmore MS, Oh SF, Kasper DL, 2023. Gut microbial fatty acid isomerization modulates intraepithelial T cells. Nature 619, 837–843. [DOI] [PubMed] [Google Scholar]
- Sowa JE, Tokarski K, 2021. Cellular, synaptic, and network effects of chemokines in the central nervous system and their implications to behavior. Pharmacol. Rep 73, 1595–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanogiannopoulos P, Kyaw TS, Guthrie BGH, Bradley PH, Lee JV, Melamed J, Malig YNA, Lam KN, Gempis D, Sandy M, Kidder W, Van Blarigan EL, Atreya CE, Venook A, Gerona RR, Goga A, Pollard KS, Turnbaugh PJ, 2022. Host and gut bacteria share metabolic pathways for anti-cancer drug metabolism. Nat. Microbiol 7, 1605–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS, 2015. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun 48, 258–264. [DOI] [PubMed] [Google Scholar]
- Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, McDonald D, Dietrich D, Ramadhar TR, Lekbua A, Mroue N, Liston C, Stewart EJ, Dubin MJ, Zengler K, Knight R, Gilbert JA, Clardy J, Lewis K, 2019. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol 4, 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y, 2004. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol 558, 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TDB, Monroy Hernandez C, Nguyen H, Wright S, Center for Systems Neurogenetics of, A., Tarantino LM, Chesler EJ, Weinstock GM, Zhou Y, Bubier JA, 2023. The microbial community dynamics of cocaine sensitization in two behaviorally divergent strains of collaborative cross mice. Genes Brain Behav 22, e12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trolese MC, Mariani A, Terao M, de Paola M, Fabbrizio P, Sironi F, Kurosaki M, Bonanno S, Marcuzzo S, Bernasconi P, Trojsi F, Aronica E, Bendotti C, Nardo G, 2020. CXCL13/CXCR5 signalling is pivotal to preserve motor neurons in amyotrophic lateral sclerosis. EBioMedicine 62, 103097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI, 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031. [DOI] [PubMed] [Google Scholar]
- Upadhyay V, Suryawanshi RK, Tasoff P, McCavitt-Malvido M, Kumar RG, Murray VW, Noecker C, Bisanz JE, Hswen Y, Ha CWY, Sreekumar B, Chen IP, Lynch SV, Ott M, Lee S, Turnbaugh PJ, 2023. Mild SARS-CoV-2 infection results in long-lasting microbiota instability. mBio 14, e0088923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, Claes S, Van Oudenhove L, Zhernakova A, Vieira-Silva S, Raes J, 2019. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol 4, 623–632. [DOI] [PubMed] [Google Scholar]
- van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O’Sullivan O, Clarke G, Stanton C, Dinan TG, Cryan JF, 2018. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol 596, 4923–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Spek A, Stewart ID, Kuhnel B, Pietzner M, Alshehri T, Gauss F, Hysi PG, MahmoudianDehkordi S, Heinken A, Luik AI, Ladwig KH, Kastenmuller G, Menni C, Hertel J, Ikram MA, de Mutsert R, Suhre K, Gieger C, Strauch K, Volzke H, Meitinger T, Mangino M, Flaquer A, Waldenberger M, Peters A, Thiele I, Kaddurah-Daouk R, Dunlop BW, Rosendaal FR, Wareham NJ, Spector TD, Kunze S, Grabe HJ, Mook-Kanamori DO, Langenberg C, van Duijn CM, Amin N, 2023. Circulating metabolites modulated by diet are associated with depression. Mol. Psychiatry 28, 3874–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hul M, Le Roy T, Prifti E, Dao MC, Paquot A, Zucker JD, Delzenne NM, Muccioli G, Clement K, Cani PD, 2020. From correlation to causality: the case of Subdoligranulum. Gut Microbes 12, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasileva SS, Yang Y, Baker A, Siskind D, Gratten J, Eyles D, 2024. Associations of the gut microbiome with treatment resistance in schizophrenia. JAMA Psychiat 81, 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman R, Madempudi RS, Neelamraju J, Ahire JJ, Vinay HR, Lal A, Thomas G, Stephen S, 2021. Effect of multi-strain probiotic formulation on students facing examination stress: a double-blind, placebo-controlled study. Probiotics Antimicrob. Proteins 13, 12–18. [DOI] [PubMed] [Google Scholar]
- von Schwartzenberg RJ, Bisanz JE, Lyalina S, Spanogiannopoulos P, Ang QY, Cai J, Dickmann S, Friedrich M, Liu SY, Collins SL, Ingebrigtsen D, Miller S, Turnbaugh JA, Patterson AD, Pollard KS, Mai K, Spranger J, Turnbaugh PJ, 2021. Caloric restriction disrupts the microbiota and colonization resistance. Nature 595, 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Chen L, Zhou R, Wang X, Song L, Huang S, Wang G, Xia B, 2014. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J. Clin. Microbiol 52, 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ, 2003. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou J, Ye J, Sun Z, He Y, Zhao Y, Ren S, Zhang G, Liu M, Zheng P, Wang G, Yang J, 2023. Multi-omics reveal microbial determinants impacting the treatment outcome of antidepressants in major depressive disorder. Microbiome 11, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weersma RK, Zhernakova A, Fu J, 2020. Interaction between drugs and the gut microbiome. Gut 69, 1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weglarz L, Dzierzewicz Z, Skop B, Orchel A, Parfiniewicz B, Wisniowska B, Swiatkowska L, Wilczok T, 2003. Desulfovibrio desulfuricans lipopolysaccharides induce endothelial cell IL-6 and IL-8 secretion and E-selectin and VCAM-1 expression. Cell. Mol. Biol. Lett 8, 991–1003. [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD, 2011. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Zhao N, Zhang C, Lam YY, Zhao L, 2021. Guild-based analysis for understanding gut microbiome in human health and diseases. Genome Med 13, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Zheng W, Song X, Bao B, Wang Y, Ramanan D, Yang D, Liu R, Macbeth JC, Do EA, Andrade WA, Yang T, Cho HS, Gazzaniga FS, Ilves M, Coronado D, Thompson C, Hang S, Chiu IM, Moffitt JR, Hsiao A, Mekalanos JJ, Benoist C, Kasper DL, 2024. Gut complement induced by the microbiota combats pathogens and spares commensals. Cell 187 (897–913), e818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY, 2015. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS, Chan V, Ling L, Joynt G, Hui DS, Chow KM, Ng SSS, Li TC, Ng RW, Yip TC, Wong GL, Chan FK, Wong CK, Chan PK, Ng SC, 2021. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 70, 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Chen QG, Chen X, Liu XT, Geng F, Zhu MM, Yan FL, Zhang ZJ, Ren QG, 2023. Intestinal dysbiosis mediates cognitive impairment via the intestine and brain NLRP3 inflammasome activation in chronic sleep deprivation. Brain Behav. Immun 108, 98–117. [DOI] [PubMed] [Google Scholar]
- Zhao H, Jin K, Jiang C, Pan F, Wu J, Luan H, Zhao Z, Chen J, Mou T, Wang Z, Lu J, Lu S, Hu S, Xu Y, Huang M, 2022. A pilot exploration of multiomics research of gut microbiome in major depressive disorders. Transl. Psychiatry 12, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P, 2016. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21, 786–796. [DOI] [PubMed] [Google Scholar]
- Zhong H, Penders J, Shi Z, Ren H, Cai K, Fang C, Ding Q, Thijs C, Blaak EE, Stehouwer CDA, Xu X, Yang H, Wang J, Wang J, Jonkers D, Masclee AAM, Brix S, Li J, Arts ICW, Kristiansen K, 2019. Impact of early events and lifestyle on the gut microbiota and metabolic phenotypes in young school-age children. Microbiome 7, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Shen X, Johnson JS, Spakowicz DJ, Agnello M, Zhou W, Avina M, Honkala A, Chleilat F, Chen SJ, Cha K, Leopold S, Zhu C, Chen L, Lyu L, Hornburg D, Wu S, Zhang X, Jiang C, Jiang L, Jiang L, Jian R, Brooks AW, Wang M, Contrepois K, Gao P, Schussler-Fiorenza Rose SM, Binh Tran TD, Nguyen H, Celli A, Hong BY, Bautista EJ, Dorsett Y, Kavathas P, Zhou Y, Sodergren E, Weinstock GM, Snyder MP, 2024b. Longitudinal profiling of the microbiome at four body sites reveals core stability and individualized dynamics during health and disease. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Johnson JS, Spakowicz D, Zhou W, Zhou Y, Sodergren E, Snyder M, Weinstock GM, 2020. Longitudinal analysis of serum cytokine levels and gut microbial abundance Links IL-17/IL-22 with clostridia and insulin sensitivity in humans. Diabetes 69, 1833–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Sailani MR, Contrepois K, Zhou Y, Ahadi S, Leopold SR, Zhang MJ, Rao V, Avina M, Mishra T, Johnson J, Lee-McMullen B, Chen S, Metwally AA, Tran TDB, Nguyen H, Zhou X, Albright B, Hong BY, Petersen L, Bautista E, Hanson B, Chen L, Spakowicz D, Bahmani A, Salins D, Leopold B, Ashland M, Dagan-Rosenfeld O, Rego S, Limcaoco P, Colbert E, Allister C, Perelman D, Craig C, Wei E, Chaib H, Hornburg D, Dunn J, Liang L, Rose SMS, Kukurba K, Piening B, Rost H, Tse D, McLaughlin T, Sodergren E, Weinstock GM, Snyder M, 2019. Longitudinal multi-omics of host-microbe dynamics in prediabetes. Nature 569, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Shen X, Johnson JS, Spakowicz DJ, Agnello M, Zhou W, Avina M, Honkala A, Chleilat F, Chen SJ, Cha K, Leopold S, Zhu C, Chen L, Lyu L, Hornburg D, Wu S, Zhang X, Jiang C, Jiang L, Jiang L, Jian R, Brooks AW, Wang M, Contrepois K, Gao P, Rose SMS, Tran TDB, Nguyen H, Celli A, Hong BY, Bautista EJ, Dorsett Y, Kavathas PB, Zhou Y, Sodergren E, Weinstock GM, Snyder MP, 2024a. Longitudinal profiling of the microbiome at four body sites reveals core stability and individualized dynamics during health and disease. Cell Host Microbe [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorkina YA, Syunyakov TS, Abramova OV, Yunes RA, Averina OV, Kovtun AS, Angelova IY, Khobta EB, Susloparova DA, Pavlichenko AV, Karpenko OA, Andreyuk DS, Tovmasyan AS, Danilenko VN, Gurina OI, Kostyuk GP, Morozova AY, 2022. Effects of diet on the gut microbiome in patients with depression. Zh. Nevrol. Psikhiatr. Im. S S Korsakova 122, 59–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed during this study are available for scientific research upon request to the corresponding author. The analysis code is publicly accessible at https://github.com/xzhou7/mentalmicrobiome.


