Abstract
AF5q31 (also called MCEF) was identified by its involvement in chromosomal translocation with the gene MLL (mixed lineage leukemia), which is associated with infant acute lymphoblastic leukemia. Several potential roles have been proposed for AF5q31 and other family genes, but the specific requirements of AF5q31 during development remain unclear. Here, we show that AF5q31 is essential for spermatogenesis. Although most AF5q31-deficient mice died in utero and neonatally with impaired embryonic development and shrunken alveoli, respectively, 13% of AF5q31-deficient mice thrived as wild-type mice did. However, the male mice were sterile with azoospermia. Histological examinations revealed the arrest of germ cell development at the stage of spermiogenesis, and virtually no spermatozoa were seen in the epididymis. AF5q31 was found to be preferentially expressed in Sertoli cells. Furthermore, mutant mice displayed severely impaired expression of protamine 1, protamine 2, and transition protein 2, which are indispensable to compact the haploid genome within the sperm head, and an increase of apoptotic cells in seminiferous tubules. Thus, AF5q31 seems to function as a transcriptional regulator in testicular somatic cells and is essential for male germ cell differentiation and survival. These results may have clinical implications in the understanding of human male infertility.
Chromosomal translocation is one of the common pathogenic mechanisms in various human malignancies, particularly in leukemias and lymphomas, and genes located at the breakpoints are involved in disease pathogenesis (21, 59, 60). The mixed lineage leukemia gene MLL (also called HRX, HTRX, and ALL-1) is frequently targeted by chromosomal rearrangements and is associated with clinically aggressive lymphoid and myeloid leukemias which are particularly prevalent in infant leukemias and treatment-related secondary leukemias (2, 18, 24, 64). MLL located on 11q23 is a human homologue of Drosophila trithorax, has a SET domain that normally functions as histone methyltransferase, and is assembled into a super-multiprotein complex with additional chromatin-remodeling components (45, 50, 70). Importantly, most of the leukemic variants of MLL lack the SET domain (7). In Drosophila, genetic evidence suggests that Trithorax controls the expression of homeobox (Hox) genes and regulates embryogenesis (39, 44, 47). In MLL-deficient mice, Hox gene expression initiates normally but is not maintained after 9.5 days postcoitus (dpc), demonstrating the importance of MLL in the maintenance of Hox gene expression (72, 73). Hox genes also play an important role in hematopoietic differentiation, and their expression levels are upregulated in the human leukemias carrying MLL rearrangements (1). An unusual feature of MLL fusion proteins is the large number and diversity of heterologous proteins that fuse with MLL. To date, the MLL locus has been found to be translocated to approximately 40 different genetic loci and at least 30 of the partner genes have been characterized (13, 31). The functions of most MLL partner genes are unknown. Although no consistent homologies or common motifs that are characteristic to all the partner gene sequences have been identified, some are classified into small subgroups according to their sequence homologies. Of interest is that the fusion partner plays an important role in determining disease features.
The (5;11)(q31;q23) translocation is associated with infant acute lymphoblastic leukemia (ALL) (63). This translocation juxtaposes the 5′ sequences of the MLL gene to the 3′ sequences of the AF5q31 gene and results in the formation of an in-frame MLL-AF5q31 fusion protein which contains the amino-terminal region of MLL, including its AT hooks, methyltransferase domain, and repression domain, and amino acids 351 to 1163 of AF5q31, including the transactivation domain in part and C-terminal homology domain. Based on the significant homology to multiple regions of the predicted AF5q31 protein, three other mammalian AF5q31 homology genes, AF4, LAF4, and FMR2, are known (2). Both AF4 and LAF4 have been independently identified as MLL partner genes in each case of pediatric ALL (19, 29, 46, 49, 65). In contrast, FMR2 has not been observed in association with chromosome translocation in leukemia, but congenital mutations in the FMR2 gene are involved in mild hereditary mental retardation (8, 22, 25). DNA binding and transcriptional properties of AF4, LAF4, and FMR2 suggest that AF5q31 and other family genes function as nuclear transcription factors (28, 41, 53, 58). Recently, AF5q31 was found to interact with positive transcription elongation factor b (P-TEFb), which activates transcription by RNA polymerase II (RNAPII), leading to the formation of progressive elongation complex (20). Although transfection studies suggested that AF5q31 acts as a repressor of RNAPII transcription, the precise role of AF5q31 in the transcriptional activity of P-TEFb is not known.
AF4 knockout mice demonstrated that AF4 is required for normal lymphopoiesis (34). In the bone marrow of the mutant mice, loss of AF4 function did not disrupt progenitor B-cell development; however, the transition from pre-B cell to the newly generated mature B cell was severely reduced and exhibited defective thymocyte development from a double-negative to a double-positive population. These findings may provide insights into lymphoid leukemogenesis by MLL-AF4. On the other hand, robotic mice carrying autosomal dominant missense mutation in the AF4 gene have been identified from a large-scale N-ethyl-N-nitrosurea (ENU) mutagenesis pool (32). As a result, newborn mice developed a severe loss of Purkinje cells of the cerebellum within several weeks after birth and showed a strange ataxic gait. But the thymic double-negative and double-positive populations were not significantly different in the mutant and control mice. Interestingly, AF4 interacts with the E3 ubiquitin ligase SIAH1 and the minimal interaction domain of AF4 to bind to SIAH1 was demonstrated to possess the PXAXVXP motif conserved within AF5q31 and other family genes (6, 57). A missense mutation V294A in the robotic mice corresponds to Val of the PXAXVXP motif, and the Val mutation of the AF4 protein has been shown to reduce the binding ability to SIAH1 protein significantly, suggesting that the phenotype of the robotic mice is caused by an increased steady-state level of AF4 protein and that all the members of the AF5q31 family are regulated by this interaction (57). Since mutation of the AF4 gene in the robotic mice occurred upstream of known translocation breakpoints, it is possible that MLL-AF4 may be more stable than AF4. However, the function of AF4 in the robotic mice would not directly account for the leukemogenic potential of MLL-AF4. Thus, there are few available data on the biological and pathological functions for AF5q31 and other family genes.
We found that AF5q31 is expressed during mouse embryogenesis at the highest level around 10.5 to 12.5 dpc and is widely expressed in adult mice, especially in Sertoli cells of the testis. This pattern suggests a specific role of AF5q31 during the differentiation of male germ cells. To gain insights into the potential role for AF5q31 in leukemogenesis and normal development, we disrupted the AF5q31 gene by homologous recombination and examined the mutant phenotype of the mice. Here, we show that AF5q31 deficiency resulted in embryonic and neonatal lethality in most mice but that some survived to grow properly except for azoospermia, thus raising the possibility that AF5q31 mutations will be found in some patients with autosomal recessive azoospermia.
MATERIALS AND METHODS
Plasmids.
To obtain the mouse AF5q31 (mAF5q31) expression construct, the DNA sequence of full-length mAF5q31 (GenBank accession number AF190449) was amplified by PCR from Ba/F3 cDNA and subcloned into pcDNA3.1 (Invitrogen) with a FLAG tag.
Antibodies.
To prepare the anti-AF5q31 antibody that can recognize both human and mouse AF5q31 proteins, we prepared polyclonal antibodies against the highly conserved transactivation domain of mAF5q31 (E4; 317 to 492 amino acids). DNA sequences corresponding to this region were amplified by PCR and subcloned into pGEX-4T (Amersham Biosciences). Anti-mAF5q31 antisera were raised in rabbits against the purified GST-mAF5q31-E4 (317- to 492-amino acid) fusion protein, depleted of anti-glutathione S-transferase (GST) antibodies, and further affinity purified on an antigen column.
To detect RNAPII, N20 antibody that reacts with both the hyperphosphorylated (IIo) and hypophosphorylated (IIa) forms of RNAPII was purchased from Santa Cruz. H5 and H14 antibodies that recognize Ser2 and Ser5 of the carboxy-terminal domain (CTD) phosphopeptides of RNAPII, respectively, were obtained from Covance Co. (Berkeley, CA). In addition, anti-α-tubulin (T-5168; Sigma) was used.
Generation of AF5q31-deficient mice.
A phage clone containing an approximately 17-kb DNA fragment was isolated from a mouse 129 SvJ λ genomic library (Stratagene) with the mAF5q31 cDNA probe. The AF5q31 targeting vector was constructed by replacing the 5.0-kb HaeII-SspI DNA fragment that contains exon II harboring the initiation codon and exon III with a 1.1-kb fragment of the neomycin-resistant gene (neo) cassette of pMC1NeoPolyA (Stratagene) in an antisense orientation. The 2.2-kb fragment of the herpes simplex virus thymidine kinase gene cassette was inserted upstream of the AF5q31 gene in an antisense orientation for negative selection. The linearized targeting plasmid DNA was electroporated into E14-1 embryonic stem (ES) cells. After double selections with 600 μg/ml G418 (Invitrogen) and 2 μM ganciclovir (Sigma), resistant clones were screened for homologous recombination by Southern blot analysis as described previously (54, 55). In brief, genomic DNA was digested with HindIII, separated by agarose gel electrophoresis, and transferred to a Hybond-N+ membrane (Amersham Biosciences). Hybridization was carried out with a 0.3-kb 3′ flanking probe. The targeting frequency was 12/384. ES cells from each of four independent AF5q31 mutant clones were injected into C57BL/6 blastocysts. The blastocysts were transferred to pseudopregnant ICR foster mothers, and chimeras derived from two independent clones transmitted the mutant allele through their germ line. All animal experiments were done according to the guidelines for animal use issued by the Committee of Animal Experiments, Institute of Medical Science, University of Tokyo.
The genotype was also determined by PCR with Ex Taq (TaKaRa, Otsu, Japan). Genomic DNAs were prepared from mouse tail snips. For the wild-type and mutant alleles of the AF5q31 gene, an antisense primer specific for the wild-type (5′-GTCTTCACGGTTCATGTTGC-3′) or mutant allele (5′-GCCCGGTTCTTTTTGTCAAG-3′, a sequence in the neo gene) was used with a common sense primer (5′-GTGGGTTATGTGCCACCAAA-3′). PCR was done at 96°C for 5 min for initial denaturing, followed by 35 cycles at 96°C for 1 min, 56°C for 1 min, and 72°C for 2 min.
Histology and immunohistochemistry.
Formalin-fixed, paraffin-embedded sections (6 μm in thickness) of embryos were stained with hematoxylin and eosin stain. Bouin-fixed, paraffin-embedded sections of testes and epidydimides were stained with hematoxylin and eosin stain. For immunohistochemistry, formalin-fixed, paraffin-embedded sections (6 μm) of testes were deparaffinized, rehydrated, quenched of endogenous peroxidase activity with 3% hydrogen peroxide, and incubated overnight at 4°C with an anti-mAF5q31-E4 antibody. After washing of the sections three times in phosphate-buffered saline, samples were incubated with anti-rabbit immunoglobulin ENVISION horseradish peroxidase (DakoCytomation). The sections were counterstained with hematoxylin.
Northern blot analysis and PCR with reverse transcription (RT-PCR).
Mouse multiple tissue blot (Clontech) was hybridized with the 32P-labeled mAF5q31 full-length cDNA probe followed by rehybridization with a mouse AF4, LAF4, and FMR2 probe and a human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe, as described previously (55, 56). Mouse embryo full-stage blot (Seegene) was hybridized with the mAF5q31 cDNA probe. The mouse AF4 and LAF4 cDNA probes were obtained by PCR amplification from a mouse thymus cDNA library and the mouse FMR2 cDNA probe from a mouse brain cDNA library. The human GAPDH cDNA probe was described previously (56). The following oligonucleotide primers specific to mouse AF4, LAF4, and FMR2 were used: for AF4, 5′-CCTGCTTCGAATCAGAGAGA-3′ (sense) and 5′-CATCCTTAGTCTGGTGAGCT-3′ (antisense); for LAF4, 5′-GGAGGAAAGAGCGAGAAAGA-3′ (sense) and 5′-CCCTCTCCATATTGCACACT-3′ (antisense); and for FMR2, 5′-GCAGTGTCACTATGAACAAG-3′ (sense) and 5′-CCAGGTGCTTGCACTGTAAA-3′ (antisense).
To confirm the gene disruption of mAF5q31, total RNAs from mouse embryonic fibroblasts (MEFs) obtained from 13.5-dpc embryos and maintained in Dulbecco's modified Eagle medium containing 10% fetal bovine serum were isolated with Trizol reagent (Invitrogen). Total RNA (3 μg) was reverse transcribed using Superscript reverse transcriptase II (Invitrogen) with random primers in a total volume of 20 μl. One μl of this reaction mixture was used as a template for PCR amplification with Ex Taq (TaKaRa) in the following condition: at 96°C for 5 min for initial denaturing, followed by 35 cycles at 96°C for 30 s, 56°C for 30 s, and 72°C for 1.5 min. The following oligonucleotide primers specific to mAF5q31 exons I to IV and GAPDH for a control were used: for AF5q31 exons I to IV, 5′-GAAATGGTTCGGGCCTAGCG-3′ (sense) and 5′-CTACACAGCTTACATCACCA-3′ (antisense), and for GAPDH, 5′-ACCACAGTCCATGCCATCAC-3′ (sense) and 5′-TCCACCACCCTGTTGCTGTA-3′ (antisense).
To assess the expression levels of several genes in testis, RT-PCR analyses were performed on total RNAs derived from the testes of 12-week-old AF5q31+/+, AF5q31+/−, and AF5q31−/− male mice and 9-week-old WBB6F1-W/Wv male mice (Japan SLC) using the same methods as in MEFs. The following oligonucleotide primers specific for TP1, TP2, Prm1, Prm2, Tpap, RT7, Hsc70t, Mcs, Pgk2, Camk4, CREM, TRF2, RARα, RXRβ, AR, FSH-R, LH-R, and GATA1 were used: for TP1, 5′-ATGTCGACCAGCCGCAAGCT-3′ (sense) and 5′-TCACAAGTGGGATCGGTAAT-3′ (antisense); for TP2, 5′-GCCTCAAAGTCACACCAGTA-3′ (sense) and 5′-ACTTGTATCTTCGCCCTGAG-3′ (antisense); for Prm1, 5′-ATGCTGCCGCAGCAAAAGCA-3′ (sense) and 5′-CACCTTATGGTGTATGAGCG-3′ (antisense); for Prm2, 5′-ATGGTTCGCTACCGAATGAG-3′ (sense) and 5′-TTAGTGATGGTGCCTCCTAC-3′ (antisense); for Tpap, 5′-GGCTCTTACCGATTAGGAGT-3′ (sense) and 5′-AGTTACCCGGCAACCGTTAA-3′ (antisense); for RT7, 5′-TGCCTGTGTGACTACAAGCT-3′ (sense) and 5′-AGTACGTCACGTCCTTCTCA-3′ (antisense); for Hsc70t, 5′-CCATGAATCCCCAGAACACT-3′ (sense) and 5′-ATGACACCTGCATCCTTGGT-3′ (antisense); for Mcs, 5′-ACCATGTTGCCCACCTAAAC-3′ (sense) and 5′-TCTCCAGAGTTTGGCCAGAT-3′ (antisense); for Pgk2, 5′-CTGTTGCTGATGAGCTCAAG-3′ (sense) and 5′-ACTCCGACCATAGAACTGTG-3′ (antisense); for Camk4, 5′-TCTCTCACACCCGAACATCA-3′ (sense) and 5′-GGTTCCACACACTGTCTTCA-3′ (antisense); for CREM, 5′-ACTTTCCTCTGATGTGCCTG-3′ (sense) and 5′-CTTGCGAGTTGCTTCTTCTG-3′ (antisense); for TRF2, 5′-TGCTTTGGAGGGAGCAAATG-3′ (sense) and 5′-AGTTCAGGTTCATAGCTGGC-3′ (antisense); for RARα, 5′-TTGAGAAGGTTCGCAAAGCG-3′ (sense) and 5′-AGGTCAGTGTGTCTTGCTCA-3′ (antisense); for RXRβ, 5′-AGACTGTACAGTGGACAAGC-3′ (sense) and 5′-TGGCAGATGTTAGTCACTGG-3′ (antisense); for AR, 5′-ACCCTATCCCAGTCCCAATT-3′ (sense) and 5′-GATGGGCAATTTTTCCTCCG-3′ (antisense); for FSH-R, 5′-CGGAACGCCATTGAACTGAG-3′ (sense) and 5′-CAAAGCTCAGTCCCATGAAG-3′ (antisense); for LH-R, 5′-TGCACTCTCCAGAGTTGTCA-3′ (sense) and 5′-TCTTCGAAACATCTGGGAGG-3′ (antisense); and for GATA1, 5′-CAGGTTTCTTTTCCTCTGGG-3′ (sense) and 5′-AAAGGACTGGGAAAGTCAGC-3′ (antisense).
To monitor the expression of AF5q31 in the juvenile mice testes at various stages, RT-PCR analyses were done on total RNAs derived from C57BL/6 male mice (Japan SLC) at various ages, using the same methods as in MEFs. The sequence within the AF5q31 exons V to VIII was amplified with the primers 5′-CGGCTATTCATACACCATGC-3′ (sense) and 5′-CTCCCTCACTGTTATGGTGT-3′ (antisense).
Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay.
Formalin-fixed, paraffin-embedded testis sections (6 μm) of 12-week-old mice were prepared, and apoptotic cells were detected in situ using ApoAlert DNA fragmentation assay kits (Clontech). The cells were counterstained with 4′,6-diamidino-2-phenylindole [DAPI].
Western blot analysis.
An equal amount of total cell lysates from MEFs (10 μg/lane) was separated in 4 to 20% gradient polyacrylamide gels. Proteins were transferred onto a nitrocellulose membrane. The blot was incubated with the primary antibody at room temperature for 1 h and with a horseradish peroxidase-conjugated secondary antibody at room temperature for 1 h. Enhanced chemiluminescence Western blotting detection reagents (Amersham Biosciences) were used for detection.
Assessment of serum hormone levels.
The blood of male AF5q31+/+, AF5q31+/−, and AF5q31−/− mice (<24 weeks) was drawn by cardiocenthesis and stored on ice for 30 min. After 10 min of centrifugation at 800 × g for 10 min, the serum was collected and stored at −80°C until analysis. The levels of serum testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) were measured by SRL Co. (Tokyo, Japan).
Fertility assessment.
The reproductive capacities of 9-week-old male AF5q31+/+, AF5q31+/−, and AF5q31−/− mice were investigated by mating one male with two 8-week-old C57BL/6j females for 2 weeks, as described previously (10, 26). Female mice were checked for vaginal plugs each morning, and litter sizes were recorded on delivery after three successive matings.
Evaluation of epididymal sperm.
The cauda epididymides were removed and minced in 0.1 ml of motile buffer (120 mM NaCl, 5 mM KCl, 25 mM NaHCO3, 1.2 mM KH2PO4, 1.2 mM MgSO4, 1.3 mM CaCl2). The tissues were incubated at 37°C for 5 min to allow sperm to disperse, as described previously (48).
Generation and purification of the recombinant proteins.
Human AF5q31 cDNA (63) was subcloned into pBacPAK8 vector (BD Biosciences) with a hemagglutinin (HA) tag on the N terminus and a FLAG tag on the C terminus. AF5q31 was expressed in Sf9 cells by using a BacPAK baculovirus expression kit (BD Biosciences) according to the manufacturer's instructions. Sf9 cells were solubilized in lysis buffer (50 mM Tris-Cl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1.0% Triton X-100) supplemented with protease inhibitor cocktails (Sigma). The extract was loaded onto an anti-FLAG M2 agarose (Sigma) column equilibrated with TBS buffer (50 mM Tris-Cl [pH 7.5], 150 mM NaCl), and bound proteins were eluted with TBS buffer supplemented with 0.2 mg/ml FLAG peptide (Sigma). Proteins in the elution were loaded onto an anti-HA 3F10 affinity matrix (Roche) column equilibrated with TBS buffer containing 0.1% NP-40, and bound proteins were eluted with HGKEN buffer (20 mM HEPES-NaOH [pH 7.9], 20% glycerol, 100 mM KCl, 0.2 mM EDTA, 0.1% NP-40) supplemented with 1 mg/ml HA peptide (Roche). Proteins in the eluate were further separated on a Mono Q column (Amersham Biosciences) equilibrated with HGKEN buffer containing 5 mM β-mercaptoethanol and 0.5 mM phenylmethylsulfonyl fluoride by elution with a linear gradient from 200 mM to 400 mM KCl. Each fraction was dialyzed against HGKEN buffer containing 1 mM dithiothreitol and 1 mM phenylmethylsulfonyl fluoride. GST-CTD and P-TEFb were purified as described previously (66, 67).
CTD kinase assay.
GST-CTD was incubated with purified P-TEFb and each recombinant AF5q31 fraction in the presence of 60 μM ATP containing [γ-32P]ATP in transcription buffer for 10 min at 30°C as described previously (66, 67). Reaction products were subjected to 4 to 20% gradient polyacrylamide gel electrophoresis followed by autoradiography.
RESULTS
High expression of AF5q31 in testis.
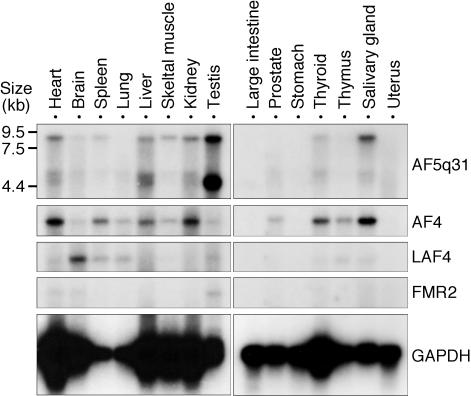
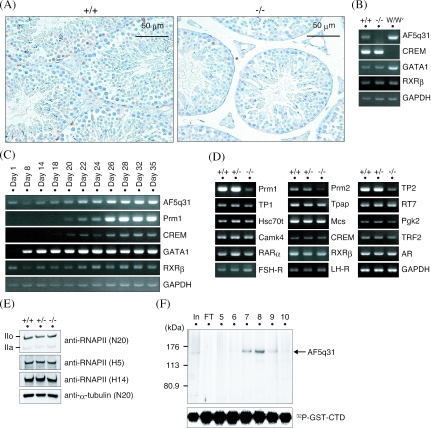
To explore the tissue distribution of AF5q31, Northern blot analysis was performed on various tissues of the adult mice. AF5q31 was present at a high level in testis and low levels in several other tissues (Fig. 1). Rehybridizations were also carried out with AF4, LAF4, and FMR2 cDNA probes. Expression of AF4 was detected in the heart, kidney, thyroid, and salivary gland at relatively high levels and at low levels in the spleen, liver, and thymus, as reported elsewhere (4). LAF4 transcript was expressed in the brain and weakly in the spleen and lung. Previously, mouse LAF4 was shown to be expressed predominantly in the thymus and the spleen of adult mice (41); however, we could not reproduce these results. Almost no signal of FMR2 expression, except in the testis, was consistent with the finding in the previous report that the expression of FMR2 occurs on or around 7.0 dpc, reaches its highest level at 10.5 to 11.5 dpc, and is very slight in other stages (9). Compared with these expression profiles, AF5q31 transcript in the testis was remarkably high.
FIG. 1.
Expression profiles of mouse AF5q31 and AF5q31 family genes in adult normal tissues. Northern blot analysis of poly(A) RNAs (2 μg/lane) from normal mouse tissues. The blot was hybridized to radioactive mouse AF5q31, AF4, LAF4, and FMR2 probes. As a control, the same blot was rehybridized with a GAPDH probe.
Targeted disruption of AF5q31.
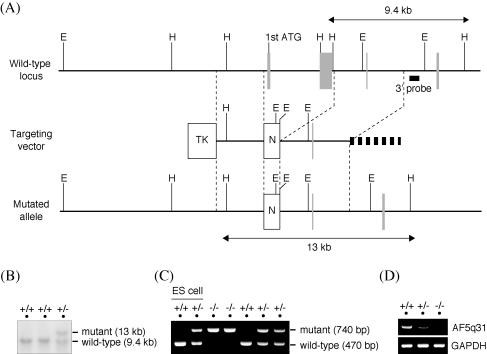
To clarify the physiological role of AF5q31, AF5q31−/− mice were generated by gene targeting. Examination of the sequences in the databases revealed that the mouse AF5q31 gene consists of at least 21 exons (coding exons II to XXI) within 70 kb of the genomic DNA. The region encoded by exons II and III carries the N-terminal homology domain and the partial transactivation domain conserved in AF5q31, AF4, LAF4, and FMR2, which consists of the N-terminal 25% of AF5q31 (2). A targeting vector was constructed by replacing exons II and III with the neo gene (Fig. 2A) and introduced into mouse ES cells. ES clones carrying the mutation were identified using Southern blots and an external 3′ probe (Fig. 2B). The blot rehybridized with a neo probe yielded only the 13-kbp band, and the EcoRI-digested genomic DNAs probed with an external 5′ probe further corroborated appropriate homologous recombination (a 21-kbp band in the wild type and a 15-kbp band in the mutant) (data not shown). After injection of the ES clones into blastocysts, generation of the chimeric mice, and backcrossing of the chimeras, AF5q31+/− mice were obtained. Genotyping of the progenies from intercrosses of the heterozygotes by PCR revealed the presence of AF5q31−/− mice (Fig. 2C). To confirm the deletion in the AF5q31 mRNA of the mutant mice, RNAs from the MEFs in AF5q31+/+, AF5q31+/−, and AF5q31−/− mice were analyzed by RT-PCR. When sequences from exons I and IV were used as primers, RT-PCR with RNAs from the AF5q31+/+ and AF5q31+/− MEFs produced a band of 973 bp, whereas no bands were detected with RNA from the AF5q31−/− MEFs (Fig. 2D). This result indicated that the AF5q31 mRNA in the mutant mice lacked the sequence for exons II and III.
FIG. 2.
Targeted disruption of the AF5q31 gene. (A) Schematic representation of the wild-type allele of mouse AF5q31 (top), the targeting vector (middle), and the mutant allele resulting from a homologous recombination (bottom). Filled boxes are exons, and open boxes are selection marker genes. H, HindIII restriction site; E, EcoRI restriction site; N, neomycin resistance gene cassette; TK, thymidine kinase gene cassette. (B) Southern blot analysis of HindIII-digested genomic DNAs (5 μg/lane) from ES clones with an external 3′ probe. The 9.4-kb and 13-kb bands represent the wild-type and targeted alleles, respectively. An external 3′ probe used to analyze is shown in panel A. (C) PCR-based genotype analysis of tail DNAs isolated from the pups of AF5q31+/− intercrosses. Three kinds of primers (see Materials and Methods) detected both the wild-type allele (470-bp band) and the targeted allele (740-bp band). As controls, parental and targeted ES cells were used. (D) RT-PCR analysis of total RNAs from AF5q31+/+, AF5q31+/−, and AF5q31−/− MEFs. The primers located on exons I and IV of the AF5q31 gene were used. RT-PCR for GAPDH confirms equivalent amounts of RNAs used for the analysis.
AF5q31 is important for embryonic development.
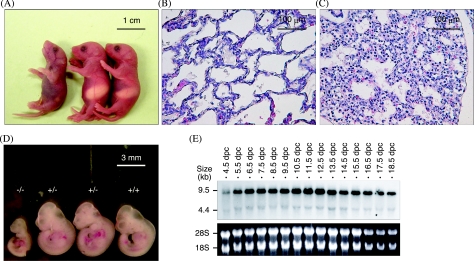
Genotype analysis of the neonates showed a decrease by 55% in the AF5q31−/− mice relative to the numbers of wild-type and heterozygous littermates, based on the Mendelian ratio, and 71% of the AF5q31−/− neonates died as early as 12 to 24 h postpartum (Table 1). It was noteworthy that neonates that would die had no milk spots and breathed abnormally (Fig. 3A). Precise histochemical analyses of the entire set of neonates revealed that the lethality of AF5q31−/− neonates was potentially caused at least in part by severely shrunken alveoli of the lung (Fig. 3C), compared with the lungs of wild-type mice (Fig. 3B).
TABLE 1.
Genotyping of staged embryos and newborn pups by AF5q31+/− intercrossing
| Embryonic stage | No. of embryos
|
Total | |||
|---|---|---|---|---|---|
| Progeny with the following genotypes:
|
Resorbed | ||||
| +/+ | +/− | −/− | |||
| 8.5 dpc | 14 | 11 | 9 (1a) | 34 | |
| 9.5 dpc | 4 (1a) | 9 | 10 | 23 | |
| 10.5 dpc | 9 (1a) | 14 (1a) | 8 (4a) | 1 | 32 |
| 12.5 dpc | 7 | 11 | 3 | 11 | 32 |
| Newborn | 44 (6b) | 115 (6b) | 24 (17b) | 183 | |
Number of growth-retarded embryos.
Number of neonates dead within 24 h of birth.
FIG. 3.
Macroscopic and microscopic analyses of AF5q31-deficient mice at different ages and the expression profiles of AF5q31 in the normal mouse embryos. (A) Gross morphology of neonatal littermates representing AF5q31+/+ (right), AF5q31+/− (center), and AF5q31−/− (left). (B and C) Histological sections of the lung from AF5q31+/+ (B) and AF5q31−/− (C) neonatal littermates stained with hematoxylin and eosin stain. (D) Gross morphology of the AF5q31+/+, AF5q31+/−, and AF5q31−/− embryos of a litter at 10.5 dpc. (E) Northern blot analysis of total RNAs (20 μg/lane) from each embryo stage of the wild-type mouse. The blot was hybridized to a radioactive AF5q31 probe. As a loading control, 18S and 28S rRNAs in total RNA are demonstrated.
When analyzed during gestation, AF5q31−/− mice accounted for 25% of all embryos at 10.5 dpc, demonstrating that disruption of the AF5q31 gene does not affect the viability of embryos until this stage (Table 1). However, growth retardations, but no obvious malformations, were macroscopically observed in 50% of the mutant embryos at 10.5 dpc (Fig. 3D), and these embryos were likely to be absorbed at 12.5 dpc, indicating that up to 50% of the mutant embryos were lethal around these periods. The expression pattern during mouse development was examined to identify the correct time at which AF5q31 expression occurs. Northern blot analysis on the RNAs from 4.5-dpc to 18.5-dpc mouse embryos revealed sustained expression of AF5q31 throughout embryogenesis, and the expression reached its highest level at 10.5 to 12.5 dpc (Fig. 3E). Hence, AF5q31 appears to be important for embryonic development in this period.
Failure of spermatogenesis in AF5q31−/− male mice.
AF5q31−/− male and female mice that survived for >2 months (13% of the AF5q31−/− mice of the C57BL/6/129 background and none of the inbred 129 background so far) seemed normal in health and behavior, and no abnormalities in any organ or tissue examined were found (data not shown), except for the testis (see below). Interestingly AF5q31−/− males were infertile whereas AF5q31−/− females were fertile. Essentially, identical results were obtained in both mouse lines derived from two independent ES cell clones. AF5q31+/− male mice exhibited normal fertility. To evaluate fertility in 9-week-old AF5q31 mutant male mice, each of the AF5q31+/+, AF5q31+/−, and AF5q31−/− mice was mated with 8-week-old C57BL/6 female mice (10, 26). Although AF5q31+/+ and AF5q31+/− male mice always gave vaginal plugs the morning after mating and impregnated their mates, some of the AF5q31−/− males failed to give vaginal plugs and all of the AF5q31−/− males could not impregnate their mates in three successive sets of 2-week pairings (Table 2). As a control, the same female mice (after 2 weeks of matings with AF5q31−/− male mice) were always impregnated after mating with C57BL/6 male mice.
TABLE 2.
Fertility assessment
| Mice | Avg. no. of litters
|
Vaginal pluga | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| AF5q31+/+ | 10 ± 0 | 6.5 ± 0.5 | 8.5 ± 0.5 | + |
| AF5q31+/− | 9 ± 1.0 | 7.5 ± 0.5 | 7 ± 0 | + |
| AF5q31−/− | 0 | 0 | 0 | ± |
+, always gave vaginal plugs; ±, some gave vaginal plugs and some did not.
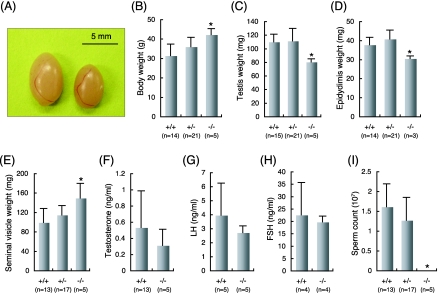
Phenotype analysis showed that there was no detectable difference in the morphology of urogenital tracts between the wild-type and mutant mice (data not shown), albeit the sizes of the testes and epididymides in AF5q31−/− mice were significantly smaller and the body weights and the sizes of seminal vesicles were larger than those of AF5q31+/+ and AF5q31+/− mice (Fig. 4A to E). Serum hormone assays showed that the levels of testosterone, LH, and FSH in AF5q31−/− mice were not significantly different from those in AF5q31+/+ mice in statistical analyses (Fig. 4F to H). Also, mRNAs for the androgen receptor (AR), LH-R, and FSH-R were equally expressed in the testes of AF5q31−/− and control littermates (see Fig. 6D). Consistent with the finding that AF5q31−/− mice were infertile, their seminal fluids were devoid of mature spermatozoa and only debris was present, indicating an arrest of spermatogenesis (Fig. 4I). The spermatozoa of the AF5q31+/− males displayed normal motility with no evident morphological abnormalities (data not shown).
FIG. 4.
Weights, hormone levels, and sperm counts in AF5q31−/− and control mice. (A) Testes from 24-week-old AF5q31+/+ (left) and AF5q31 −/− (right) male mice. (B to E) Weights of body and urogenital tracts of 12-week-old AF5q31+/+, AF5q31+/−, and AF5q31−/− male mice. (F to H) Serum testosterone, LH, and FSH levels in AF5q31+/+ and AF5q31−/− male mice. (I) Numbers of sperm cells prepared from 12-week-old AF5q31+/+, AF5q31+/−, and AF5q31−/− male mice. The data are given as averages. Error bars represent standard errors. Statistical significance (*, P < 0.01) in each assay was assessed using Student's t test between the wild-type and AF5q31−/− mice.
FIG. 6.
Mechanism of defective spermatogenesis in AF5q31-deficient mice. (A) Expression of AF5q31 in testes. Immunohistochemical staining was performed with an anti-mAF5q31-E4 antibody on sections of the testes from 12-week-old AF5q31+/+ and AF5q31−/− mice. Sections were counterstained with hematoxylin. Brown areas represent the positive signals. (B) RT-PCR analyses of AF5q31 expression using total RNAs isolated from the testes of 12-week-old AF5q31+/+ and AF5q31−/− male mice and 9-week-old W/Wv male mice. RT-PCR for GAPDH confirms the equivalent amounts of RNAs used for the analysis. (C) Expression of AF5q31 during juvenile testis development in mice. RT-PCR analyses of AF5q31 exons V to VIII and several marker genes in testis are demonstrated. RT-PCR for GAPDH confirms the equivalent amounts of RNAs used for the analysis. (D) Expression of spermatogenesis- and spermiogenesis-related genes in the testes of 12-week-old AF5q31+/+, AF5q31+/−, and AF5q31−/− male mice. RT-PCR for GAPDH confirms the equivalent amounts of RNAs used for the analysis. (E) RNAPII CTD phosphorylation in AF5q31+/+, AF5q31+/−, and AF5q31−/− MEFs. Whole-cell extracts (10 μg/lane) were immunoblotted with the indicated antibodies. As a control, anti-α-tubulin was used to monitor the loading amounts. (F) In vitro kinase assay of P-TEFb in the presence or absence of AF5q31. Chromatography of purified HA-AF5q31-Flag on a Mono Q column revealed the presence of full-length AF5q31 (140 kDa). Each fraction (4 μl) on the Mono Q column was analyzed by SDS-PAGE and silver staining (upper panel). The lane marked “In” represents a part of the material before loading the column, and the lane marked “FT” indicates the flowthrough of the Mono Q column. Equal aliquots from each fraction were added to the kinase reaction mixture containing P-TEFb and GST-CTD and resolved by SDS-PAGE. Phosphorylated GST-CTD was detected by autoradiography (lower panel).
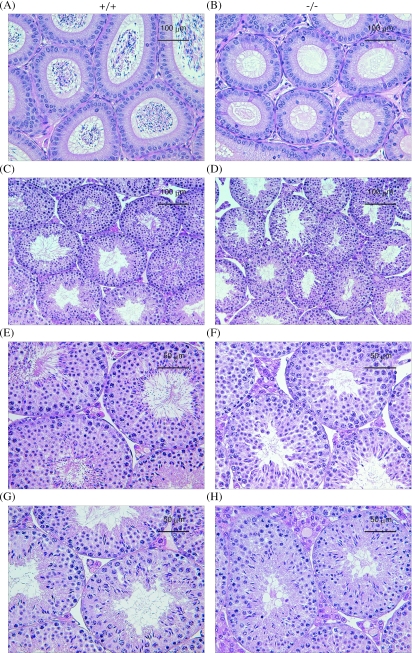
To verify the defect in the spermatogenesis of AF5q31−/− mice, testes were histologically analyzed. As expected, no sperm were found in the cauda epididymides of the AF5q31−/− mice, in contrast to those of the wild-type mice, which accounts for the infertility of the mutant mice (Fig. 5A and B). Detailed histological analysis revealed that round spermatids in the seminiferous tubules of the AF5q31−/− mice differentiated until at least step 11 but failed to undergo normal morphological change to elongated spermatids and to be released as spermatozoa within the germinal epithelium, while somatic Sertoli cells appeared morphologically normal (Fig. 5D, F, and H) and the morphology of seminiferous tubules in the mutant mice was indistinguishable from that of the wild-type mice. In contrast, most stages of the spermatogenic cycles in wild-type mice were represented (Fig. 5C, E, and G). Thus, spermatogenesis is arrested at the stage of spermiogenesis in AF5q31−/− mice.
FIG. 5.
Histology of epididymides and seminiferous tubules of AF5q31+/+ and AF5q31−/− male mice. The epididymal (A and B) and testicular (C to H) sections from 24-week-old AF5q31+/+ (A, C, E, and G) and AF5q31−/− (B, D, F, and H) male mice were stained with hematoxylin and eosin stain.
Mechanism of infertility in AF5q31−/− mice.
Immunohistochemical analysis on testes with a purified anti-AF5q31 antibody disclosed that AF5q31 was expressed preferentially in Sertoli cells, weakly in germ cells, and barely in Leydig cells (Fig. 6A). Consistent with this finding is that AF5q31 expression in RT-PCR analysis was elevated in c-kit mutant W/Wv male mice which harbor greatly reduced numbers of germ cells (38), compared with that in the mice with the normal c-kit gene (Fig. 6B). This pattern in RT-PCR analysis is similar to that of GATA1 which is expressed only in Sertoli cells in the testis (71) and is the opposite of that of the cyclic AMP-responsive element modulator (CREM), which is exclusively expressed in postmeiotic germ cells in the testis (5, 51) (Fig. 6B). Furthermore, early expression of AF5q31 during testis development also supports the preferential expression of AF5q31 in Sertoli cells (Fig. 6C). As Sertoli cells are known to regulate spermatogenesis through the interactions with germ cells (23, 62), we determined if the transcription of some of spermatogenesis-related genes would be deregulated in AF5q31−/− mice by RT-PCR assays (Fig. 6D). Four genes which have critical roles in transcriptional regulation, CREM, TBP-related factor 2 (TRF2), retinoic acid receptor α (RARα), and retinoid X receptor β (RXRβ), were normally expressed in the testes of AF5q31−/− mice (5, 36, 40, 43, 51, 74). Furthermore, testis-specific cytoplasmic poly(A) polymerase (Tpap), sperm outer dense fiber protein (RT7), heat shock protein Hsc70t, mitochondria capsule selenoprotein (Mcs), and phosphoglycerate kinase-2 (Pgk2), which are known to be expressed in spermiogenesis, were not significantly changed, except for a slight decrease of Mcs in the mutant testes (35). After meiosis, histones are replaced by protamines (protamines 1 and 2 [Prm1 and Prm2, respectively]) through transition proteins (transition proteins 1 and 2 [TP1 and TP2, respectively]) in order to package the haploid genome within the sperm head in mammals (61). Intriguingly, expression levels of TP2, Prm1, and Prm2 were drastically decreased and that of TP1 was slightly decreased in AF5q31−/− testes. But the expression levels of Ca2+/calmodulin-dependent protein kinase IV (Camk4), which is expressed in spermatids and phosphorylates Prm2, did not differ among AF5q31+/+, AF5q31+/−, and AF5q31−/− mice (68, 69).
One report demonstrated that AF5q31 is associated with P-TEFb and may contribute to regulate RNAPII processivity by phosphorylation of the CTD (20). To monitor RNAPII phosphorylation in MEFs derived from AF5q31+/+, AF5q31+/−, and AF5q31−/− embryos, we did Western blotting with antibodies N20, H5, and H14 that recognize both the IIo and IIa RNAPII, Ser2, and Ser5 CTD phosphopeptides of RNAPII, respectively. Although the IIo form predominantly existed in MEFs, the proportion of the IIo to IIa form was not distinctly changed among AF5q31+/+, AF5q31+/−, and AF5q31−/− MEFs (Fig. 6E). The reason for this may relate to the compensation by other factors, including AF4, LAF4, and FMR2, in the absence of AF5q31. To assess the effect of AF5q31 on P-TEFb, an in vitro kinase assay was performed using reconstitution proteins. To obtain a sufficient quantity of AF5q31 for further biochemical studies, whole-cell lysates of Sf9 cells expressing epitope-tagged AF5q31 (N-terminal HA tag and C-terminal FLAG tag) were purified by immunoaffinity chromatography using anti-Flag and anti-HA antibody columns, successively. Epitope-tagged AF5q31 proteins were allowed to bind to a Mono Q column and were then eluted with a linear gradient from 200 mM to 400 mM KCl (Fig. 6F, upper panel). Fractions peaking from 320 to 380 mM KCl (fractions 7 to 9) were found to contain AF5q31. The activities of each eluate were compared by the CTD in vitro kinase assay (66, 67). However, the CTD phosphorylations corresponding to fractions 7 to 9 were not significantly changed from those corresponding to the other fractions (Fig. 6F, lower panel). These results suggested that AF5q31 regulates spermiogenesis through the modulation of tissue-specific gene expression in Sertoli cells rather than affecting general transcriptional machinery.
Germ cell apoptosis in AF5q31−/− mice.
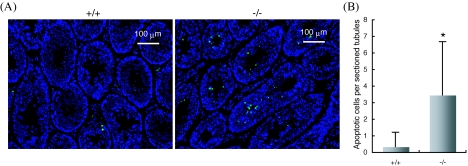
To further clarify why AF5q31−/− mice were infertile and azoospermatic, the frequency of apoptotic cells in testes was compared between AF5q31+/+ and AF5q31−/− mice by using a TUNEL assay (Fig. 7A). This assay revealed a 6.5-fold increase in apoptotic germ cells in seminiferous tubules in 12-week-old mutant mice, yet these were barely detectable in wild-type littermates (Fig. 7B). Hence, AF5q31 appears to be essential in both the differentiation program and the survival of germ cells.
FIG. 7.
Germ cell apoptosis in AF5q31+/+ and AF5q31−/− mice. (A) Apoptotic cells detected by an in situ TUNEL assay in testis sections from 12-week-old AF5q31+/+ (left) and AF5q31−/− (right) mice. TUNEL-positive cells were seen with fluorescein isothiocyanate (green). All the cells were visualized with DAPI (blue). (B) Quantification of apoptotic germ cells in the seminiferous tubules of 12-week-old AF5q31+/+ and AF5q31−/− mice. In each testis, TUNEL-positive (apoptotic) nuclei in more than 100 randomly sectioned seminiferous tubules were counted and averaged. Error bars represent standard errors. Statistical significance (*, P < 0.01) was assessed by Student's t test.
DISCUSSION
Incomplete penetrance of the embryonic and neonatal lethality observed in AF5q31-deficient mice indicates that the loss of AF5q31 does not cause a complete and uniform block of embryogenesis at a given point but that AF5q31 possesses versatile roles during embryogenesis. Since AF5q31 and AF4 are widely expressed during embryogenesis and in the adult tissues of mice, it is possible that AF4 functionally compensates for the lack of AF5q31 in most tissues (4, 33). Presently, it is unclear why the embryonic and neonatal death occurs and whether the incomplete penetrance of this phenotype results from heterogeneity in the genetic background of the mutant mice.
Spermatogenesis is a multistep process from spermatogonia, which are the stem cells of the germ cell lineage, to spermatozoa (14). Sertoli cells play major roles in supporting spermatogenesis, which involves the complex interaction of germ cells and Sertoli cells within the seminiferous tubules (23, 62), and Leydig cells produce the testosterone. The expression of AF5q31 in Sertoli cells without the expression of other family genes in the testis suggests an indispensable role for AF5q31 in the testis. It should be kept in mind that serum levels of testosterone, LH, and FSH and expression levels of AR, LH-R, and FSH-R did not show any significant difference between the wild-type and AF5q31−/− mice. Thus, azoospermia in AF5q31−/− mice seems to be caused by functional defects in testicular somatic cells, particularly Sertoli cells. Several reports suggested that abnormal Sertoli cells were impaired regarding the ability to assist the normal maturation and release of spermatids in the deficient mice for the nuclear receptors and related cofactors such as RARα, RXRβ, AR, and Cnot7 (10, 15, 30, 36, 40, 48). It is possible that AF5q31 functions as a coregulator of these transcription factors in spermatogenesis.
Human infertility affects 10 to 15% of couples, with an approximately equal contribution from both partners (16). In a large number of male infertility patients, the cause of the infertility might be related to disturbances in the replacement of histones by protamines during spermatogenesis. Previous reports stated that sperm from sterile males shows abnormal protein contents, with anomalously elevated levels of histones and/or an altered protamine 1/2 ratio (3, 11, 17). In mice and humans, genes encoding Prm1, Prm2, and TP2 are clustered together on chromosome 16 (52). In addition, these three genes lie in the same orientation to one another and are coordinately expressed in a haploid-specific manner during spermatogenesis. Notwithstanding the subtle decrease of TP1 expression, the levels of TP2, Prm1, and Prm2 were dramatically reduced in AF5q31−/− mice. Previous studies demonstrated that the transcription of transition proteins and protamines initiates shortly after the completion of meiosis in round spermatids (after step 7 in spermiogenesis) and ceases in elongating spermatids (step 11) with a global repression of transcription (37, 42). In addition, the haplo-insufficient chimeras of Prm1 and Prm2 were infertile, displaying an abnormal nuclear condensation (12). Thus, the reduced levels of TP2, Prm1, and Prm2 may be the cause of spermiogenesis arrest in AF5q31−/− mice.
Selective decreases in the levels of mRNAs of TP2, Prm1, and Prm2 among a set of postmeiotic genes in germ cells raise the possibility that AF5q31 also directly regulates the transcription of these genes. In fact, AF5q31 is weakly expressed in germ cells. It remains to be determined if Sertoli cells and germ cells are independently affected by the lack of AF5q31 or whether germ cells are secondarily affected, or both. Clarification of a potential role for AF5q31 in regulating the expression levels of TP2, Prm1, and Prm2 may provide new insights into the mechanisms of human male infertility.
ALLs are characterized by the clonal proliferation, accumulation, and tissue infiltration of neoplastic cells (21). The majority of cases of ALL demonstrate abnormal karyotypes, either in chromosome number or as structural changes such as translocations, inversions, or deletions. As a consequence of translocations between chromosomes 5 and 11, the reciprocal fusion gene is generated and it encodes the MLL-AF5q31 fusion protein, which is expressed in the leukemic blasts (63). It is unknown whether the fusion protein can act as a dominant negative product on AF5q31 function in the leukemic blasts. However, the fact that AF5q31−/− mice did not show any hematological abnormalities suggests that the dominant negative effects of this fusion protein on AF5q31 in leukemogenesis are less likely. It is more likely that MLL-AF5q31 fusion leads to constitutive activation of the MLL target genes (1, 27). Clarification of the AF5q31-mediated gene regulation in testes will also help us to elucidate the molecular mechanism by which the fusion converts normal MLL into the leukemogenic form.
Acknowledgments
We are grateful to T. Nakamura and T. Noce for useful technical advice and discussions; to M. Sakaki, N. Kakuta, N. Iwamori, J. Kato, and members of the H. Handa laboratory for excellent technical support; and to S. Mori, T. Nakajima, H. Onoda, and N. Oyaizu for invaluable histological analysis. We also thank members of the T. Kitamura laboratory for helpful suggestions.
The Division of Hematopoietic Factors is supported by the Chugai Pharmaceutical Company, Ltd.
REFERENCES
- 1.Armstrong, S. A., J. E. Staunton, L. B. Silverman, R. Pieters, M. L. den Boer, M. D. Minden, S. E. Sallan, E. S. Lander, T. R. Golub, and S. J. Korsmeyer. 2002. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat. Genet. 30:41-47. [DOI] [PubMed] [Google Scholar]
- 2.Ayton, P. M., and M. L. Cleary. 2001. Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins. Oncogene 20:5695-5707. [DOI] [PubMed] [Google Scholar]
- 3.Balhorn, R., S. Reed, and N. Tanphaichitr. 1988. Aberrant protamine 1/protamine 2 ratios in sperm of infertile human males. Experientia 44:52-55. [DOI] [PubMed] [Google Scholar]
- 4.Baskaran, K., F. Erfurth, G. Taborn, N. G. Copeland, D. J. Gilbert, N. A. Jenkins, P. M. Iannaccone, and P. H. Domer. 1997. Cloning and developmental expression of the murine homolog of the acute leukemia proto-oncogene AF4. Oncogene 15:1967-1978. [DOI] [PubMed] [Google Scholar]
- 5.Blendy, J. A., K. H. Kaestner, G. F. Weinbauer, E. Nieschlag, and G. Schutz. 1996. Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature 380:162-165. [DOI] [PubMed] [Google Scholar]
- 6.Bursen, A., S. Moritz, A. Gaussmann, S. Moritz, T. Dingermann, and R. Marschalek. 2004. Interaction of AF4 wild-type and AF4.MLL fusion protein with SIAH proteins: indication for t(4;11) pathobiology? Oncogene 23:6237-6249. [DOI] [PubMed] [Google Scholar]
- 7.Canaani, E., P. C. Nowell, and C. M. Croce. 1995. Molecular genetics of 11q23 chromosome translocations. Adv. Cancer Res. 66:213-234. [DOI] [PubMed] [Google Scholar]
- 8.Chakrabarti, L., S. J. Knight, A. V. Flannery, and K. E. Davies. 1996. A candidate gene for mild mental handicap at the FRAXE fragile site. Hum. Mol. Genet. 5:275-282. [DOI] [PubMed] [Google Scholar]
- 9.Chakrabarti, L., J. Bristulf, G. S. Foss, and K. E. Davies. 1998. Expression of the murine homologue of FMR2 in mouse brain and during development. Hum. Mol. Genet. 7:441-448. [DOI] [PubMed] [Google Scholar]
- 10.Chang, C., Y. T. Chen, S. D. Yeh, Q. Xu, R. S. Wang, F. Guillou, H. Lardy, and S. Yeh. 2004. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc. Natl. Acad. Sci. USA 101:6876-6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chevaillier, P., N. Mauro, D. Feneux, P. Jouannet, and G. David. 1987. Anomalous protein complement of sperm nuclei in some infertile men. Lancet 2:806-807. [DOI] [PubMed] [Google Scholar]
- 12.Cho, C., W. D. Willis, E. H. Goulding, H. Jung-Ha, Y. C. Choi, N. B. Hecht, and E. M. Eddy. 2001. Haploinsufficiency of protamine-1 or -2 causes infertility in mice. Nat. Genet. 28:82-86. [DOI] [PubMed] [Google Scholar]
- 13.Collins, E. C., and T. H. Rabbitts. 2002. The promiscuous MLL gene links chromosomal translocations to cellular differentiation and tumour tropism. Trends Mol. Med. 8:436-442. [DOI] [PubMed] [Google Scholar]
- 14.Cooke, H. J., and P. T. Saunders. 2002. Mouse models of male infertility. Nat. Rev. Genet. 3:790-801. [DOI] [PubMed] [Google Scholar]
- 15.De Gendt, K., J. V. Swinnen, P. T. Saunders, L. Schoonjans, M. Dewerchin, A. Devos, K. Tan, N. Atanassova, F. Claessens, C. Lecureuil, W. Heyns, P. Carmeliet, F. Guillou, R. M. Sharpe, and G. Verhoeven. 2004. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc. Natl. Acad. Sci. USA 101:1327-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Kretser, D. M., and H. W. Baker. 1999. Infertility in men: recent advances and continuing controversies. J. Clin. Endocrinol. Metab. 84:3443-3450. [DOI] [PubMed] [Google Scholar]
- 17.de Yebra, L., J. L. Ballesca, J. A. Vanrell, L. Bassas, and R. Oliva. 1993. Complete selective absence of protamine P2 in humans. J. Biol. Chem. 268:10553-10557. [PubMed] [Google Scholar]
- 18.Djabali, M., L. Selleri, P. Parry, M. Bower, B. D. Young, and G. A. Evans. 1992. A trithorax-like gene is interrupted by chromosome 11q23 translocations in acute leukaemias. Nat. Genet. 2:113-118. [DOI] [PubMed] [Google Scholar]
- 19.Domer, P. H., S. S. Fakharzadeh, C. S. Chen, J. Jockel, L. Johansen, G. A. Silverman, J. H. Kersey, and S. J. Korsmeyer. 1993. Acute mixed-lineage leukemia t(4;11)(q21;q23) generates an MLL-AF4 fusion product. Proc. Natl. Acad. Sci. USA 90:7884-7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estable, M. C., M. H. Naghavi, H. Kato, H. Xiao, J. Qin, A. Vahlne, and R. G. Roeder. 2002. MCEF, the newest member of the AF4 family of transcription factors involved in leukemia, is a positive transcription elongation factor-b-associated protein. J. Biomed. Sci. 9:234-245. [DOI] [PubMed] [Google Scholar]
- 21.Faderl, S., H. M. Kantarjian, M. Talpaz, and Z. Estrov. 1998. Clinical significance of cytogenetic abnormalities in adult acute lymphoblastic leukemia. Blood 91:3995-4019. [PubMed] [Google Scholar]
- 22.Gecz, J., A. K. Gedeon, G. R. Sutherland, and J. C. Mulley. 1996. Identification of the gene FMR2, associated with FRAXE mental retardation. Nat. Genet. 13:105-108. [DOI] [PubMed] [Google Scholar]
- 23.Griswold, M. D. 1995. Interactions between germ cells and Sertoli cells in the testis. Biol. Reprod. 52:211-216. [DOI] [PubMed] [Google Scholar]
- 24.Gu, Y., T. Nakamura, H. Alder, R. Prasad, O. Canaani, G. Cimino, C. M. Croce, and E. Canaani. 1992. The t(4;11) chromosome translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell 71:701-708. [DOI] [PubMed] [Google Scholar]
- 25.Gu, Y., Y. Shen, R. A. Gibbs, and D. L. Nelson. 1996. Identification of FMR2, a novel gene associated with the FRAXE CCG repeat and CpG island. Nat. Genet. 13:109-113. [DOI] [PubMed] [Google Scholar]
- 26.Guerif, F., V. Cadoret, M. Plat, M. Magistrini, J. Lansac, M. T. Hochereau-De Reviers, and D. Royere. 2002. Characterization of the fertility of Kit haplodeficient male mice. Int. J. Androl. 25:358-368. [DOI] [PubMed] [Google Scholar]
- 27.Hess, J. L. 2004. MLL: a histone methyltransferase disrupted in leukemia. Trends Mol. Med. 10:500-507. [DOI] [PubMed] [Google Scholar]
- 28.Hillman, M. A., and J. Gecz. 2001. Fragile XE-associated familial mental retardation protein 2 (FMR2) acts as a potent transcription activator. J. Hum. Genet. 46:251-259. [DOI] [PubMed] [Google Scholar]
- 29.Hiwatari, M., T. Taki, T. Taketani, M. Taniwaki, K. Sugita, M. Okuya, M. Eguchi, K. Ida, and Y. Hayashi. 2003. Fusion of an AF4-related gene, LAF4, to MLL in childhood acute lymphoblastic leukemia with t(2;11)(q11;q23). Oncogene 22:2851-2855. [DOI] [PubMed] [Google Scholar]
- 30.Holdcraft, R. W., and R. E. Braun. 2004. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development 131:459-467. [DOI] [PubMed] [Google Scholar]
- 31.Huret, J. L., P. Dessen, and A. Bernheim. 2001. An atlas of chromosomes in hematological malignancies. Example: 11q23 and MLL partners. Leukemia 15:987-989. [DOI] [PubMed] [Google Scholar]
- 32.Isaacs, A. M., P. L. Oliver, E. L. Jones, A. Jeans, A. Potter, B. H. Hovik, P. M. Nolan, L. Vizor, P. Glenister, A. K. Simon, I. C. Gray, N. K. Spurr, S. D. Brown, A. J. Hunter, and K. E. Davies. 2003. A mutation in Af4 is predicted to cause cerebellar ataxia and cataracts in the robotic mouse. J. Neurosci. 23:1631-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isnard, P., D. Depetris, M. G. Mattei, P. Ferrier, and M. Djabali. 1998. cDNA cloning, expression and chromosomal localization of the murine AF-4 gene involved in human leukemia. Mamm. Genome 9:1065-1068. [DOI] [PubMed] [Google Scholar]
- 34.Isnard, P., N. Core, P. Naquet, and M. Djabali. 2000. Altered lymphoid development in mice deficient for the mAF4 proto-oncogene. Blood 96:705-710. [PubMed] [Google Scholar]
- 35.Kashiwabara, S., J. Noguchi, T. Zhuang, K. Ohmura, A. Honda, S. Sugiura, K. Miyamoto, S. Takahashi, K. Inoue, A. Ogura, and T. Baba. 2002. Regulation of spermatogenesis by testis-specific, cytoplasmic poly(A) polymerase TPAP. Science 298:1999-2002. [DOI] [PubMed] [Google Scholar]
- 36.Kastner, P., M. Mark, M. Leid, A. Gansmuller, W. Chin, J. M. Grondona, D. Decimo, W. Krezel, A. Dierich, and P. Chambon. 1996. Abnormal spermatogenesis in RXR beta mutant mice. Genes Dev. 10:80-92. [DOI] [PubMed] [Google Scholar]
- 37.Kierszenbaum, A. L., and L. L. Tres. 1975. Structural and transcriptional features of the mouse spermatid genome. J. Cell Biol. 65:258-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurohmaru, M., Y. Kanai, and Y. Hayashi. 1992. A cytological and cytoskeletal comparison of Sertoli cells without germ cell and those with germ cells using the W/Wv mutant mouse. Tissue Cell 24:895-903. [DOI] [PubMed] [Google Scholar]
- 39.Kuzin, B., S. Tillib, Y. Sedkov, L. Mizrokhi, and A. Mazo. 1994. The Drosophila trithorax gene encodes a chromosomal protein and directly regulates the region-specific homeotic gene fork head. Genes Dev. 8:2478-2490. [DOI] [PubMed] [Google Scholar]
- 40.Lufkin, T., D. Lohnes, M. Mark, A. Dierich, P. Gorry, M. P. Gaub, M. LeMeur, and P. Chambon. 1993. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc. Natl. Acad. Sci. USA 90:7225-7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma, C., and L. M. Staudt. 1996. LAF-4 encodes a lymphoid nuclear protein with transactivation potential that is homologous to AF-4, the gene fused to MLL in t(4;11) leukemias. Blood 87:734-745. [PubMed] [Google Scholar]
- 42.Mali, P., A. Kaipia, M. Kangasniemi, J. Toppari, M. Sandberg, N. B. Hecht, and M. Parvinen. 1989. Stage-specific expression of nucleoprotein mRNAs during rat and mouse spermiogenesis. Reprod. Fertil. Dev. 1:369-382. [DOI] [PubMed] [Google Scholar]
- 43.Martianov, I., G. M. Fimia, A. Dierich, M. Parvinen, P. Sassone-Corsi, and I. Davidson. 2001. Late arrest of spermiogenesis and germ cell apoptosis in mice lacking the TBP-like TLF/TRF2 gene. Mol. Cell 7:509-515. [DOI] [PubMed] [Google Scholar]
- 44.Mazo, A. M., D. H. Huang, B. A. Mozer, and I. B. Dawid. 1990. The trithorax gene, a trans-acting regulator of the bithorax complex in Drosophila, encodes a protein with zinc-binding domains. Proc. Natl. Acad. Sci. USA 87:2112-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milne, T. A., S. D. Briggs, H. W. Brock, M. E. Martin, D. Gibbs, C. D. Allis, and J. L. Hess. 2002. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol. Cell 10:1107-1117. [DOI] [PubMed] [Google Scholar]
- 46.Morrissey, J., D. C. Tkachuk, A. Milatovich, U. Francke, M. Link, and M. L. Cleary. 1993. A serine/proline-rich protein is fused to HRX in t(4;11) acute leukemias. Blood 81:1124-1131. [PubMed] [Google Scholar]
- 47.Mozer, B. A., and I. B. Dawid. 1989. Cloning and molecular characterization of the trithorax locus of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 86:3738-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamura, T., R. Yao, T. Ogawa, T. Suzuki, C. Ito, N. Tsunekawa, K. Inoue, R. Ajima, T. Miyasaka, Y. Yoshida, A. Ogura, K. Toshimori, T. Noce, T. Yamamoto, and T. Noda. 2004. Oligo-astheno-teratozoospermia in mice lacking Cnot7, a regulator of retinoid X receptor beta. Nat. Genet. 36:528-533. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura, T., H. Alder, Y., Gu, R. Prasad, O. Canaani, N. Kamada, R. P. Gale, B. Lange, W. M. Crist, and P. C. Nowell. 1993. Genes on chromosomes 4, 9, and 19 involved in 11q23 abnormalities in acute leukemia share sequence homology and/or common motifs. Proc. Natl. Acad. Sci. USA 90:4631-4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura, T., T. Mori, S. Tada, W. Krajewski, T. Rozovskaia, R. Wassell, G. Dubois, A. Mazo, C. M. Croce, and E. Canaani. 2002. ALL-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol. Cell 10:1119-1128. [DOI] [PubMed] [Google Scholar]
- 51.Nantel, F., L. Monaco, N. S. Foulkes, D. Masquilier, M. LeMeur, K. Henriksen, A. Dierich, M. Parvinen, and P. Sassone-Corsi. 1996. Spermiogenesis deficiency and germ-cell apoptosis in CREM-mutant mice. Nature 380:159-162. [DOI] [PubMed] [Google Scholar]
- 52.Nelson, J. E., and S. A. Krawetz. 1993. Linkage of human spermatid-specific basic nuclear protein genes. Definition and evolution of the P1→P2→TP2 locus. J. Biol. Chem. 268:2932-2936. [PubMed] [Google Scholar]
- 53.Nilson, I., M. Reichel, M. G. Ennas, R. Greim, C. Knorr, G. Siegler, J. Greil, G. H. Fey, and R. Marschalek. 1997. Exon/intron structure of the human AF-4 gene, a member of the AF-4/LAF-4/FMR-2 gene family coding for a nuclear protein with structural alterations in acute leukaemia. Br. J. Haematol. 98:157-169. [DOI] [PubMed] [Google Scholar]
- 54.Nosaka, T., J. M. van Deursen, R. A. Tripp, W. E. Thierfelder, B. A. Witthuhn, A. P. McMickle, P. C. Doherty, G. C. Grosveld, and J. N. Ihle. 1995. Defective lymphoid development in mice lacking Jak3. Science 270:800-802. [DOI] [PubMed] [Google Scholar]
- 55.Nosaka, T., S. Morita, H. Kitamura, H. Nakajima, F. Shibata, Y. Morikawa, Y. Kataoka, Y. Ebihara, T. Kawashima, T. Itoh, K. Ozaki, E. Senba, K. Tsuji, F. Makishima, N. Yoshida, and T. Kitamura. 2003. Mammalian twisted gastrulation is essential for skeleto-lymphogenesis. Mol. Cell. Biol. 23:2969-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nosaka, T., T. Kawashima, K. Misawa, K. Ikuta, A. L.-F. Mui, and T. Kitamura. 1999. STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J. 18:4654-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oliver, P. L., E. Bitoun, J. Clark, E. L. Jones, and K. E. Davies. 2004. Mediation of Af4 protein function in the cerebellum by Siah proteins. Proc. Natl. Acad. Sci. USA 101:14901-14906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prasad, R., T. Yano, C. Sorio, T. Nakamura, R. Rallapalli, Y. Gu, D. Leshkowitz, C. M. Croce, and E. Canaani. 1995. Domains with transcriptional regulatory activity within the ALL1 and AF4 proteins involved in acute leukemia. Proc. Natl. Acad. Sci. USA 92:12160-12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rabbitts, T. H. 1994. Chromosomal translocations in human cancer. Nature 372:143-149. [DOI] [PubMed] [Google Scholar]
- 60.Rowley, J. D. 2001. Chromosome translocations: dangerous liaisons revisited. Nat. Rev. Cancer 1:245-250. [DOI] [PubMed] [Google Scholar]
- 61.Sassone-Corsi, P. 2002. Unique chromatin remodeling and transcriptional regulation in spermatogenesis. Science 296:2176-2178. [DOI] [PubMed] [Google Scholar]
- 62.Siu, M. K., and C. Y. Cheng. 2004. Dynamic cross-talk between cells and the extracellular matrix in the testis. Bioessays 26:978-992. [DOI] [PubMed] [Google Scholar]
- 63.Taki, T., H. Kano, M. Taniwaki, M. Sako, M. Yanagisawa, and Y. Hayashi. 1999. AF5q31, a newly identified AF4-related gene, is fused to MLL in infant acute lymphoblastic leukemia with ins(5;11)(q31;q13q23). Proc. Natl. Acad. Sci. USA 96:14535-14540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tkachuk, D. C., S. Kohler, and M. L. Cleary. 1992. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell 7:691-700. [DOI] [PubMed] [Google Scholar]
- 65.von Bergh, A. R., H. B. Beverloo, P. Rombout, E. R. van Wering, M. H. van Weel, G. C. Beverstock, P. M. Kluin, R. M. Slater, and E. Schuuring. 2002. LAF4, an AF4-related gene, is fused to MLL in infant acute lymphoblastic leukemia. Genes Chromosomes Cancer 35:92-96. [DOI] [PubMed] [Google Scholar]
- 66.Wada, T., T. Takagi, Y. Yamaguchi, A. Ferdous, T. Imai, S. Hirose, S. Sugimoto, K. Yano, G. A. Hartzog, F. Winston, S. Buratowski, and H. Handa. 1998. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev. 12:343-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wada, T., T. Takagi, Y. Yamaguchi, D. Watanabe, and H. Handa. 1998. Evidence that P-TEFb alleviates the negative effect of DSIF on RNA polymerase II-dependent transcription in vitro. EMBO J. 17:7395-7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu, J. Y., and A. R. Means. 2000. Ca(2+)/calmodulin-dependent protein kinase IV is expressed in spermatids and targeted to chromatin and the nuclear matrix. J. Biol. Chem. 275:7994-7999. [DOI] [PubMed] [Google Scholar]
- 69.Wu, J. Y., T. J. Ribar, D. E. Cummings, K. A. Burton, G. S. McKnight, and A. R. Means. 2000. Spermiogenesis and exchange of basic nuclear proteins are impaired in male germ cells lacking Camk4. Nat. Genet. 25:448-452. [DOI] [PubMed] [Google Scholar]
- 70.Yokoyama, A., Z. Wang, J. Wysocka, M. Sanyal, D. J. Aufiero, I. Kitabayashi, W. Herr, and M. L. Cleary. 2004. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol. Cell. Biol. 24:5639-5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yomogida, K., H. Ohtani, H. Harigae, E. Ito, Y. Nishimune, J. D. Engel, and M. Yamamoto. 1994. Developmental stage- and spermatogenic cycle-specific expression of transcription factor GATA-1 in mouse Sertoli cells. Development 120:1759-1766. [DOI] [PubMed] [Google Scholar]
- 72.Yu, B. D., J. L. Hess, S. E. Horning, G. A. Brown, and S. J. Korsmeyer. 1995. Altered Hox expression and segmental identity in Mll-mutant mice. Nature 378:505-508. [DOI] [PubMed] [Google Scholar]
- 73.Yu, B. D., R. D. Hanson, J. L. Hess, S. E. Horning, and S. J. Korsmeyer. 1998. MLL, a mammalian trithorax-group gene, functions as a transcriptional maintenance factor in morphogenesis. Proc. Natl. Acad. Sci. USA 95:10632-10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang, D., T. L. Penttila, P. L. Morri, M. Teichmann, and R. G. Roeder. 2001. Spermiogenesis deficiency in mice lacking the Trf2 gene. Science 292:1153-1155. [DOI] [PubMed] [Google Scholar]